Introduction
Gastric cancer, one of the leading causes of
cancer-associated mortalities worldwide, is the fourth and the
fifth most common cancer in males and females, respectively
(1). In 2012, Nagini suggested that,
in spite of the advances in diagnosis and therapeutic methods,
numerous patients with gastric cancer succumb to invasion and
metastasis, with only a <20% 5-year survival rate for advanced
gastric cancer (2). The diverse risk
factors that contribute to the development of gastric cancer
include, among others, precancerous lesions, genetic factors and
Helicobacter pylori infection (3). However, the reasons for the very poor
prognosis of advanced gastric cancer are currently not understood.
Therefore, the mechanisms involved in gastric cancer metastasis
require to be urgently investigated.
Forkhead box M1 (FoxM1), a transcription factor
characterized by a conserved winged-helix DNA-binding domain, was
previously known as forkheaddrosophila homolog like 16,
HNF-3/fork-head homolog 11, Trident and membrane palmitoylated
protein 2 in the literature (4).
Emerging data suggest that FoxM1 is considered to be a key
regulator in the cell cycle at the G1-S and G2-M phases, by
controlling the expression of essential genes and the progression
of mitosis (5). Recently, numerous
studies on FoxM1 have suggested that FoxM1 is implicated in
angiogenesis, invasion and metastasis, indicating that FoxM1 may be
oncogenic and play a significant role in tumorigenesis (6,7). Based on
the published literature, the expression of FoxM1 in multiple
cancers is strongly positive, and markedly contributes to the
progression of cancers such as pancreatic, colorectal and breast
cancer (6–8). Notably, the study by Li et al
reports that the expression of FoxM1 in tissue and cell lines of
gastric cancer is higher than that in normal tissues, and is a
favorable biomarker for the prognosis of patients with gastric
cancer (9). Furthermore, the above
study offers prominent evidence that enforced expression of FoxM1
in mice enhanced the tumorigenic and metastatic abilities of human
gastric cancer cells, whereas attenuated FoxM1 expression did the
opposite, indicating that FoxM1 occupies an important position in
the progression of gastric cancer (10). Additionally, it has been reported that
the expression of vascular endothelial growth factor is directly
regulated by FoxM1 at the level of transcription, contributing
critically to the angiogenesis of gastric cancer (10). Furthermore, it has been previously
demonstrated that reduced FoxM1 expression inhibits the ability of
proliferation and invasion of nasopharyngeal carcinoma cells by
modulating the epithelial-to-mesenchymal transition (EMT) (11). In contrast, elevated expression of
FoxM1 increases the invasion of hepatocellular carcinoma cells by
suppressing the expression of epithelial cell markers and acquiring
the EMT phenotype (12). Due to the
critical role of FoxM1 in multiple tumors, it is essential to
determine the precise mechanisms and functions of FoxM1 in tumors,
particularly in gastric cancer, which is rarely studied.
E-cadherin, also termed cadherin 1, is a pivotal
membrane-spanning glycoprotein of epithelial cells, containing an
extracellular structure consisting of five cadherin repeats
(13). Accumulating evidence has
demonstrated that E-cadherin functions in several aspects of
cellular activity, including cell communication, cell signalling,
cell recognition, tissue morphogenesis, oncogenesis and, in
particular, intercellular adhesion (13). Notably, little is known about the role
of E-cadherin in EMT, which induces the invasion and metastasis of
cancer cells (14). Yu et al
have verified that a reduction in E-cadherin expression is
associated with the loss of epithelial phenotypes, which play a
critical role in the process of EMT (15). Furthermore, patients with loss of
E-cadherin in breast cancer have a higher proportion of mesenchymal
phenotype tumor cells circulating compared with patients with
normal E-cadherin expression, demonstrating that E-cadherin may
also be responsible for the diffusion of circulating tumor cells
with a mesenchymal phenotype (16).
Despite the large body of research on the role of E-caherin in the
development of EMT, the underlying regulatory mechanism(s) of
E-caherin remain(s) largely unknown, particularly in the case of
gastric cancer.
The present study aims to clarify the clinical
significance of FoxM1 and E-cadherin and to establish any
correlation between FoxM1 and E-cadherin, which may help to
establish the role of FoxM1 in EMT, invasion and metastasis of
gastric cancer.
Materials and methods
Tissue microarray
All gastric cancer tissue microarrays used in the
present study were purchased from US Biomax, Inc. (Rockville, MD,
USA), including 70 cases of gastric cancer tissues and 5 cases of
normal gastric mucosa. Each core of the tissue microarray
represents a sample. US Biomax, Inc. also provided patient
information concerning age, gender, clinical stage, histological
grade and pathological diagnosis.
