Introduction
Morvan's syndrome (MoS) is a rare, complex
neurological disorder characterized by neuromyotonia,
neuropsychiatric features (insomnia, confusion, amnesia and
hallucinations), dysautonomia (hyperhidrosis, severe constipation,
drooling and cardiac arrhythmias) and neuropathic pain (1). It was first described in 1890 by the
French physician Augustin Marie Morvan as ‘chorée fibrillaire’
(2); since then, only ~60 cases have
been reported (1,3–24).
Anti-voltage-gated potassium channel (VGKC)-complex antibodies are
present in the serum of the vast majority of MoS patients,
suggesting an autoimmune aetiology (1). Although these antibodies are directed
against leucine-rich, glioma inactivated 1 (LGI-1) protein,
contactin-associated protein-2 (Caspr-2) or commonly both,
anti-Caspr-2 antibodies are predominant and are always associated
with thymoma (1). In fact, patients
with MoS may have an associated underlying tumour, such as thymoma
(most common), lung cancer, sigmoid cancer, testicular cancer and
lymphoma (1). Cases without an
associated tumour, usually experiencing a good clinical response to
immunotherapy, have also been described (1). Of note, MoS patients with thymomas and
myasthenia gravis have also been reported (5).
The present study describes a case of paraneoplastic
MoS associated with thymoma in which the characteristic symptoms of
MoS occurred following the surgical treatment of the tumour
recurrence. Written informed consent was obtained from the
patient.
Case report
In January 2010, a 35-year-old Caucasian male
patient was admitted to The Regina Elena National Cancer Institute
(Rome, Italy) and diagnosed with stage IVA thymoma, according to
the Masaoka staging system (25). The
patient was subsequently administered 3 cycles of intravenous
neoadjuvant chemotherapy consisting of 50 mg/m2 (92.5 mg
total) cisplatin, 50 mg/m2 (92.5 mg total) doxorubicin
and 500 mg/m2 (925 mg total) cyclophosphamide on day 1
every 3 weeks. Six months later, the patient underwent thymectomy,
right lung resection, partial diaphragmatic pleurectomy and
pericardio-phrenectomy, as well as partial neuroablation of the
right phrenic nerve. For the pathological evaluation, the tumour
tissue was formalin-fixed and paraffin embedded. Sections (5-µm
thick) were cut from the paraffin blocks of tumour tissue, placed
on glass slides, hydrated by decreasing alcohol percentages and
stained with Mayer's heematoxylin and eosin. Slides were then
dehydrated, rinsed with xylol and covered by mounting medium.
Finally, the slides were covered by coverslips and examined using
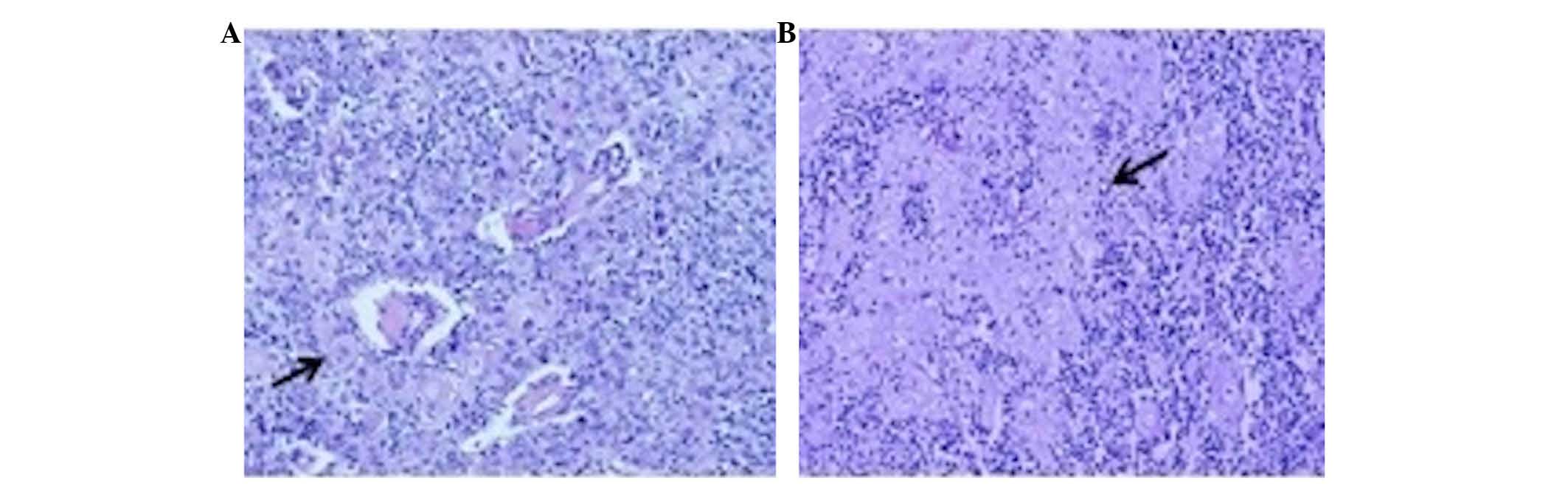
light microscopy. The pathological evaluation revealed a type B2-B3
thymoma with focal squamous differentiation, according to the World
Health Organization criteria (Fig. 1)
(26) In addition, prominent areas of
keratinization were present (Fig. 1).
Following R2 resection (presence of macroscopic residual tumour),
