Introduction
The burden of cervical cancer, which is the main
type of cancer related to human papillomavirus (HPV) infection, is
substantially higher in Central and Eastern Europe compared with
the rest of Europe, with increasing trends of incidence and
mortality in several countries. However, several gaps in knowledge
exist concerning the incidence and associated mortality of other
HPV-related cancers. The same is true for HPV prevalence and type
distribution among women with HPV-related cancers other than
cervical cancer and in the general female population (1).
Limited data are available regarding HPV incidence
in Romania and in terms of mortality due to cervical cancer,
Romania ranks first in Europe, recording 6.3-fold more deaths from
cervical cancer than the mean registered number in other European
countries. Although vaccination campaigns were launched by health
officials in Romania, the acceptance rate remained low and thus the
programs were discontinued. A successful vaccination program
requires a high rate of acceptance and accurate information for
health professionals and parents (2).
In 2014, Salavastru et al published an epidemiological study
stating that genital infection with HPV has become one of the most
frequently viral sexually transmitted diseases. The infection may
remain asymptomatic, may take the form of external genital warts or
may give raise to cervical cancers. The aim of that study was to
assess the frequency of patients with genital warts, obtaining data
from five tertiary referral dermatological units in Romania, and to
compare the results with outpatient data reported by all Romanian
hospitals (3). The study reported
data obtained for the year 2012, with 952 patients (731 females and
221 males) with 26 males under 20 years of age and 251 female
patients in the age group of 0–20 years. In the overall population
(males and females combined) the total number of genital warts
cases registered at the hospital emergency rooms in the five
centers, was approximately 30%. In that epidemiological study
though, no data were obtained regarding HPV cervical or urethral
genotyping, the incidence of high-risk HPV strains versus low-risk
strains and the possible effects on cervical cancer (3).
In this study, in order to establish the actual
high-risk strain distribution in a female population, we assessed
the incidence of HPV strains through HPV genotyping in patients
with genital warts to highlight the particular pattern of this
tumorigenesis related-virus in order to obtain a better
understanding of the possible effects in time upon
carcinogenesis.
Materials and methods
A total of 713 female patients with genital warts,
treated at Medicover Healthcare Centre (Bucharest, Romania), from
January to December, 2015 comprised the patient group and cervical
swabs were obtained from these patients. All patients provided
written informed consent prior to obtaining the samples, and this
study was approved by the Ethics Committee of Carol Medical Center,
Bucharest, Romania. Cervical cells were collected in
PreservCyt® Solution (Cytyc Corp., Boxborough, MA, USA)
and then analyzed. HPV genotyping was assessed using LINEAR
ARRAY® HPV Genotyping test (Roche Diagnostics,
Indianapolis, IN, USA). Briefly, the test detects HPV by the
amplification of target DNA using polymerase chain reaction (PCR)
and nucleic acid hybridization as follows:
The standard kit was developed according to
previously published protocols (4).
Briefly, the samples were prepared for PCR by standard protocols by
digestion with proteinase K and 1% Laureth-12, spun and heated to
95°C for 10 min residual protease denaturation. The samples were
centrifuged and 5 µl was used for each PCR assay. PCR amplification
was performed according to the manufacturer's recommendations.
Briefly, a working master mix was prepared and added into each
reaction tube. Subsequently, 50 µl of each processed sample was
added to the appropriate amplification tubes containing the working
master mix. The PCR cycling conditions were as indicated by the
manufacturer. Quality control consisted of 2 µl of the amplicon
electrophoresed on a 1.5% Flash gel (Lonza Inc., Rockland, ME, USA)
and if correct banding was present, genotyping followed. HPV
genotyping was performed according to the manufacturer's
recommendations. A total of 75 µl of denatured amplicon was added
to each standard labeled strip, and several incubation and washing
cycles were performed at 53°C for 30 min. Subsequently, 4 ml of
conjugate were added to each well containing a strip, followed by
incubation for 30 min. After washing, 4 ml of working substrate
were added to each well, followed by incubation. After the strips
were dried, the HPV genotyping calls were made using the detectable
hybridization bands provided by the manufacturer. The test detects
37 anogenital HPV DNA genotypes [6, 11, 16, 18, 26, 31, 33, 35, 39,
40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68,
69, 70, 71, 72, 73 (MM9), 81, 82 (MM4), 83 (MM7), 84 (MM8), IS39
and CP6108].