Tissue immunohistochemistry
The expression of FoxM1 and E-cadherin in gastric
cancer tissue microarrays was analyzed using rabbit anti-FoxM1
polyclonal antibody (cat. no. sc-500; 1:100; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and mouse anti-E-cadherin
monoclonal antibody (cat. no. 610182; 1:200; BD Biosciences,
Franklin Lakes, NJ, USA). Standard immunohistochemical procedures
were implemented as follows: The samples on slides embedded with
paraffin and formalin were firstly dewaxed in xylene and rehydrated
in alcohol gradients of 100, 95, 85 and 75%. The slides were then
heated to 95°C for 30 min to retrieve antigens in the tissues. The
activity of endogenous peroxidase was blocked by employing 3%
hydrogen peroxide for 10 min. Part of the tissue was covered with
normal serum (Proteintech Group, Inc., Rosemont, IL, USA) at room
temperature for 30 min. Then, the samples on the slides were
incubated with antibodies against FoxM1 and E-cadherin overnight at
4°C. The slides were subsequently incubated with
peroxidase-conjugated AffiniPure goat anti-rabbit (cat. no.
SA00001-2; 1:500) and goat ant-mouse (cat. no. SA00001-1; 1:500) Ig
G (H+L) (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA,
USA) for 1 h at room temperature. Next, the samples were stained
with 3,3-diaminobenzidine and counterstained with hematoxylin. The
procedure of dehydration was implemented, and finally covers slips
were applied.
Immunohistochemistry score
The staining scores were evaluated independently by
two investigators, who were blinded to the information on the