the patient underwent another 2 cycles of 1-day chemotherapy,
administered intravenously every 3 weeks, including: Cisplatin, 50
mg/m2 (92.5 mg); doxorubicin, 50 mg/m2 (92.5
mg); and cyclophosphamide, 500 mg/m2 (925 mg). The
results were consolidated by the adjuvant tridimensional conformal
radiotherapy with a total dose of 50 Gy (2 Gy/day, 5 days a
week).
Two years later, following a period of clinical
stability, the patient presented with local intrathoracic
recurrence and underwent wedge resection of multiple lung nodules.
Assessment of the recurrent tumour did not reveal any marked
changes in tumour histology, confirming a B2-B3 thymoma with focal
squamous differentiation. Four weeks after the second surgery, the
patient experienced generalized and continuous muscle
fasciculations, asthenia, four-limb paresthesias and dysesthesias
with mechanical allodynia. Furthermore, the patient reported
painful muscular contractions of the legs, hyperhidrosis, weight
loss (~13 kg over 2 months), drooling, generalized itching,
impotence, tachycardia, refractory insomnia, agitation, confusion
and hallucinations. Upon examination, irregular myoclonic jerks of
the four limbs, particularly the upper limbs, were observed. Tendon
reflexes were brisk. Plantars were downgoing. Serial
electrocardiograms demonstrated sinus tachycardia.
Electroencephalogram revealed only diffuse slowing of background
rhythm. Nerve conduction studies and electromyography revealed
normal motor and sensory nerve conduction velocities, multiple
repetitive compound motor action potentials to single stimulation,
no decremental response to 2-Hz stimulation of the left axillary
nerve, and doublets, triplets, multiplets and continuous
neuromyotonic discharges in multiple muscles, including the tongue
and facial muscles, without evidence of denervation. These findings
were compatible with the diagnosis of neuromyotonia. Brain magnetic
resonance imaging scan displayed no obvious findings.
Anti-acetylcholine receptor antibodies, anti-striated muscle
antibodies, anti-30-kDa titin fragment antibodies were all
negative. Genetic analysis for mutations in the gene coding for
caveolin-3 yielded negative results. Anti-VGKC-complex antibodies
were increased (333.3 pmol/l; normal range <100 pmol/l), and
both anti-LGI-1 and anti-Caspr-2 antibodies were detected in the
serum.
The patient underwent 3 cycles of intravenous
administration of immunoglobulins (0.4 g/kg/day for 5 days every 4
weeks) with little clinical and electrophysiological improvement.
The 36-Item Short-Form Health Survey from the Medical Outcome Study
(27) was used to evaluate the
quality of life of the patient, who reported a subjective
improvement. Evaluation of the levels of anti-VGKC-complex
antibodies was repeated after 6 months, and their value (456.2
pmol/l) remained increased. Both anti-LGI-1 and anti-Caspr-2
antibodies were still present.