Statistical analysis was performed using ANOVA and a
value of P<0.05 was considered to indicate a statistically
significant difference. The results are presented as a percentage
of the distribution and age incidence in various groups using the
Prism and Excel programs.
Results
According to the literature (1–3), there is
a panel of HPV high-risk strains, namely 16, 18, 31, 33, 35, 39,
45, 51, 52, 56, 58, 59 and 68, while the panel comprising 26, 40,
42, 53, 54, 55, 61, 62, 64, 66 and 67 is considered low-risk. In
the patients enrolled in our study who were diagnosed with genital
warts, we found that although there was a clear-cut difference
between the groups with the high- versus the low-risk strains,
there are also a group of patients (not quite negligible) that had
both strain types.
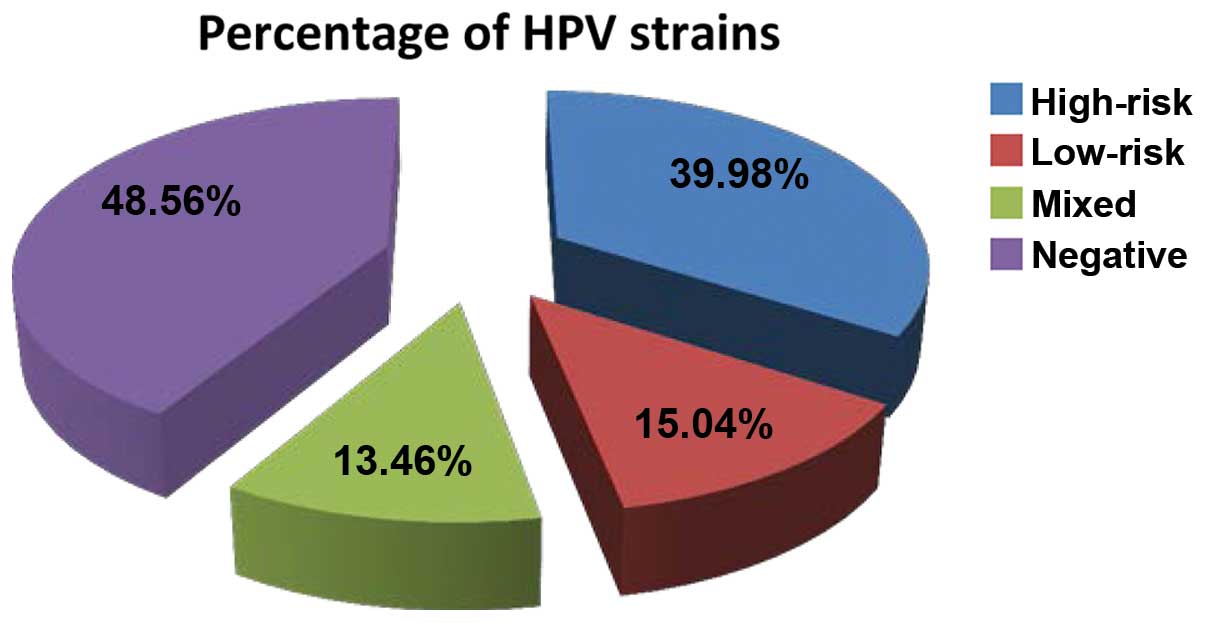
When assessing the samples of the patient group
(Fig. 1), approximately half of the
females examined tested as negative for any HPV strains, while the
low-risk strains only were found in approximately 15% of all cases.
Almost 85% of the study population tested positive either for the
high-risk strains or for mixed strains (high + low-risk strains).
This last subpopulation of tested females comprised the group that
had exhibited a risk pattern, and in terms of numbers, this covered
almost half of the population, this latter subpopulation being the
main group with a significant risk of developing cancerous
lesions.