microarrays. Based on the staining intensity and number of positive
cells, FoxM1 and E-cadherin samples were sorted into three groups,
namely, a negative group, a weak positive group and a strong
positive group. The staining intensity was categorized into four
grades as follows: No staining, 0; light staining, 1; moderate
staining, 2; and dark staining, 3. The percentage of positive cells
was classified into five groups as follows: <10%, 0; 10–25%, 1;
25–50%, 2; 50–75%, 3; and >75%, 4. The overall scores of ≤3, 3–6
and >6 were termed as negative, weakly positive and strongly
positive, respectively.
Statistical analysis
The differences between FoxM1 and E-cadherin
expression and clinicopathological parameters from the tissue
microarray specimens were determined by the Wilcoxon rank-sum test.
The correlation between FoxM1 and E-cadherin expression was
determined by the Spearmans rank correlation test (r,P). P<0.05
was considered to indicate a statistically significant difference.
SPSS version 17.0. (SPSS, Inc., Chicago, IL, USA) was used for the
analysis.
Results
Expression of FoxM1 in gastric cancer
samples and its direct association with clinicopathological
features
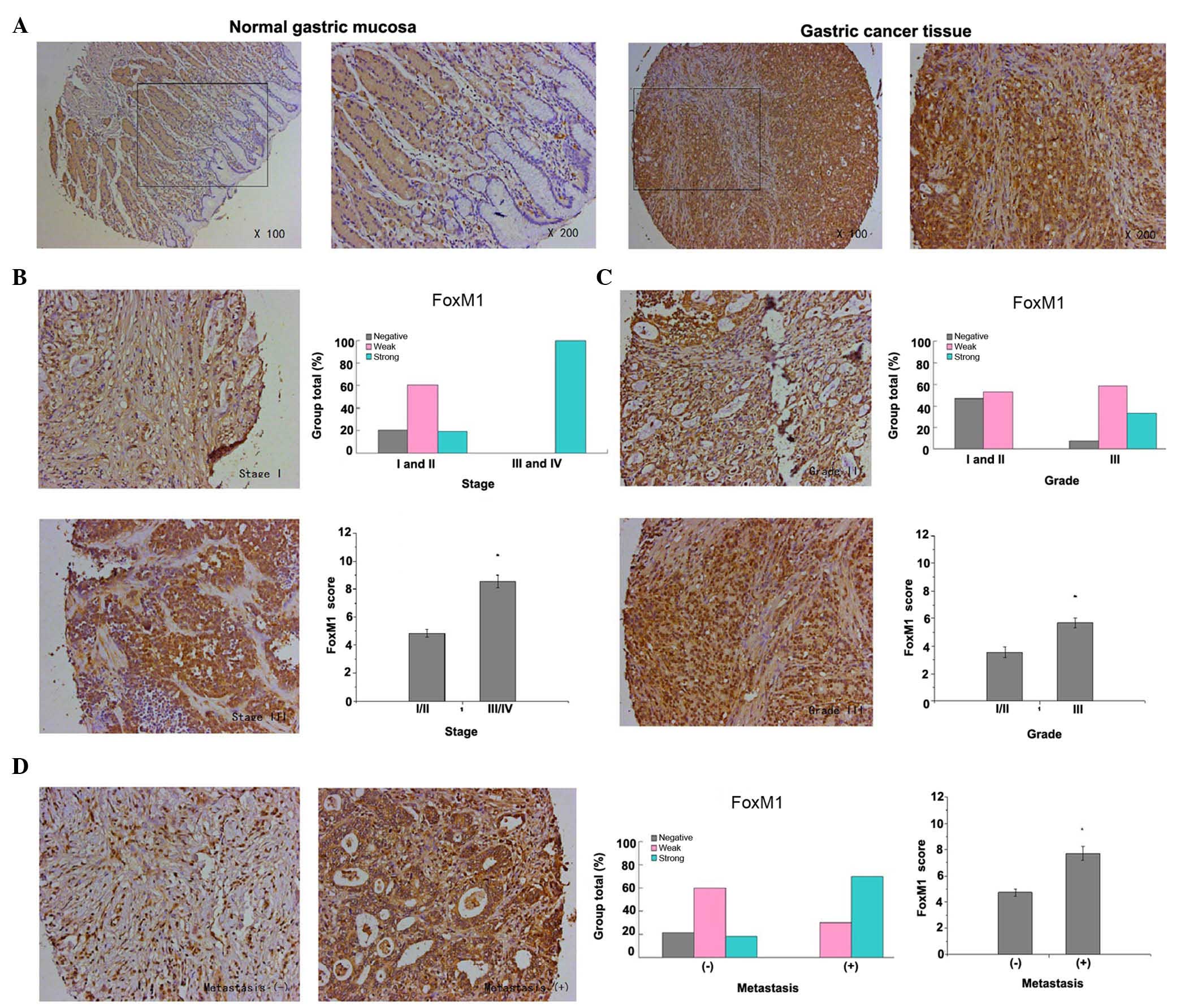
FoxM1 expression in gastric cancer was detected
primarily in the cytoplasm and nuclear compartment. The results
demonstrated that the staining of FoxM1 in normal gastric mucosa
was negative, whereas the staining of FoxM1 in the majority of
gastric cancer tissues was strong or weak positive (P<0.001,
Fig. 1A). Furthermore, the present