In November 2013, at the last follow-up, the
patient's condition was relatively stable compared with that in May
2013 after the immunoglobulin therapy. The symptoms of excessive
sweating, lower limb dysesthesias and some myoclonic jerks of the
upper limbs remained. The patient was then lost to follow-up due to
relocation to another city. There are no literature data regarding
the prognosis of patients with thymoma and MoS, but patients with
advanced thymoma have been reported to have a 5-year survival rate
of 30–50% (28).
Discussion
Since the first description of MoS in 1890, only a
limited number of MoS cases (~60) have been reported in the
literature (1,3–24).
Currently, MoS is recognized as a rare combination of peripheral
nerve hyperexcitability, dysautonomia and encephalopathy.
Anti-VGKC-complex antibodies are present in 79% of cases of MoS,
and are usually directed against LGI-1 and Caspr-2 (1). LGI-1 is a key hippocampal protein of
synaptic organization that is associated with the subunits of
potassium voltage-gated channel subfamily A member 1, connecting
pre- and post-synaptic proteins [a disintegrin and
metalloproteinase (Adam)23 and Adam22, respectively] to form a
bridge (29). Anti-LGI-1-positive
patients usually have a better clinical prognosis than
anti-LGI-1-negative patients, which is likely to be dependent on
the tumour status (1,30). Caspr-2 is an axonal trans-membrane
protein of the neurexin superfamily that binds to contactin-2
(31). It is expressed in the
juxta-paranodal region (nodes of Ranvier), hippocampus and
cerebellum (32). An association has
been reported between high levels of Caspr-2 and a poor prognosis
and higher risk of cancer (1,30). Of note, thymectomy and thymoma
chemotherapy may act as disease triggers, suggesting that thymic
tumours may also harbour the antigenic targets, in particular,
Caspr-2 (33).
Clinically, MoS is characterized by peripheral nerve
involvement, with neuromyotonia, neuropathic pain, areflexia and a
stocking-type sensory loss (1).
Insomnia, spatial and temporal disorientation, confusion, amnesia,
hallucinations, agitation, epileptic seizures, abnormal behaviours,
autonomic disturbance with hyperhidrosis, pruritus, drooling,
severe constipation, urinary incontinence, excessive lacrimation,
cardiac arrhythmias, weight loss, skin lesions or itching, and
hyponatremia due to syndrome of inappropriate antidiuretic hormone
secretion, comprise all known possible symptoms (1,33).
The MoS case described in the present report is a
clinically and neurophysiologically typical case of paraneoplastic
MoS, albeit the description of a B2-B3 thymoma with prominent
squamous differentiation represents an unexpected association with
MoS; no previous report of such a case was found in the literature.
Furthermore, in the present patient, the symptoms of MoS arose a
few weeks after the surgery performed for the treatment of local
tumour recurrence, whereas the onset of paraneoplastic MoS most
commonly occurs prior to the diagnosis of the underlying tumour,
and is improved following its treatment (1).
The mechanism through which thymoma triggers
autoimmunity has been a matter of debate for several years, and a
few different explanations have been proposed (34–36). These
theories refer to the failure of positive and negative selection of
T-lymphocytes in the thymus, resulting in the alteration of the
development of T-cells, producing self-reactive lymphocytes
(34). To the best of our knowledge,
this is the first case report of MoS associated with a thymoma with
prominent squamous differentiation. We can speculate that the late
onset of symptoms in the present patient may be triggered by an
increase in the serum level of anti-VGKC antibody caused by surgery
or by thymoma recurrence itself, confirming that thymic tumours may
also harbour antigenic targets, particularly Caspr-2 (35,36).
References
|
1
|
Irani SR, Pettingill P, Kleopa KA, Schiza
N, Waters P, Mazia C, Zuliani L, Watanabe O, Lang B, Buckley C and
Vincent A: Morvan syndrome: Clinical and serological observations
in 29 cases. Ann Neurol. 72:241–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morvan A: De la chorèe fibrillaire.
Gazzette Hebdomadaire de Mèdecine et de Chirurgie. 27:173–200.
