Introduction
Bladder cancer remains a common malignant disease in
the USA, with an estimated incidence of ~73,500 cases in 2012, and
accounts for almost 15,000 mortalities per year (1). Approximately 75% of newly diagnosed
urothelial bladder cancers (UBCs) are non-invasive, but have a high
rate of recurrence and progression despite local therapy (2). This frequency, coupled with the
relapsing nature of UBC, means that UBC poses an enormous burden on
healthcare systems (2,3). Therefore, it is of enormous importance
to identify novel effective therapeutics or chemoprevention to halt
and delay the recurrence and progression of UBC.
N-(4-hydroxyphenyl) retinamide (4-HPR) is one of the
most studied retinoid analogs for use in cancer prevention
(4–6).
4-HPR inhibits the growth of breast, prostate and ovarian cancers
in animal models (4–6). Compared with other vitamin A analogues,
4-HPR-induced cell differentiation is weak, its cancer
chemopreventive effect is remarkable and its anti-cancer activity
is apparent (7). In addition, 4-HPR
has exhibited potent preventive effects in a rodent mammary tumor
model and less toxicity than retinoic acid, and has also been
tested in a large breast cancer prevention trial (8). Therefore, 4-HPR may exert a
chemo-preventive effect on UBC growth.
Matrix metalloproteinases (MMPs) are a super-family
of proteolytic enzymes capable of degrading the extracellular
matrix (ECM) and basement membrane (9). Tumor invasion, metastasis and
angiogenesis require controlled degradation of the ECM, and
increased expression of MMPs is associated with tumor invasion and
metastasis of malignant tumors with different histogenetic origin
(10,11). Of all the MMPs described so far, MMP-2
(also known as gelatinase A or type IV collagenase) has been
reported to be closely linked to cancer (12). The principal activity of MMP-2 is the
hydrolysis of gelatin and type IV collagen, which are the main
structural components of basement membranes (13). Furthermore, sulforaphane inhibits the
migration of human bladder cancer cells via the
cyclooxygenase-2/MMP-2 signaling pathway (14).
Wingless-type mouse mammary tumor virus integration
site family (Wnt) proteins play essential roles in developmental
and physiological processes by regulating various cell functions,
including proliferation, differentiation, apoptosis, survival,
adhesion, migration and polarity (15). Wnt5a is a member of the Wnt family of
secreted glycoproteins, which are involved in tumor progression and
malignant initiation (16). The
non-canonical Wnt signaling consists of at least the Wnt/c-Jun
N-terminal kinase (JNK) and Wnt/Ca2+ signaling pathways,
which are considered to have crucial functions in the regulation of
cell migration and polarity (17).
Wnt5a is a representative Wnt protein that activates non-canonical
Wnt signaling (17). It has been
proposed that Wnt5a signaling is activated by the Wnt/JNK signaling
pathway, which regulates convergent extension movements in
Xenopus gastrulation, and/or inhibition of the
β-catenin/transcription factor signaling pathway (18). JNK is known to play a role in cell
migration by phosphorylation of paxillin Ser178, a focal adhesion
molecule (19). In addition, Wnt5a
signaling is correlated with infiltrative activity in human glioma
by inducing cellular migration and MMP-2 expression (20). Therefore, the present authors
hypothesized that 4-HPR inhibits migration and invasion of bladder
cancer cells via Wnt5a/JNK or Wnt5a/MMP-2 signaling.
Materials and methods
Cell cultures
The EJ cell line was purchased from the Cancer
Institute and Hospital of the Chinese Academy of Medical Sciences
(Beijing, China), and cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
foetal calf serum (Gibco; Thermo Fisher Scientific, Inc.). 4-HPR
was purchased from Sigma-Aldrich (St. Louis, MO, USA), dissolved in
dimethyl sulfoxide (DMSO) as 10-mM stock solutions and stored in
the dark at −20°C. Lipofectamine 2000 reagent was purchased from
Invitrogen (Thermo Fisher Scientific, Inc.). The inhibitor of JNK
SP600125 (Cayman Chemical Company, Ann Arbor, MI, USA) was applied
to the culture medium at a final concentration of 20 µM.
Cell proliferation, apoptosis,
chemotaxis and invasion assays
All proliferation and viability assays were based on
the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) method. Cells were seeded in a 96-well plate at a density of
2,000 cells/well. The cells were treated with 0, 2.5, 5 and 10 µM
4-HPR, and allowed to grow for 24, 48, 72 and 96 h. At the end of
the experiment, the media were removed, and DMSO was added with MTT
solubilization solution. Absorbance was measured at 595 nm.
Matrigel invasion assays were used to assess the effect of 4-HPR in
EJ cells. The 8-µm pore size polycarbonate nucleopore filter
inserts in a 24-well transwell chamber (BD Biosciences, Franklin
Lakes, NJ, USA) were coated with 60 µg/filter Matrigel (BD
Biosciences). 4-HPR-treated EJ cells at a density of 40,000
cells/well were seeded into the upper part of the Matrigel-coated
filter, and RPMI-1640 medium supplemented with 10% heat-inactivated
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) was
added to the lower part. After 48 h, the cells that had migrated
through the Matrigel and the 8-µm pore-size membrane were fixed,
stained and counted under a light microscope. Migration assays
adopted the same method that invasion assays, with the exception of
the use of uncoated filters, and were conducted for 24 h.