study revealed that high expression of FoxM1 in gastric cancer was
closely associated with advanced tumor stage, poor tumor
differentiation and lymph node (or distant) metastasis. The
expression of FoxM1 in advanced tumors (stages III and IV) was
higher in comparison with that in early tumors (stages I and II)
(P<0.001, Fig. 1B). Additionally,
the expression of FoxM1 in well-differentiated (grade I) and
moderately-differentiated (grade II) gastric cancer was lower
compared with that in poorly-differentiated (grade III) gastric
cancer (P=0.002, Fig. 1C). In
addition, the staining of FoxM1 in samples with lymph node (or
distant) metastasis was strongly positive, in contrast to that in
samples with no metastasis (P<0.001, Fig. 1D)
Expression of E-cadherin in gastric
cancer samples and its direct association with clinicopathological
features
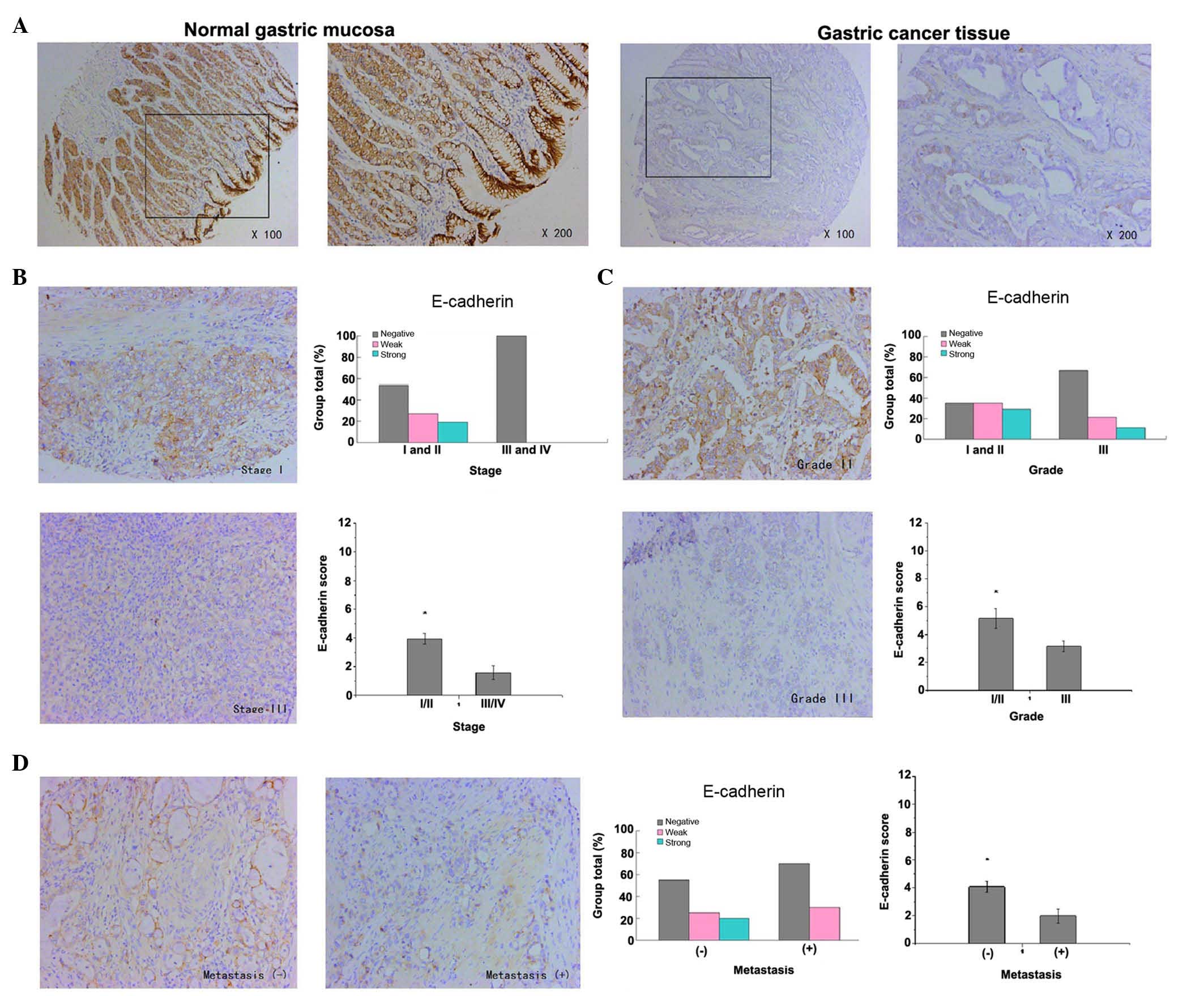
The expression of E-cadherin in gastric cancer was
detected mainly in the membrane of epithelial cells. The results
revealed that the staining of E-cadherin in normal gastric mucosa
was strongly positive, whereas the staining of E-cadherin in the
majority of gastric cancer tissue samples was weakly positive or
negative (P<0.001, Fig. 2A). These
results indicate that the low expression of E-cadherin in gastric
cancer was closely correlated with advanced tumor stages, poor
tumor differentiation and lymph node (or distant) metastasis. The
expression of E-cadherin in advanced tumors (stages III and IV) was
lower in comparison with that in early tumors (stages I and II)
(P=0.029, Fig. 2B).