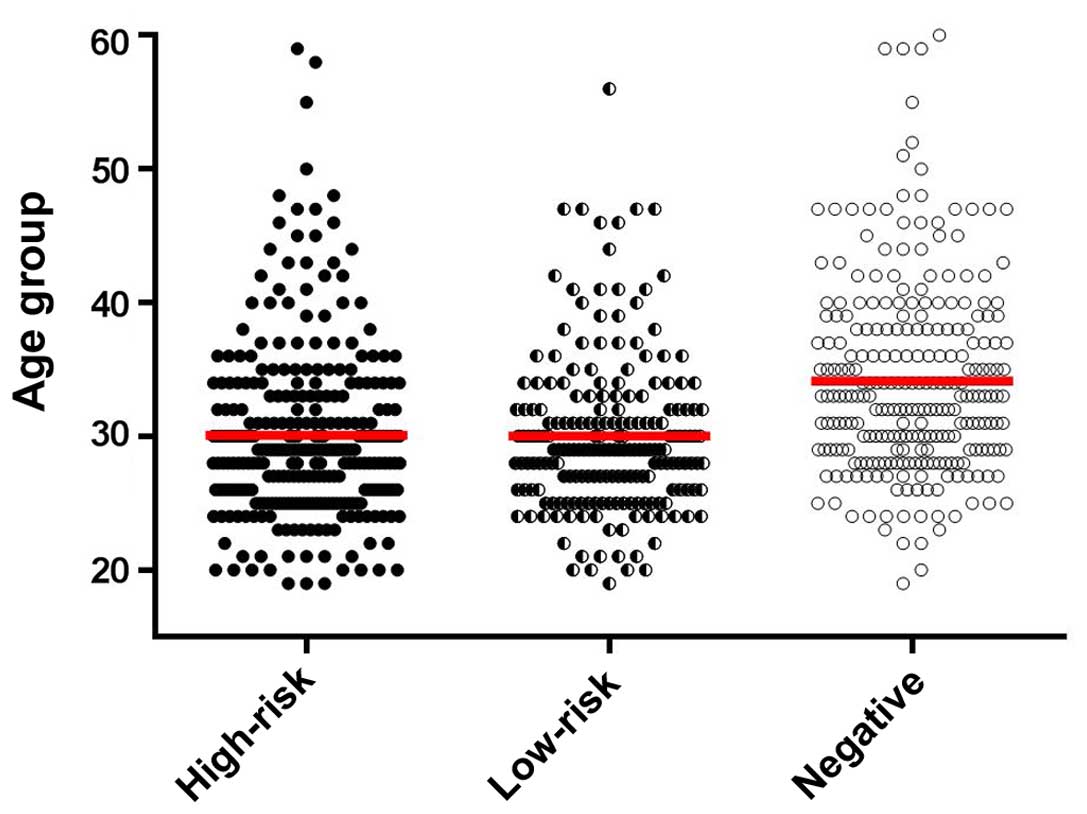
When assessing the age distribution of all the
tested female patients (Fig. 2), we
found that as regards the mean age of the low- and high-risk female
patient groups, there was no statistically significant difference,
while in the group that tested negative there is a clear-cut
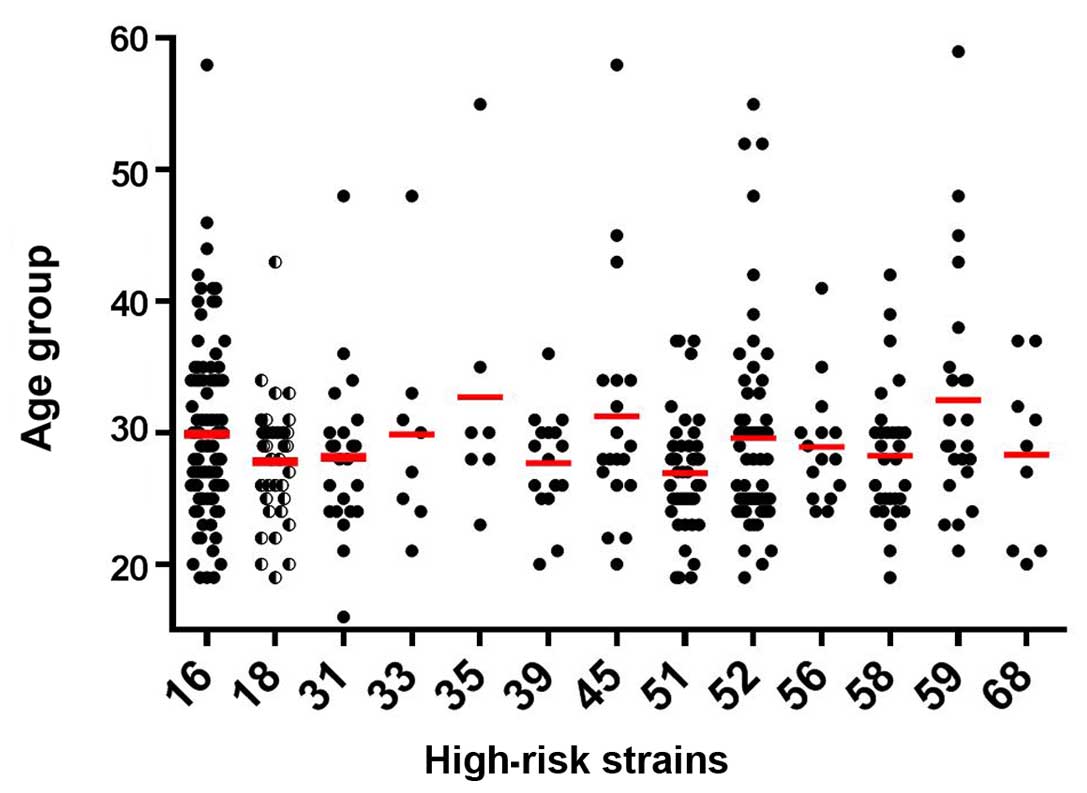
increase as in mean age. When examining the statistical
significance as regards the predisposition for cancer associated
with high-risk HPV in terms of female age (Fig. 3) we observed that there was a clear
tendency towards the high-risk HPV strains in patients <35 years
of age, with no actual preponderance among the different high-risk
strains.
Discussion
Over the past years, the healthcare system and
pharmaceutical industry are facing major challenges due to the
economic and social changes (5). In
this context, in 2013, two analyses developed under the aegis of
the Center for Disease and Control and Prevention (CDC) highlighted
the severe human and economic burden associated with sexually
transmitted infections (STIs) in the United States (6,7). These two
studies estimated the number of new STI cases per year in the
United States to be approximately 20 million, and estimated the
total lifetime direct medical costs for these new cases of STIs to
reach to almost 16 billion USD. While most of these STIs will not
cause harm, some have the potential to cause serious health
problems, particularly if not diagnosed and treated at an early
stage (8). In our study, in our
female population, the high-risk HPV strains were found in the
patients who were <35 years of age. While the vast majority of
HPV infections will self heal within 2 years and cause no harm,