Protein extraction and western blot
analyses
Proteins were obtained from EJ cells after 48-h
culture in the absence or presence of 4-HPR, or after 24 h in the
presence of SP600125. The cells were then lysed in
radioimmunoprecipitation assay buffer containing protease
inhibitors. Protein concentration was determined with the DC
Protein Assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Equal amounts of samples were resolved by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, transferred to
nitrocellulose membranes and probed at 4°C overnight with the
following anti-human antibodies: Mouse monoclonal anti-Wnt5a
antibody (ab130163; 1:1,000; Abcam, Cambridge, UK), rabbit
monoclonal anti-MMP-2 (13132; 1:1,000; Cell Signaling Technology,
Inc., Danvers, MA, USA), rabbit monoclonal anti-JNK (9252; 1:1,000;
Cell Signaling Technology, Inc.) and rabbit monoclonal
anti-phospho-JNK on Thr183/Tyr185 (4668; 1:1,000, Cell Signaling
Technology, Inc.).
After washing, the blots were incubated for 1 h at
room temperature with horseradish peroxidase (HRP)-conjugated
secondary antibodies (RPN2232; GE Healthcare Life Sciences,
Chalfont, UK), and specific complexes were revealed by enhanced
chemiluminescence (GE Healthcare Life Sciences). An
anti-glyceraldehyde 3-phosphate dehydrogenase antibody conjugated
to HRP (Novus Biologicals, LLC, Littleton, CO, USA) was utilized as
a loading control for all samples.
Gene knockdown
For gene knockdown, small interfering RNA (siRNA)
duplexes (Shanghai GenePharma Co., Ltd., Shanghai, China) targeting
Wnt5a (5′-GACCUGGUCUACAUCGACCTT-3′ and 5′-CCGCGAGCGGGAGCGCAT-3′)
and scrambled sequence Wnt5a siRNA (5′-GGUCCAGUCAGCCACUCUATT-3′)
were transfected into EJ cells by using Lipofectamine 2000 reagent.
Knockdown efficiency was evaluated 48 h after transfection by
measuring the protein levels in the cell lysates.
Statistical analysis
The normality and homogeneity of variance were
tested prior to statistical analysis with Prism 5 using the
Kolmogorov-Smirnov test. Data were expressed as the mean ± standard
deviation, unless otherwise noted. The differences between groups
were analyzed using a two-tailed Student's t-test and the
null hypothesis was rejected at the 0.05 level. For all statistical
tests, two-tailed P<0.05 was considered to indicate a
statistically significant difference.
Results
4-HPR decreases cell proliferation,