Additionally, the expression of E-cadherin in well-differentiated
(grade I) and moderately-differentiated (grade II) gastric cancer
was higher compared with that in poorly-differentiated (grade III)
gastric cancer (P=0.050, Fig. 2C). In
addition, the expression of E-cadherin in samples that had not
metastasized was strongly higher than that in samples with lymph
node (or distant) metastasis (P=0.037, Fig. 2D)
Correlation between FoxM1 and
E-cadherin expression
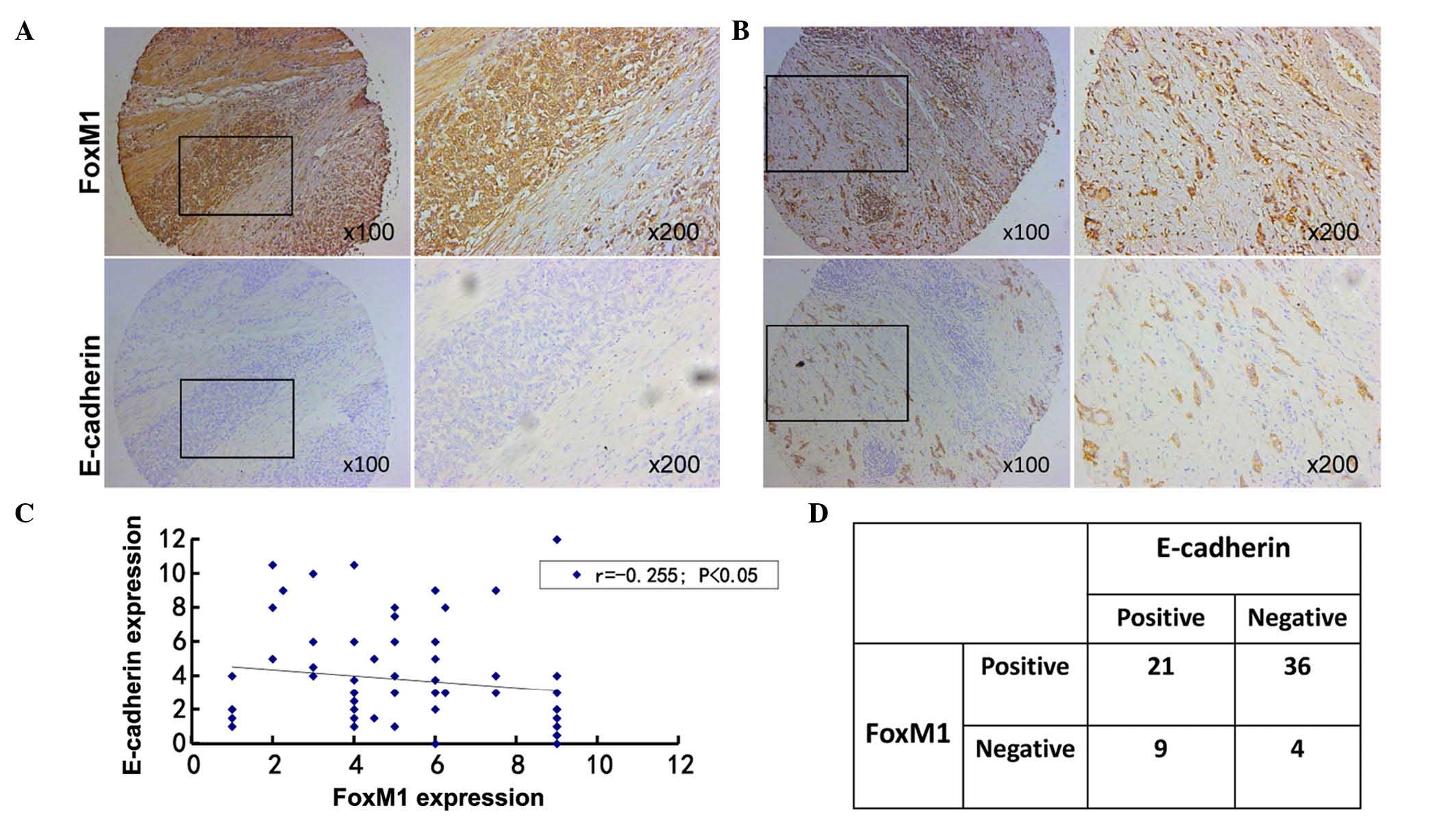
To clarify the association between FoxM1 and
E-cadherin expression in gastric cancer, the results of
immunohistochemical staining were analyzed. Among all the primary
gastric cancer tissues, 36 cases with positive FoxM1 expression and
negative E-cadherin expression were identified. There were 9 cases
with negative FoxM1 expression and positive E-cadherin expression.
The correlation between FoxM1 and E-cadherin expression in gastric
cancer was negative (r=−0.255, P=0.035). Additionally, the
staining of FoxM1 in normal gastric mucosa was negative, while the
staining of E-cadherin was positive in the same samples. Based on
these results, a negative association appears to exist between
FoxM1 and E-cadherin expression, since the staining of E-cadherin
was negative in samples that were strongly positive for FoxM1
(Fig. 3), whereas the staining of
E-cadherin was moderately positive in those samples that exhibited
moderately- positive staining for FoxM1 (Fig. 3).