some of these infections will take hold and potentially lead to
serious disease, including cervical cancer (8,9). Although
there is also no generally accepted definition of HPV persistence
(10), the most commonly used
definition is two or more HPV DNA-positive tests during the
follow-up (11,12).
HPV16 and 18 are the predominant oncogenic
genotypes, involved in the development of 70% of global cervical
cancer cases (13,14), with the exception of women infected
with HIV, in whom HPV58 is reported to be the second most dominant
strain behind HPV16 (15). In our
study, no HIV+ subjects were enrolled and the
predominant strain in female subjects was strain 16, followed by
strains 52, 51 and 18. It has been reported that the majority of
young females with oral HPV infection have cervical HPV infection
with type concordance and dominance of HPV16 (16,17). In
this aspect, the continuation of our study will be the
identification of high-risk strains in both cervical and oral
sites. As the literature is citing extremely diverse associations,
different results in studies may be due to the different
populations, biological sampling methods and the assays used
(16,18).
However, for neoplastic transformation to take
place, apart from HPV infection, a number of putative viral, host
and environmental co-factors, such as ionizing irradiation, UV
exposure, as well as mechanical and chemical stresses are required
(19). As we found in our female
population, there was a significant proportion of subjects
exhibiting a combination of high- and low-risk HPV strains; thus,
we cannot rule out the possibility of the association of low-risk
strains with the risk of neoplastic transformation.