migration and invasion
The action of 4-HPR on bladder cancer cell
proliferation was assessed by treating EJ cells with a range of
4-HPR concentrations. All the concentrations tested inhibited cell
growth, with statistically significant differences only after 96-h
exposure (Fig. 1A). Migration of
cancer cells is one of the key factors responsible for cancer
metastasis (10). To metastasize,
cancer cells must migrate from the original growth site, invade
surrounding tissues and locate to other parts of the body through
the blood or the lymphatic system (10). The effect of 4-HPR on migration and
invasion of EJ cells upon exposure (48 h) to micromole
concentrations of 4-HPR was then examined. All the concentrations
tested inhibited cell migration and invasion, particularly when
cells were treated with 4-HPR at 10 µM (Fig. 1B and C).
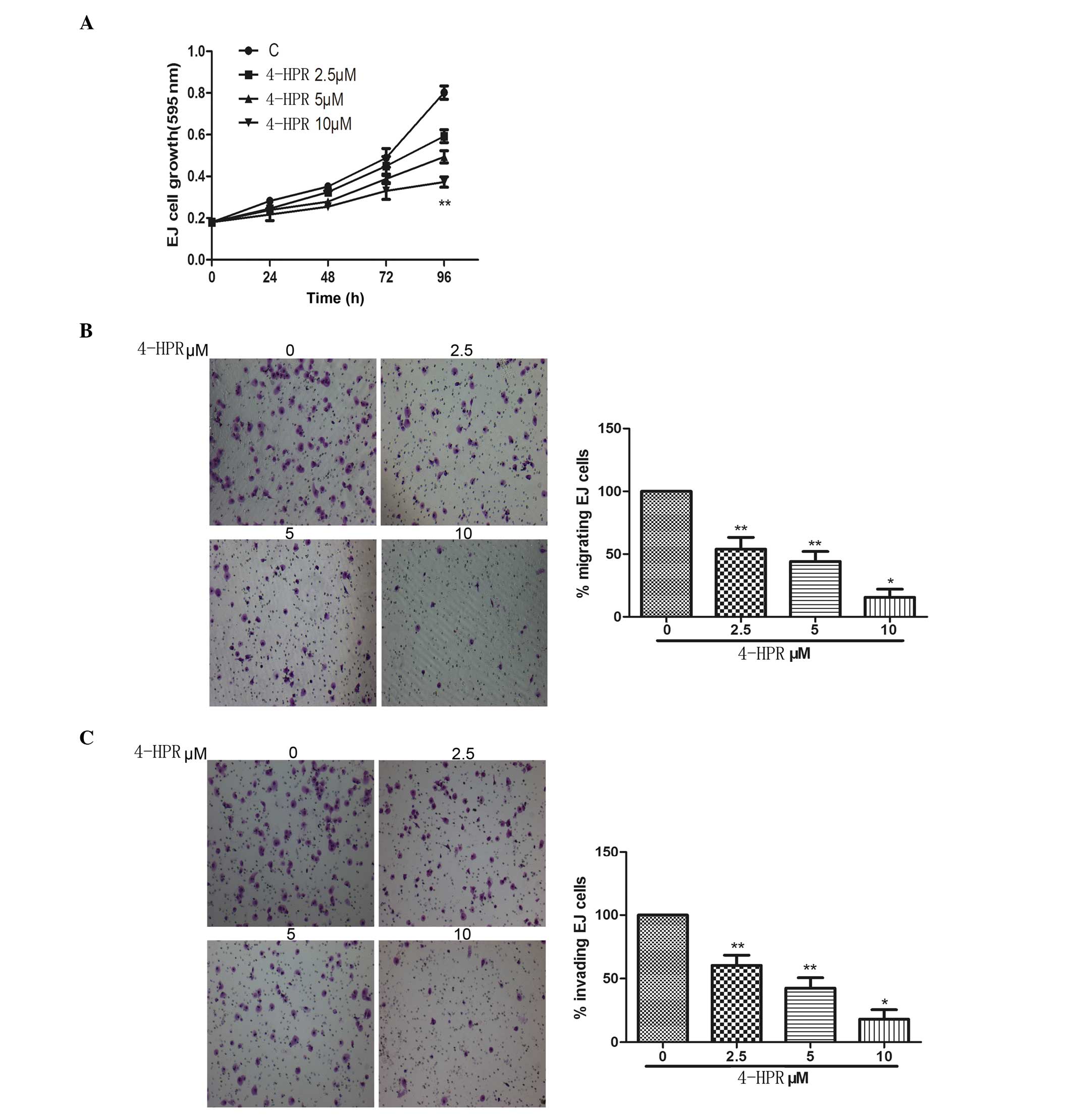 | Figure 1.Bladder cancer cell growth, migration
and invasion were inhibited by increasing doses of 4-HPR. (A) Cell
proliferation, as evaluated by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay,
was significantly inhibited at all the 4-HPR concentrations tested
after 96-h exposure. (B) Cell motility through uncoated filters was
measured 24 h after plating in the absence or presence of the
indicated concentrations of 4-HPR for 48 h. Then, cells in the
lower chamber were stained with crystal violet and photographed
with a light microscope (magnification, ×100), followed by
calculation of the percentage of migrating cells. (C) Invasion
assays adopted the same method than migration assays, with the
exception of the use of coated filters for 48 h. Data were
expressed as the mean ± standard deviation from three independent
experiments. *P<0.05, **P<0.01 vs. control. C, control;
4-HPR, N-(4-hydroxyphenyl) retinamide. |
4-HPR inhibits the migration and
invasion of EJ cells by increasing the expression of Wnt5a
Previous studies indicate that Wnt5a inhibits cell
growth, migration and invasiveness of thyroid and colorectal cancer
cells (21,22), indicating that Wnt5a may act as a
tumor suppressor. Accordingly, the present study examined whether
or not expression of Wnt5a can be induced following 4-HPR
stimulation. As shown in Fig. 2A,
treatment of EJ cells with 4-HPR for 48 h upregulated the
expression of Wnt5a in a dose-dependent manner, as detected by
western blot analysis of extracts from EJ cells.