Discussion
The present study aimed to determine the clinical
significance of FoxM1 and E-cadherin expression, and to uncover any
critical correlation between FoxM1 and E-cadherin in driving the
progression of gastric cancer. On the basis of the present results,
there is a clear association between FoxM1, E-cadherin and
clinicopathological features. Additionally, a negative correlation
between FoxM1 expression and E-cadherin expression was identified
in gastric cancer. Based on the aforementioned findings, it is
possible to suggest that the dysregulated expression of FoxM1 may
be responsible for E-cadherin expression, which may be a critical
contributor to the EMT of gastric cancer. The negative correlation
between FoxM1 and E-cadherin suggests that there may be a
FoxM1/E-cadherin signaling pathway that ultimately results in the
metastasis of gastric cancer.
FoxM1, a transcription factor belonging to the Fox
protein superfamily, is involved in the complicated process of
temporal and spatial gene expression during embryonic development
and tissue homeostasis (17). Lines
of newly-presented evidence have demonstrated that FoxM1 is a key
regulator, not only playing a central role in controlling gene
expression in both the G1-S and G2-M phases, but is also essential
for mitotic progression and stability of chromosomes (17). Furthermore, FoxM1 has been implicated
in different aspects of the progression of various cancers,
including cell proliferation, tumor angiogenesis, migration,
invasion, EMT and metastasis (18).
According to the literature, the expression of FoxM1 is
particularly high in pancreatic cancer (6), breast cancer (7), colorectal cancer (8) and hepatocellular carcinoma (12), as well as in a large number of other
types of tumor cells (19),
indicating that FoxM1 greatly contributes to the development and
progression of numerous human tumors. Clinical evidence reported by
Chu et al (20) revealed that
the expression of FoxM1 protein was much higher in colorectal
cancer in comparison with that in the surrounding normal tissue.
Additionally, high FoxM1 expression was closely correlated with
advanced tumor-node-metastasis (TNM) stage, lymph node metastasis
and low 5-year survival rate. In our previous studies, it was
demonstrated that the high expression of FoxM1 in pancreatic cancer
was significantly correlated with poor tumor differentiation,
advanced TNM stage and lymph node metastasis, thus manifesting the
key role of FoxM1 in the progression of pancreatic cancer (6). Similarly, the present study revealed
that high expression of FoxM1 was strongly correlated with poor
tumor differentiation, advanced tumor stage and lymph node (or
distant) metastasis, thus revealing the critical role of FoxM1 in
the development, invasion and metastasis of gastric cancer.
Accumulating evidence suggests that FoxM1 plays a critical role in
the migration, invasion and metastasis of different type of
cancers, including pancreatic cancer (21), lung adenocarcinoma (22) and prostate cancer (23), by acquiring the EMT phenotype. Our
previous study detected the expression of FoxM1 in different
pancreatic cancer cell lines, and the expression of FoxM1 in
pancreatic cancer cell lines with pronounced metastatic ability was
observed to be higher compared with that in pancreatic cancer cell
lines with poor metastatic ability (21). Kong et al demonstrated that
reduced FoxM1 expression can reverse the mesenchymal phenotype of
non-small cell lung cancer (NSCLC) cell lines into the epithelial
phenotype (24). In addition, the
migratory ability of NSCLC cell lines was decreased upon
transfection with small interfering RNA targeting FoxM1 (24). Overall, the above observations further
strengthen the hypothesis that FoxM1 may function as a tumor
promoter in gastric cancer.
It is well known that E-cadherin, which is mainly
expressed by epithelial cells and is responsible for cell-cell
adhesion in tissues, plays a critical role in the maintenance of
cell morphology and homeostasis (25). Several lines of evidence have
demonstrated that E-cadherin, termed a tumor suppressor, inhibits
the migration, invasion and dissemination of tumors by affecting
the EMT, which is acknowledged as the key mechanism underlying
metastasis of various tumor cells, including hepatocellular
(26), breast (27) and colorectal cancer (28). Zhai et al (29) observed that decreased expression of
E-cadherin was critically correlated with various
clinicopathological features of hepatocellular cancer, including
late disease stages, poor tumor differentiation and lymph node
metastasis. Consistent with these observations, in the present
study, reduced expression of E-cadherin was also strongly
associated with advanced tumor stage, poor tumor differentiation
and lymph node metastasis, indicating that E-cadherin detection may
be a significant prognostic hallmark in gastric cancer. Based on
the published literature, there are numerous potential mechanisms
involved in migration, invasion and metastasis of tumors. For
example, it was stated by Zhou et al that the activation of
the estrogen receptor β1 can upregulate the expression of
E-cadherin in breast cancer and inhibit its migration and invasion
(27). However, little is known
concerning the regulatory mechanisms involved in invasion and
metastasis and their association with FoxM1 and E-cadherin in
gastric cancer. In the current study, a negative correlation was
detected between FoxM1 and E-cadherin in tissue and cell lines of
gastric cancer. Furthermore, a previous study by Wierstra (30) revealed that the transcription factor
FoxM1c can directly combine with the promoter of the EMT-associated
gene E-cadherin in mice and humans. Therefore, we hypothesize that
FoxM1 can directly bind to the promoter of E-cadherin, thus
inducing changes in EMT, which promotes invasion and metastasis in
gastric cancer.