1890.
|
|
3
|
Robain O and Attal C: Morvan's analgesic
panaris in a 7-year-old child. Arch Fr Pediatr. 31:405–410.
1974.(In French). PubMed/NCBI
|
|
4
|
Murri L, Bonuccelli U, Iudice A and
Simonetti C: Sleep disturbances in a case of Morvan's chorea
(author's transl). Riv Patol Nerv Ment. 97:350–356. 1976.(In
Italian). PubMed/NCBI
|
|
5
|
Lee EK, Maselli RA, Ellis WG and Agius MA:
Morvan's fibrillary chorea: A paraneoplastic manifestation of
thymoma. J Neurol Neurosurg Psychiatry. 65:857–862. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jaben EA and Winters JL: Plasma exchange
as a therapeutic option in patients with neurologic symptoms due to
antibodies to voltage-gated potassium channels: A report of five
cases and review of the literature. J Clin Apher. 27:267–273. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Otani S, Kawamura M, Sasaki H, Nakazawa S,
Ohara H, Shimada T, Kamitani T, Asato Y, Suzuki M and Sekizuka E:
Case report; A case of Morvan syndrome: a paraneoplastic
manifestation of angioimmunoblastic T-cell lymphoma. Nihon Naika
Gakkai Zasshi. 101:3519–3521. 2012.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Loukaides P, Schiza N, Pettingill P,
Palazis L, Vounou E, Vincent A and Kleopa KA: Morvan's syndrome
associated with antibodies to multiple components of the
voltage-gated potassium channel complex. J Neurol Sci. 312:52–56.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abou-Zeid E, Boursoulian LJ, Metzer WS and
Gundogdu B: Morvan syndrome: A case report and review of the
literature. J Clin Neuromuscul Dis. 13:214–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ong E, Viaccoz A, Ducray F, Pérol M,
Cavillon G, Rogemond V, Honnorat J and Petiot P: Dramatic
improvement after rituximab in a patient with paraneoplastic
treatment-refractory Morvan syndrome associated with anti-CASPR2
antibodies. Eur J Neurol. 20:e96–e97. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lukas RV, Rezania K, Malec M and Salgia R:
Teaching Video NeuroImages: Myokymia and nerve hyperexcitability as
components of Morvan syndrome due to malignant thymoma. Neurology.
80:e552013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sharma S and Sharma P: Morvan syndrome:
After scrotal sac drainage and chemical instillation in hydrocele.
Neurol India. 61:300–302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Ren H, Ren M, Cui F, Yang F, Chen Z,
Cui L and Huang X: Morvan syndrome plus thyroid dysfunction: A case
with chronic mercury exposure. Neurol India. 62:218–219. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abgrall G, Demeret S, Rohaut B,
Leu-Semenescu S and Arnulf I: Status dissociatus and disturbed
dreaming in a patient with Morvan syndrome plus myasthenia gravis.
Sleep Med. 16:894–896. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Benedetti L, Franciotta D, Zoccarato M,
Beronio A, Godani M, Schirinzi E, Siciliano G, Ciarmiello A and Del
Sette M: Post-therapy normalization of brain FDG-PET in Morvan's
syndrome. J Neurol Sci. 353:175–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ehler E and Meleková A: Neuromyotonia with
polyneuropathy, prominent psychoorganic syndrome, insomnia, and
suicidal behavior without antibodies: A case report. J Med Case
Rep. 9:1012015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baas G Horta: Intravenous immunoglobulin
therapy in Morvan syndrome secondary to recurrent thymic carcinoma.
Medwave. 15:e63232015.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baiardi S, Provini F, Avoni P, Pasquinelli
M and Liguori R: Immunotherapy of oneiric stupor in Morvan
syndrome: Efficacy documented by actigraphy. Neurology.
84:2457–2459. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Laurencin C, André-Obadia N, Camdessanché
JP, Mauguière F, Ong E, Vukusic S, Peter-Derex L, Meyronet D,
Bouhour F, Vial C, et al: Peripheral small fiber dysfunction and
neuropathic pain in patients with Morvan syndrome. Neurology.