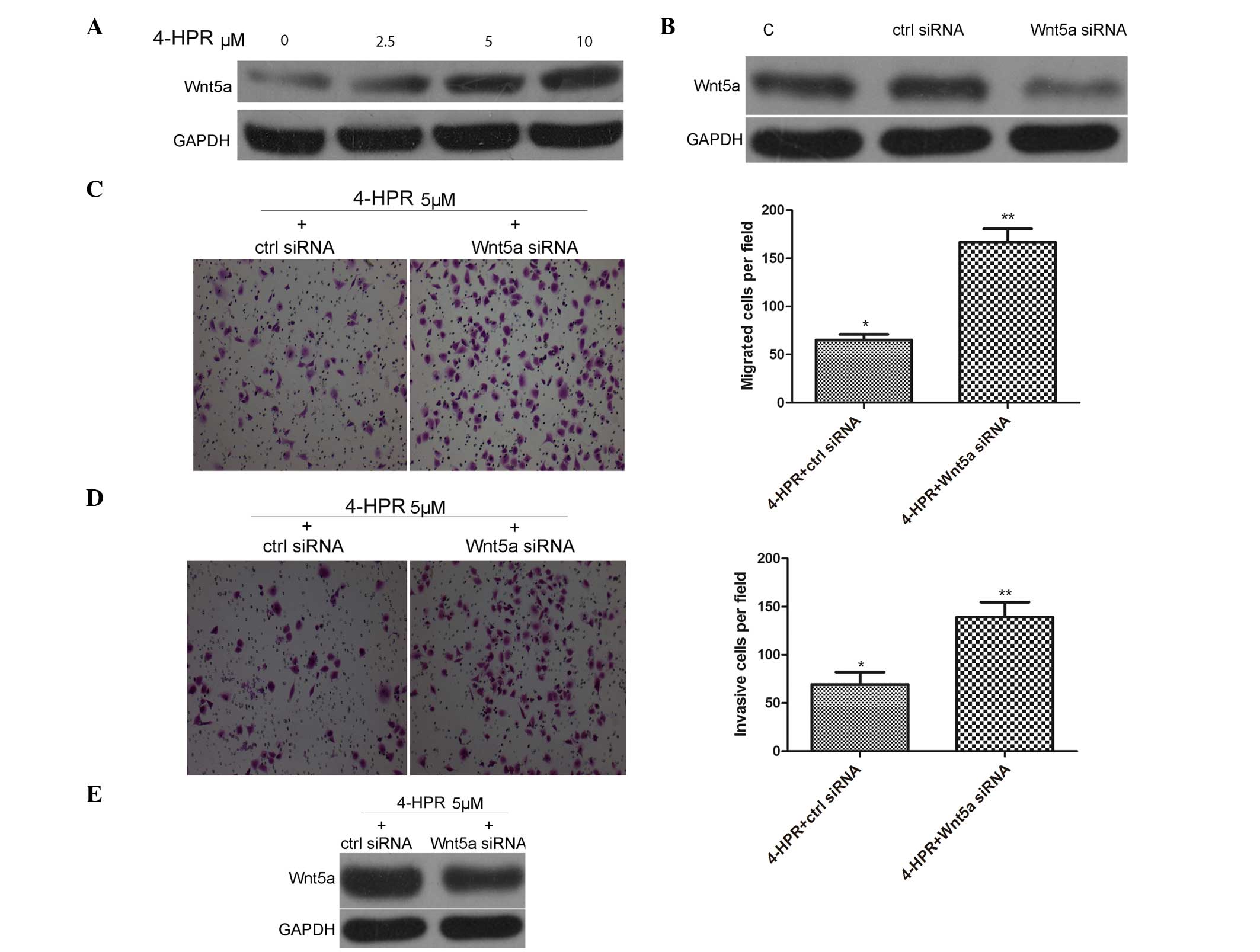 | Figure 2.4-HPR inhibits the migration and
invasion of EJ cells by increasing the expression of Wnt5a. (A) EJ
cells were incubated with the indicated concentrations of 4-HPR for
48 h, and the protein levels of Wnt5a were measured by western
blotting. GAPDH was used as an internal control. (B) EJ cells were
transfected with ctrl siRNA and Wnt5a siRNA, and the protein levels
of Wnt5a were measured by western blotting. GAPDH was used as an
internal control. (C and D) EJ cells were transfected with ctrl
siRNA and Wnt5a siRNA for 24 h. After incubation with 4-HPR for 48
h, the cells were analyzed by (C) migration and (D) invasion
assays, as described in Fig. 1B and C
(magnification, ×100). (E) EJ cells were transfected with ctrl
siRNA and Wnt5a siRNA for 24 h. After incubation with 4-HPR for 48
h, the protein levels of Wnt5a were measured by western blotting.
GAPDH was used as an internal control. Data were expressed as the
mean ± standard deviation from three independent experiments. *P
<0.05, **P<0.01 vs. control. 4-HPR, N-(4-hydroxyphenyl)
retinamide; siRNA, small interfering RNA; Wnt5a, wingless-type
mouse mammary tumor virus integration site family, member 5a;
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; C, control; ctrl,
control. |
To analyze the efficiency of gene knockdown of
Wnt5a, siRNAs were used to knockdown Wnt5a expression in EJ cells,
followed by western blot assays. siRNA against human Wnt5a knocked
down Wnt5a expression by >60%, as assessed by immunoblotting in
EJ cells (Fig. 2B). To confirm
whether Wnt5a activity is involved in the 4-HPR-induced migration
and invasion of EJ cells, EJ cells were pre-treated with 5 µM 4-HPR
for 48 h, and then transfected with control siRNA or Wnt5a siRNA.
As shown in Fig. 2C-E, knocking down
Wnt5a expression with Wnt5a siRNA could inhibit the migration and
invasion of EJ cells, and decrease the expression of Wnt5a,
compared with control siRNA transfection. Collectively, these data
support the idea that 4-HPR inhibits the migration and invasion of
bladder cancer cells through high expression of Wnt5a.
4-HPR inhibits the migration and
invasion of EJ cells by stimulating Wnt5a activity and causing the
phosphorylation of JNK on Thr183/Tyr185
As shown in Fig. 3A,
treatment of EJ cells with 4-HPR for 48 h upregulated the
phosphorylation of JNK in a dose-dependent manner, as detected by
western blot analysis of extracts from EJ cells. To further confirm
whether the phosphorylation of JNK could be induced following Wnt5a
stimulation, EJ cells were exposed to 4-HPR for 48 h to stimulate
the activity of Wnt5a, and the phosphorylation of JNK was induced
upon stimulation with 4-HPR, as assessed by western blot analysis.