Taken together, the present findings have
demonstrated the expression pattern of FoxM1 and E-cadherin in
gastric cancer and adjacent normal tissue, and have also uncovered
the correlation between FoxM1, E-cadherin and clinicopathological
parameters in gastric cancer. The present results strongly suggest
that FoxM1 and E-cadherin play a vital role in the development and
progression of gastric cancer. The positive correlation between
FoxM1 and E-cadherin in tissues and cell lines of gastric cancer
indicate that there may be a FoxM1/E-cadherin signaling pathway
contributing to the invasion and metastasis of gastric cancer,
which may be a promising molecular target for the treatment of
gastric cancer in the future.
Acknowledgements
The present study was partly supported by grant no.
13PJD024 (awarded to C.H.) from the Shanghai Municipal Human
Resources and Social Security Bureau (Shanghai, China), grant no.
XYQ2013092 (awarded to C.H.) from the Shanghai Health and Family
Planning Commission (Shanghai, China) and grant no. 14411966800
(awarded to C.H.) from the Shanghai Municipal Science and
Technology Commission (Shanghai, China).
References
|
1
|
Cho JY: Molecular diagnosis for
personalized target therapy in gastric cancer. J Gastric Cancer.
13:129–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagini S: Carcinoma of the stomach: A
review of epidemiology, pathogenesis, molecular genetics and
chemoprevention. World J Gastrointest Oncol. 4:156–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yatsuya H, Toyoshima H, Mizoue T, Kondo T,
Tamakoshi K, Hori Y, Tokui N, Hoshiyama Y, Kikuchi S, Sakata K, et
al: Family history and the risk of stomach cancer death in Japan:
Differences by age and gender. Int J Cancer. 97:688–694. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Halasi M and Gartel AL: Targeting FOXM1 in
cancer. Biochem Pharmacol. 85:644–652. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alvarez-Fernández M and Medema RH: Novel
functions of FoxM1: From molecular mechanisms to cancer therapy.
Front Oncol. 3:302013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang C, Xie D, Cui J, Li Q, Gao Y and Xie
K: FOXM1c promotes pancreatic cancer epithelial-to-mesenchymal
transition and metastasis via upregulation of expression of the
urokinase plasminogen activator system. Clin Cancer Res.
20:1477–1488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang C, Chen H, Tan G, Gao W, Cheng L,
Jiang X, Yu L and Tan Y: FOXM1 promotes the epithelial to
mesenchymal transition by stimulating the transcription of Slug in
human breast cancer. Cancer Lett. 340:104–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li D, Wei P, Peng Z, Huang C, Tang H, Jia
Z, Cui J, Le X, Huang S and Xie K: The critical role of
dysregulated FOXM1-PLAUR signaling in human colon cancer
progression and metastasis. Clin Cancer Res. 19:62–72. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li X, Tang D, Yao Y, Qi W and Liang J:
Clinical significance and positive correlation of FoxM1 and Her-2
expression in gastric cancer. Clin Exp Med. 14:447–455. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Q, Zhang N, Jia Z, Le X, Dai B, Wei D,
Huang S, Tan D and Xie K: Critical role and regulation of
transcription factor FoxM1 in human gastric cancer angiogenesis and
progression. Cancer Res. 69:3501–3509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu C, Chen L, Yie L, Wei L, Wen T, Liu Y
and Chen H: Targeting FoxM1 inhibits proliferation, invasion and
migration of nasopharyngeal carcinoma through the
epithelialto-mesenchymal transition pathway. Oncol Rep.