HPV is a leading cause of anogenital malignancies
and a role of HPV in the etiology of oro-pharyngeal cancers has
been demonstrated. The frequency of oral HPV infection in patients
with genital warts and the association between concomitant genital,
anal and oral infection is unclear. Kofoed et al recruited a
total of 201 men and women with genital wart-like lesions. Swab
samples were obtained from the genital warts and the anal canal and
an oral rinse was collected. Anal HPV was found in 46.2% and oral
HPV in 10.4% of the participants. The concordance between anal and
genital wart HPV types was 78.1%, while the concordance between
oral and genital wart types was 60.9%. A lower concordance of 21.7%
was observed between the anal and oral HPV types. Significantly
more women than men had multiple HPV types and anal HPV (20). In our study, all enrolled patients
were diagnosed with genital warts.
In conclusion, in this study >700 female subjects
were tested for high- and low-risk genital HPV. We found that the
high-risk strains were mainly identified in the patients <35
years of age. The incidence of HPV in the human cancer domain is
still a subject of intensive investigation. There are areas in the
field of HPV infection in which there are still ongoing debates and
conclusions have not yet been reached. For example, there is still
debate as to the involvement of stresses in triggering
dermatological conditions (21)
and/or the involvement of stresses in triggering an HPV+
lesion to evolve into neoplasia (22–24).
Chemically-induced pro-tumoral factors, drugs and environmental
milieu (25), represent another
domain that could explain HPV+-triggering conditions.
Last but not least, the omics domain (26) may bring additional data into this
field, as there are data showing a pro-tumoral association of
HPV+ lesions with miRNA profiles and/or particular
proteomics patterns (27).
Acknowledgements
The authors would like to thank the Medicover
Healthcare Centre (Bucharest, Romania) for granting us access to
their database.
References
|
1
|
Škamperle M, Kocjan BJ, Maver PJ, Seme K
and Poljak M: Human papillomavirus (HPV) prevalence and HPV type
distribution in cervical, vulvar, and anal cancers in central and
eastern Europe. Acta Dermatovenerol Alp Pannonica Adriat. 22:1–5.
2013.PubMed/NCBI
|
|
2
|
Voidăzan S, Tarcea M, Morariu SH, Grigore
A and Dobreanu M: Human papillomavirus vaccine - knowledge and
attitudes among parents of children aged 10–14 years: A
cross-sectional study, Tirgu Mures, Romania. Cent Eur J Public
Health. 24:29–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salavastru CM, Niculescu MC, Zota A,
Nicola G, Morariu HS, Solovan C, Patrascu V, Popovici G, Vladuta R,
Panduru M, et al: Epidemiological aspects of genital warts in
romania - a 2012 retrospective survey. Maedica (Buchar). 9:144–150.
2014.PubMed/NCBI
|
|
4
|
Gravitt PE, Peyton CL, Alessi TQ, Wheeler
CM, Coutlée F, Hildesheim A, Schiffman MH, Scott DR and Apple RJ:
Improved amplification of genital human papillomaviruses. J Clin
Microbiol. 38:357–361. 2000.PubMed/NCBI
|
|
5
|
Raţiu MP, Purcărea I, Popa F, Purcărea VL,
Purcărea TV, Lupuleasa D and Boda D: Escaping the economic turn
down through performing employees, creative leaders and growth
driver capabilities in the Romanian pharmaceutical industry.
Farmacia. 59:119–130. 2011.
|
|
6
|
Satterwhite CL, Torrone E, Meites E, Dunne
EF, Mahajan R, Ocfemia MC, Su J, Xu F and Weinstock H: Sexually
transmitted infections among US women and men: Prevalence and
incidence estimates, 2008. Sex Transm Dis. 40:187–193. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Owusu-Edusei K Jr, Chesson HW, Gift TL,
Tao G, Mahajan R, Ocfemia MC and Kent CK: The estimated direct
medical cost of selected sexually transmitted infections in the
United States, 2008. Sex Transm Dis Mar. 40:197–201. 2013.
View Article : Google Scholar
|
|
8
|
Workowski KA and Berman S: Centers for
Disease Control and Prevention (CDC): Sexually transmitted diseases
treatment guidelines, 2010. MMWR Recomm Rep. 59(RR-12): 1–110.