In addition, the phosphorylation of JNK was enhanced by 4-HPR, and
this 4-HPR-induced enhancement was abrogated by Wnt5a siRNA
(Fig. 3B). Additionally, knocking
down Wnt5a expression abrogated the 4-HPR-induced migration and
invasion of EJ cells, further demonstrating the critical roles of
phosphorylation of JNK, induced by Wnt5a signaling, in the
migration and invasion of EJ cells (Fig.
3C and D).
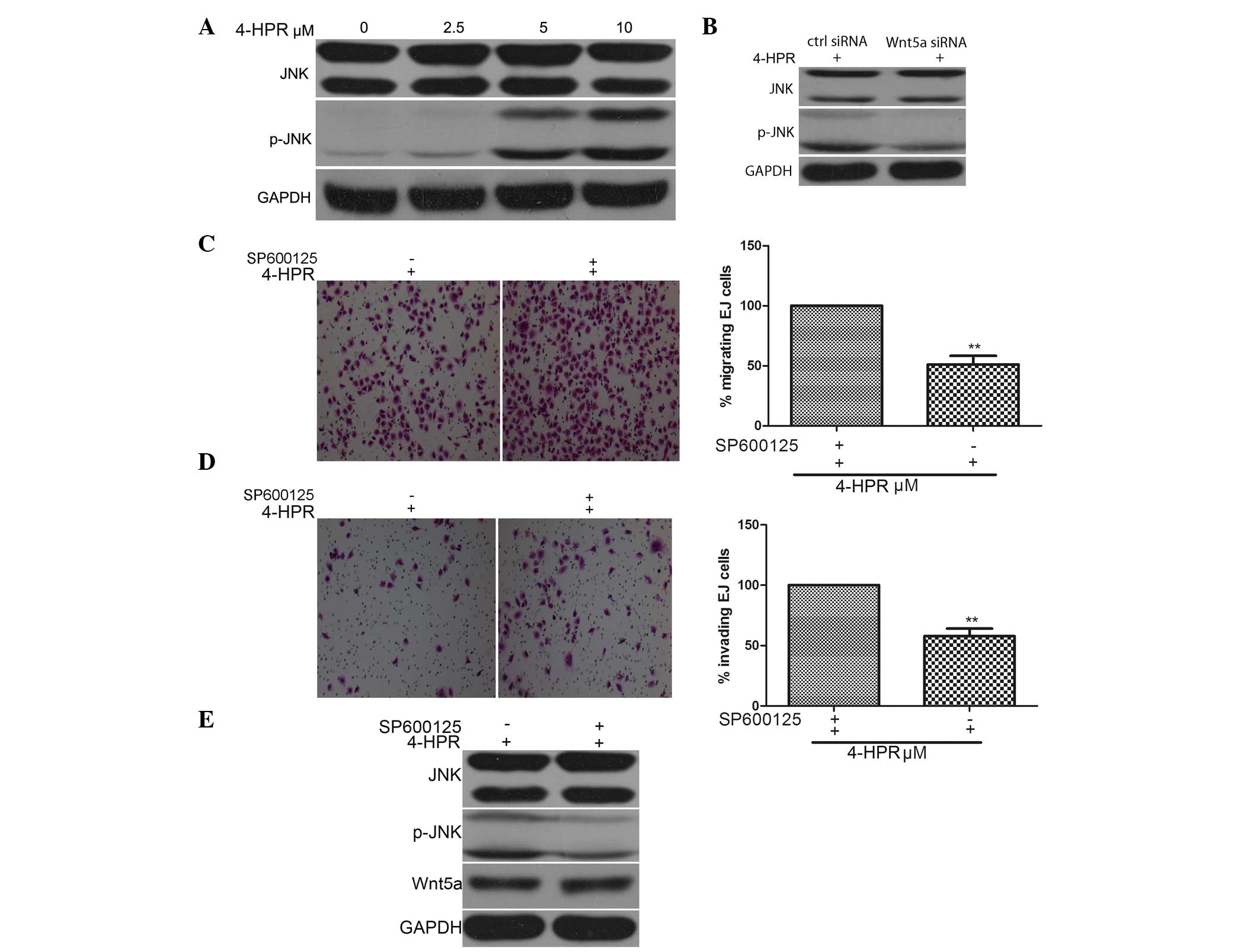 | Figure 3.4-HPR inhibits the migration and
invasion of EJ cells by stimulating Wnt5a activity and JNK
phosphorylation. (A) EJ cells were incubated with the indicated
concentrations of 4-HPR for 48 h, and the protein levels of JNK and
pJNK were measured by western blotting. GAPDH was used as an
internal control. (B) EJ cells were treated with 5 µM 4-HPR for 48
h, and then transfected with control or Wnt5a small interfering
RNA. Cell lysates were assayed for JNK and pJNK by western blotting
with anti-JNK, anti-pJNK and anti-GAPDH antibodies. (C-E) EJ cells
were treated with SP600125, an inhibitor of JNK, and then incubated
with 5 µM 4-HPR for 48 h. Cell migration and invasion were measured
by (C) migration and (D) invasion assays (magnification, ×100), (E)
and the protein extracts were analyzed by western blotting for JNK
and pJNK. Data were expressed as the mean ± standard deviation from
three independent experiments. *P<0.05, **P<0.01 vs. control.