33:2402–2410. 2015.PubMed/NCBI
|
|
12
|
Meng FD, Wei JC, Qu K, Wang ZX, Wu QF, Tai
MH, Liu HC, Zhang RY and Liu C: FoxM1 overexpression promotes
epithelial-mesenchymal transition and metastasis of hepatocellular
carcinoma. World J Gastroenterol. 21:196–213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhatt T, Rizvi A, Batta SP, Kataria S and
Jamora C: Signaling and mechanical roles of E-cadherin. Cell Commun
Adhes. 20:189–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang SW, Ping YF, Jiang YX, Luo X, Zhang
X, Bian XW and Yu PW: ATG4A promotes tumor metastasis by inducing
the epithelial-mesenchymal transition and stem-like properties in
gastric cells. Oncotarget. Jun 4–2016.(Epub ahead of print).
|
|
15
|
Yu H, Shen Y, Hong J, Xia Q, Zhou F and
Liu X: The contribution of TGF-β in Epithelial-Mesenchymal
Transition (EMT): Down-regulation of E-cadherin via snail.
Neoplasma. 62:1–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Markiewicz A, Wełnicka-Jaśkiewicz M,
Seroczyńska B, Skokowski J, Majewska H, Szade J and Żaczek AJ:
Epithelial-mesenchymal transition markers in lymph node metastases
and primary breast tumors-relation to dissemination and
proliferation. Am J Transl Res. 6:793–808. 2014.PubMed/NCBI
|
|
17
|
Bella L, Zona S, de Moraes G Nestal and
Lam EW: FOXM1: A key oncofoetal transcription factor in health and
disease. Semin Cancer Biol. 29:32–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang C, Du J and Xie K: FOXM1 and its
oncogenic signaling in pancreatic cancer pathogenesis. Biochim
Biophys Acta. 1845:104–116. 2014.PubMed/NCBI
|
|
19
|
Koo CY, Muir KW and Lam EW: FOXM1: From
cancer initiation to progression and treatment. Biochim Biophys
Acta. 1819:28–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chu XY, Zhu ZM, Chen LB, Wang JH, Su QS,
Yang JR, Lin Y, Xue LJ, Liu XB and Mo XB: FOXM1 expression
correlates with tumor invasion and a poor prognosis of colorectal
cancer. Acta Histochem. 114:755–762. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang C, Qiu Z, Wang L, Peng Z, Jia Z,
Logsdon CD, Le X, Wei D, Huang S and Xie K: A novel FoxM1-caveolin
signaling pathway promotes pancreatic cancer invasion and
metastasis. Cancer Res. 72:655–665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei P, Zhang N, Wang Y, Li D, Wang L, Sun
X, Shen C, Yang Y, Zhou X and Du X: FOXM1 promotes lung
adenocarcinoma invasion and metastasis by upregulating SNAIL. Int J
Biol Sci. 11:186–198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Yao B, Wang Y, Zhang M, Fu S, Gao
H, Peng R, Zhang L and Tang J: Increased FoxM1 expression is a
target for metformin in the suppression of EMT in prostate cancer.
Int J Mol Med. 33:1514–1522. 2014.PubMed/NCBI
|
|
24
|
Kong FF, Zhu YL, Yuan HH, Wang JY, Zhao M,
Gong XD, Liu F, Zhang WY, Wang CR and Jiang B: FOXM1 regulated by
ERK pathway mediates TGF-β1-induced EMT in NSCLC. Oncol Res.
22:29–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van Roy F: Beyond E-cadherin: Roles of
other cadherin superfamily members in cancer. Nat Rev Cancer.
14:121–134. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakagawa H, Hikiba Y, Hirata Y,
Font-Burgada J, Sakamoto K, Hayakawa Y, Taniguchi K, Umemura A,
Kinoshita H, Sakitani K, et al: Loss of liver E-cadherin induces
sclerosing cholangitis and promotes carcinogenesis. Proc Natl Acad
Sci USA. 111:1090–1095. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou Y, Ming J, Xu Y, Zhang Y and Jiang J:
ERβ1 inhibits the migration and invasion of breast cancer cells
through upregulation of E-cadherin in a Id1-dependent manner.
Biochem Biophys Res Commun. 457:141–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang W, Zhu Y, Gao J, Fu J, Liu C, Liu Y,
Song C, Zhu S, Leng Y, Wang G, et al: MicroRNA-29a promotes
colorectal cancer metastasis by regulating matrix metalloproteinase
2 and E-cadherin via KLF4. Br J Cancer. 110:450–458. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhai X, Zhu H, Wang W, Zhang S, Zhang Y
and Mao G: Abnormal expression of EMT-related proteins, S100A4,
vimentin and E-cadherin, is correlated with clinicopathological
features and prognosis in HCC. Med Oncol. 31:9702014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wierstra I: The transcription factor
FOXM1c binds to and transactivates the promoter of the tumor
suppressor gene E-cadherin. Cell Cycle. 10:760–766. 2011.
View Article : Google Scholar : PubMed/NCBI
|