2010.PubMed/NCBI
|
|
9
|
Koshiol J, Lindsay L, Pimenta JM, Poole C,
Jenkins D and Smith JS: Persistent human papillomavirus infection
and cervical neoplasia: A systematic review and meta-analysis. Am J
Epidemiol. 168:123–137. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Louvanto K, Rintala MA, Syrjänen KJ,
Grénman SE and Syrjänen SM: Genotype-specific persistence of
genital human papillomavirus (HPV) infections in women followed for
6 years in the Finnish Family HPV Study. J Infect Dis. 202:436–444.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kjær SK, Frederiksen K, Munk C and Iftner
T: Long-term absolute risk of cervical intraepithelial neoplasia
grade 3 or worse following human papillomavirus infection: Role of
persistence. J Natl Cancer Inst. 102:1478–1488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Syrjänen K, Shabalova I, Naud P,
Kozachenko V, Derchain S, Zakharchenko S, Roteli-Martins C,
Nerovjna R, Longatto-Filho A, Kljukina L, et al: NIS and LAMS Study
Research Groups: Risk estimates for persistent high-risk human
papillomavirus infections as surrogate endpoints of progressive
cervical disease critically depend on reference category: Analysis
of the combined prospective cohort of the New Independent States of
the Former Soviet Union and Latin American Screening studies. Int J
STD AIDS. 22:315–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li N, Franceschi S, Howell-Jones R,
Snijders PJ and Clifford GM: Human papillomavirus type distribution
in 30,848 invasive cervical cancers worldwide: Variation by
geographical region, histological type and year of publication. Int
J Cancer. 128:927–935. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mammas IN and Spandidos DA: Fighting
against human papillomavirus: the 25-year old contribution of the
University of Crete School of Medicine. J BUON. 20:17–21.
2015.PubMed/NCBI
|
|
15
|
Clifford GM, Gonçalves MA and Franceschi
S: HPV and HIV Study Group: Human papillomavirus types among women
infected with HIV: A meta-analysis. AIDS. 20:2337–2344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du J, Nordfors C, Ährlund-Richter A,
Sobkowiak M, Romanitan M, Näsman A, Andersson S, Ramqvist T and
Dalianis T: Prevalence of oral human papillomavirus infection among
youth, Sweden. Emerg Infect Dis. 18:1468–1471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giraldo P, Gonçalves AK, Pereira SA,
Barros-Mazon S, Gondo ML and Witkin SS: Human papillomavirus in the
oral mucosa of women with genital human papillomavirus lesions. Eur
J Obstet Gynecol Reprod Biol. 126:104–106. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Termine N, Giovannelli L, Matranga D,
Caleca MP, Bellavia C, Perino A and Campisi G: Oral human
papillomavirus infection in women with cervical HPV infection: New
data from an Italian cohort and a metanalysis of the literature.
Oral Oncol. 47:244–250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Marco F: Oxidative stress and HPV
carcinogenesis. Viruses. 5:708–731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kofoed K, Sand C, Forslund O and Madsen K:
Prevalence of human papillomavirus in anal and oral sites among
patients with genital warts. Acta Derm Venereol. 94:207–211. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Căruntu C, Grigore C, Căruntu A,
Diaconeasa A and Boda D: The role of stress in skin disease. Intern
Med. 8:73–84. 2011.
|
|
22
|
Fang CY, Miller SM, Bovbjerg DH, Bergman
C, Edelson MI, Rosenblum NG, Bove BA, Godwin AK, Campbell DE and
Douglas SD: Perceived stress is associated with impaired T-cell
response to HPV16 in women with cervical dysplasia. Ann Behav Med.
35:87–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spandidos DA: A unified theory for the
development of cancer. Biosci Rep. 6:691–708. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Spandidos DA: The cancer story. Cancer
Biol Ther. 3:1184–1186. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neagu M, Caruntu C, Constantin C, Boda D,
Zurac S, Spandidos DA and Tsatsakis AM: Chemically induced skin
carcinogenesis: Updates in experimental models (Review). Oncol Rep.
35:2516–2528. 2016.PubMed/NCBI
|
|
26
|
Boda D: Cellomics as integrative omics for
cancer. Curr Proteomics. 10:237–245. 2013. View Article : Google Scholar
|
|
27
|
Wu DW, Chuang CY, Lin WL, Sung WW, Cheng
YW and Lee H: Paxillin promotes tumor progression and predicts
survival and relapse in oral cavity squamous cell carcinoma by
microRNA-218 targeting. Carcinogenesis. 35:1823–1829. 2014.
View Article : Google Scholar : PubMed/NCBI
|