4-HPR, N-(4-hydroxyphenyl) retinamide; siRNA, small interfering
RNA; Wnt5a, wingless-type mouse mammary tumor virus integration
site family, member 5a; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase; ctrl, control; JNK, c-Jun N-terminal kinase; pJNK,
phosphorylated c-Jun N-terminal kinase. |
Next, the effect of phosphorylation of JNK on the
migration and invasion of EJ cells was examined. For this purpose,
EJ cells were pre-treated with the JNK inhibitor SP600125 for 30
min, and then exposed to 4-HPR for 48 h. The migration and invasion
of EJ cells were inhibited by 4-HPR, and this 4-HPR-induced
inhibition was abrogated by the presence of the JNK inhibitor
SP600125, while the 4-HPR-induced phosphorylation of JNK was also
abrogated by SP600125 (Fig. 3E).
These data indicate that 4-HPR stimulates the phosphorylation and
subsequent activity of JNK via the Wnt5a signaling pathway, leading
to the inhibition of migration and invasion in EJ bladder cancer
cells.
4-HPR inhibits the migration and
invasion of bladder cancer cells through the suppression of
MMP-2
The evidence for MMPs as active contributors to
cancer progression comes from previous animal studies (23). In previous transplantation assays,
relatively benign cancer cells acquired malignant properties when
MMP expression was upregulated (23).
Conversely, highly malignant cells became less aggressive when MMP
expression or activity was reduced (12). It is well established that MMP-2 is
involved in bladder cancer migration and invasion. In the present
study, 4-HPR decreased the expression of MMP-2 in a dose-dependent
manner, when the migration and invasion of EJ cells were inhibited
(Fig. 4A). Furthermore, this
suppressed expression of MMP-2 was abrogated by a suppressed
expression of Wnt5a, compared with control siRNA transfection
(Fig. 4B). These results indicate
that 4-HPR inhibits the migration and invasion of EJ cells through
inhibiting MMP-2 expression, which is regulated by Wnt5a
signaling.
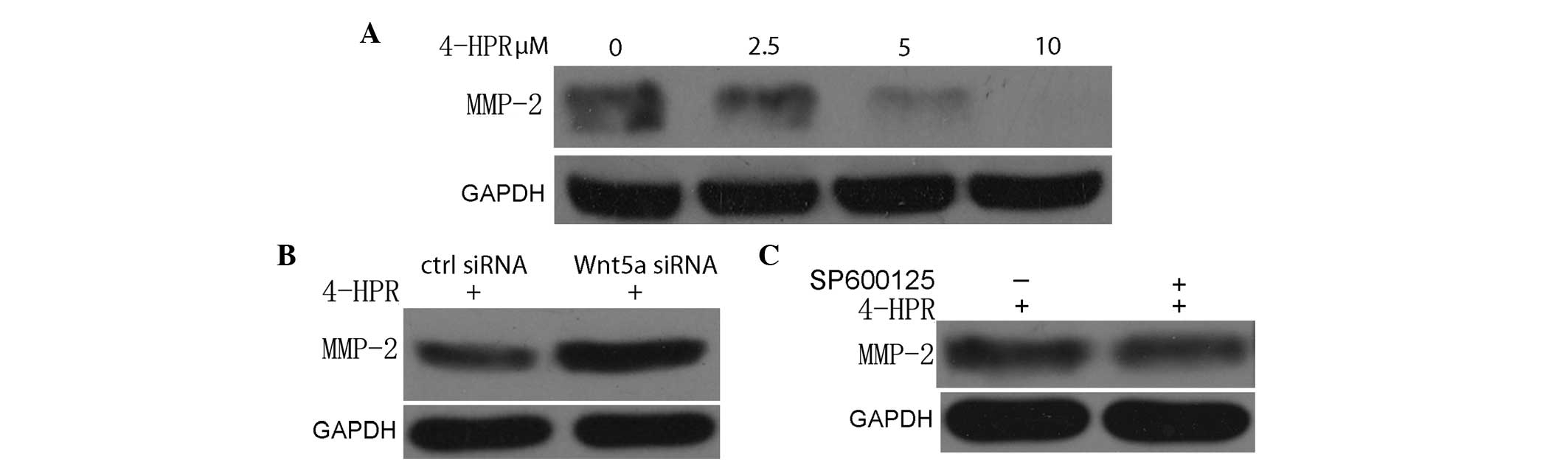 | Figure 4.4-HPR inhibits the migration and
invasion of bladder cancer cells through suppression of MMP-2. (A)
EJ cells were incubated with the indicated concentrations of 4-HPR
for 48 h, and the protein levels of MMP-2 were measured by western
blotting. GAPDH was used as an internal control. (B) EJ cells were
treated with 5 µM 4-HPR for 48 h, and then transfected with control
or Wnt5a small interfering RNA. Cell lysates were assayed for MMP-2
by western blotting with an anti-MMP-2 antibody. (C) EJ cells were
treated with SP600125, an inhibitor of c-Jun N-terminal kinase, and
then incubated with 5 µM 4-HPR for 48 h. Next, the protein extracts
were analyzed by western blotting for MMP-2. 4-HPR,
N-(4-hydroxyphenyl) retinamide; siRNA, small interfering RNA;
Wnt5a, wingless-type mouse mammary tumor virus integration site
family, member 5a; GAPDH, glyceraldehyde 3-phosphate dehydrogenase;
ctrl, control; MMP-2, matrix metalloproteinase-2. |
The present study demonstrated that 4-HPR inhibited
the migration and invasion of EJ cells through the suppression of
MMP-2, which was regulated by Wnt5a signaling, although the
suppressed phosphorylation of JNK did not appear to have a direct
effect on the expression of MMP-2, as assessed by western blot
analysis.