85:2076–2078. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lotan I, Djaldetti R, Hellman MA and
Benninger F: Atypical case of Morvan's syndrome. J Clin Neurosci.
25:132–134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Macaron G, El Rassy E and Koussa S: Morvan
syndrome secondary to thymic carcinoma in a patient with systemic
lupus erythematosus. Case Rep Neurol Med.
2016:91424862016.PubMed/NCBI
|
|
22
|
Mather H: A complicated case of metastatic
thymoma. BMJ Support Palliat Care. 6:116–118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang L, Lu Q, Guan HZ, Mei JH, Ren HT,
Liu MS, Peng B and Cui LY: A Chinese female Morvan patient with
LGI1 and CASPR2 antibodies: A case report. BMC Neurol. 16:372016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maskery M, Chhetri SK, Dayanandan R, Gall
C and Emsley HC: Morvan syndrome: A case report with patient
narrative and video. Neurohospitalist. 6:32–35. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Detterbeck FC, Nicholson AG, Kondo K, Van
Schil P and Moran C: The Masaoka-Koga stage classification for
thymic malignancies: Clarification and definition of terms. J
Thorac Oncol. 6(7 Suppl 3): S1710–S17168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Travis WD, Brambilla E, Müller-Hermelink
HK and Harris CC: World Health Organization Classification of
Tumours. Pathology and Genetics of Tumours of the Lung, Pleura,
Thymus and Heart (3rd). IARC Press. (Lyon). 2004.
|
|
27
|
Ware JE Jr and Sherbourne CD: The MOS
36-item short-form health survey (SF-36). I. Conceptual framework
and item selection. Med Care. 30:473–483. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eng TY, Fuller CD, Jagirdar J, Bains Y and
Thomas CR Jr: Thymic carcinoma State of the art review. Int J
Radiat Oncol Biol Phys. 59:654–664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kegel L, Aunin E, Meijer D and Bermingham
JR: LGI proteins in the nervous system. ASN Neuro. 5:167–181. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Irani SR, Alexander S, Waters P, Kleopa
KA, Pettingill P, Zuliani L, Peles E, Buckley C, Lang B and Vincent
A: Antibodies to Kv1 potassium channel-complex proteins
leucine-rich, glioma inactivated 1 protein and contactin-associated
protein-2 in limbic encephalitis, Morvan's syndrome and acquired
neuromyotonia. Brain. 133:2734–2748. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arroyo EJ, Xu YT, Zhou L, Messing A, Peles
E, Chiu SY and Scherer SS: Myelinating Schwann cells determine the
internodal localization of Kv1.1, Kv1.2, Kvbeta2, and Caspr. J
Neurocytol. 28:333–347. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Poliak S, Gollan L, Martinez R, Custer A,
Einheber S, Salzer JL, Trimmer JS, Shrager P and Peles E: Caspr2, a
new member of the neurexin superfamily, is localized at the
juxtaparanodes of myelinated axons and associates with K+ channels.
Neuron. 24:1037–1047. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Plantone D, Renna R and Koudriavtseva T:
Neurological diseases associated with autoantibodies targeting the
voltage-gated potassium channel complex: Immunobiology and clinical
characteristics. Neuroimmunol Neuroinflamm. 3:69–78. 2016.
|
|
34
|
Bernard C, Frih H, Pasquet F, Kerever S,
Jamilloux Y, Tronc F, Guibert B, Isaac S, Devouassoux M,
Chalabreysse L, et al: Thymoma associated with autoimmune diseases:
85 cases and literature review. Autoimmun Rev. 15:82–92. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cottrell DA, Blackmore KJ, Fawcett PRW,
Birchall D, Vincent A, Barnard S and Walls TJ: Sub-acute
presentation of Morvan's syndrome after thymectomy. J Neurol
Neurosurg Psychiatry. 75:1504–510. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lai M, Huijbers MG, Lancaster E, Graus F,
Bataller L, Balice-Gordon R, Cowell JK and Dalmau J: Investigation
of LGI1 as the antigen in limbic encephalitis previously attributed
to potassium channels: A case series. Lancet Neurol. 9:776–785.
2010. View Article : Google Scholar : PubMed/NCBI
|