Discussion
4-HPR has potent chemo-preventive and
anti-metastatic effects in several animal models (24–26), and
is currently under investigation on clinical trials for use as a
preventive agent for ovarian carcinoma, lung carcinoma and breast
neoplasia (27,28). In the present study, 4-HPR inhibited
the migration and invasion of EJ cells in a dose-dependent manner.
MMPs were prime candidates for invasion and metastasis, since MMP
family members collectively degrade all the structural components
of the ECM (29). In addition, MMPs
are upregulated in virtually all human and animal tumors, as well
as in the majority of tumor cell lines (30). Previous studies indicate that 4-HPR
inhibits breast cancer cell invasion through inhibiting MMP-9
expression (31). The present results
indicate that 4-HPR inhibits the expression of MMP-2, suggesting
that 4-HPR can inhibit cell migration and invasion by suppressing
MMP-2 expression.
Wnt5a, as one of the most highly investigated
non-canonical Wnts, has been implicated in almost all aspects of
cancer development (32). Wnt5a plays
essential roles in developmental and physiological processes by
regulating several cell functions, including proliferation,
differentiation, apoptosis, survival, adhesion, migration and
polarity (21). A primary observation
of the present study is that 4-HPR inhibits the migration and
invasion of EJ bladder cancer cells, which was fully abolished by
Wnt5a siRNA, confirming that 4-HPR inhibits the migration and
invasion of EJ bladder cancer cells via stimulation of Wnt5a. In
addition, it has already been reported that mouse Wnt5a is capable
of activating JNK in cultured cells (33). The present findings suggest that 4-HPR
stimulates the phosphorylation of JNK and its activity via the
Wnt5a signaling pathway, leading to inhibited migration and
invasion of EJ bladder cancer cells. In addition, the present data
support the idea that 4-HPR inhibits MMP-2 activity via the Wnt5a
signaling pathway, leading to inhibited migration and invasion of
EJ bladder cancer cells.
In conclusion, the present study reports that 4-HPR
stimulates the activation of Wnt5a in bladder cancer, and
demonstrates that 4-HPR inhibits the migration and invasion of
bladder cancer cells though stimulating Wnt5a activity, causing the
downregulation of MMP-2 expression and enhancing enhancement JNK
phosphorylation. Thus, 4-HPR may be a potent anti-invasive and
anti-metastatic agent that works via the Wnt5a/JNK and Wnt5a/MMP-2
signaling pathways in bladder cancer cells. Therefore, 4-HPR is a
potential chemotherapeutic candidate with a broad spectrum of
anticancer activities. Despite these findings, further studies on
the mechanism of Wnt5a/JNK and Wnt5a/MMP-2 signaling, as well as
future experiments using in vivo models, are required.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (Beijing, China; grant
no. 303162520).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sievert KD, Amend B, Nagele U, Schilling
D, Bedke J, Horstmann M, Hennenlotter J, Kruck S and Stenzl A:
Economic aspects of bladder cancer: What are the benefits and
costs? World J Urol. 27:295–300. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Babjuk M, Oosterlinck W, Sylvester R,
Kaasinen E, Böhle A, Palou-Redorta J and Rouprêt M: European
Association of Urology (EAU): EAU guidelines on non-muscle-invasive
urothelial carcinoma of the bladder, the 2011 update. Eur Urol.
59:997–1008. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dowlatshahi K, Mehta RG, Thomas CF, Dinger
NM and Moon RC: Therapeutic effect of N-(4-hydroxyphenyl)
retinamide on N-methyl-N-nitrosourea-induced rat mammary cancer.
Cancer Lett. 47:187–192. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Formelli F and Cleris L: Synthetic
retinoid fenretinide is effective against a human ovarian carcinoma
xenograft and potentiates cisplatin activity. Cancer Res.
53:5374–5376. 1993.PubMed/NCBI
|
|
6
|
Pollard M and Luckert PH: The inhibitory
effect of 4-hydroxyphenyl retinamide (4-HPR) on metastasis of
prostate adenocarcinoma-III cells in Lobund-Wistar rats. Cancer
Lett. 59:159–163. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kurie JM1, Lee JS, Khuri FR, Mao L, Morice
RC, Lee JJ, Walsh GL, Broxson A, Lippman SM, Ro JY, et al:
N-(4-hydroxyphenyl)retinamide in the chemoprevention of squamous
metaplasia and dysplasia of the bronchial epithelium. Clin Cancer
Res. 6:2973–2979. 2000.PubMed/NCBI
|
|
8
|
Costa A, Formelli F, Chiesa F, Decensi A,
De Palo G and Veronesi U: Prospects of chemoprevention of human
cancers with the synthetic retinoid fenretinide. Cancer Res. 54(7
Suppl): 2032s–2037s. 1994.PubMed/NCBI
|
|
9
|
Stamenkovic I: Matrix metalloproteinases
in tumor invasion and metastasis. Semin Cancer Biol. 10:415–433.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Basset P, Okada A, Chenard MP, Kannan R,
Stoll I, Anglard P, Bellocq JP and Rio MC: Matrix
metalloproteinases as stromal effectors of human carcinoma
progression: Therapeutic implications. Matrix Biol. 15:535–541.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johnsen M, Lund LR, Rømer J, Almholt K and
Danø K: Cancer invasion and tissue remodeling: Common themes in
proteolytic matrix degradation. Curr Opin Cell Biol. 10:667–671.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Srivastava P, Kapoor R and Mittal RD:
Association of single nucleotide polymorphisms in promoter of
matrix metalloproteinase-2, 8 genes with bladder cancer risk in
Northern India. Urol Oncol. 31:247–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shan Y, Zhang L, Bao Y, Li B, He C, Gao M,
Feng X, Xu W, Zhang X and Wang S: Epithelial-mesenchymal
transition, a novel target of sulforaphane via COX-2/MMP2, 9/Snail,
ZEB1 and miR-200c/ZEB1 pathways in human bladder cancer cells. J
Nutr Biochem. 24:1062–1069. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wong GT, Gavin BJ and McMahon AP:
Differential transformation of mammary epithelial cells by Wnt
genes. Mol Cell Biol. 14:6278–6286. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishita M, Enomoto M, Yamagata K and
Minami Y: Cell/tissue-tropic functions of Wnt5a signaling in normal
and cancer cells. Trends Cell Biol. 20:346–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oishi I, Suzuki H, Onishi N, Takada R,
Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, et
al: The receptor tyrosine kinase Ror2 is involved in non-canonical
Wnt5a/JNK signaling pathway. Genes Cells. 8:645–654. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang C, Rajfur Z, Borchers C, Schaller MD
and Jacobson K: JNK phosphorylates paxillin and regulates cell
migration. Nature. 424:219–223. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kikuchi A, Yamamoto H, Sato A and
Matsumoto S: Wnt5a: Its signaling, functions and implication in
diseases. Acta Physiol (Oxf). 204:17–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dejmek J, Dejmek A, Säfholm A, Sjölander A
and Andersson T: Wnt-5a protein expression in primary dukes B colon
cancers identifies a subgroup of patients with good prognosis.
Cancer Res. 65:9142–9146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kremenevskaja N, von Wasielewski R, Rao
AS, Schöfl C, Andersson T and Brabant G: Wnt-5a has tumor
suppressor activity in thyroid carcinoma. Oncogene. 24:2144–2154.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Coussens LM and Werb Z: Matrix
metalloproteinases and the development of cancer. Chem Biol.
3:895–904. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Green A, Shilkaitis A and Christov K:
4-(hydroxyphenyl) retinamide selectively inhibits the development
and progression of ductal hyperplastic lesions and carcinoma in
situ in mammary gland. Carcinogenesis. 20:1535–1540. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shaker MR, Yang G, Timme TL, Park SH,
Kadmon D, Ren C, Ji X, Lee HM, Sehgal I, Anzano M, et al: Dietary
4-HPR suppresses the development of bone metastasis in vivo in a
mouse model of prostate cancer progression. Clin Exp Metastasis.
18:429–438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hursting SD, Perkins SN, Phang JM and
Barrett JC: Diet and cancer prevention studies in p53-deficient
mice. J Nutr. 131(11 Suppl): 3092S–3094S. 2001.PubMed/NCBI
|
|
27
|
Cai J and Jones DP: Superoxide in
apoptosis. Mitochondrial generation triggered by cytochrome c loss.
J Biol Chem. 273:11401–11404. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Levi MS, Borne RF and Williamson JS: A
review of cancer chemopreventive agents. Curr Med Chem.
8:1349–1362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McCawley LJ and Matrisian LM: Matrix
metalloproteinases: multifunctional contributors to tumor
progression. Mol Med Today. 6:149–156. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Coussens LM, Fingleton B and Matrisian LM:
Matrix metalloproteinase inhibitors and cancer: Trials and
tribulations. Science. 295:2387–2392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kang H, Lee M, Choi KC, Shin DM, Ko J and
Jang SW: N-(4-hydroxyphenyl) retinamide inhibits breast cancer cell
invasion through suppressing NF-KB activation and inhibiting matrix
metalloproteinase-9 expression. J Cell Biochem. 113:2845–2855.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nusse R: Wnt signaling in disease and in
development. Cell Res. 15:28–32. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamanaka H, Moriguchi T, Masuyama N,
Kusakabe M, Hanafusa H, Takada R, Takada S and Nishida E: JNK
functions in the non-canonical Wnt pathway to regulate convergent
extension movements in vertebrates. EMBO Rep. 3:69–75. 2002.
View Article : Google Scholar : PubMed/NCBI
|


















