Introduction
Of all types of cancer, lung cancer is the
malignancy with the greatest incidence and associated mortality,
worldwide (GLOBOCAN 2012, v1.0). The poor chances of survival for
lung cancer patients are often due to a late diagnosis (1). A variety of genetic and epigenetic
factors contributing to the development of lung cancer have been
discovered, yet a valid marker set for the early detection of
disease has not been established. Methylation of promoter regions
is a known cause for transcriptional repression (2) and can, therefore, contribute to
carcinogenesis and tumor progression (2). This epigenetic mechanism of gene
silencing has already been described in lung cancer for several
genes, including cyclin-dependent kinase inhibitor 2A,
O-6-methylguanine-DNA methyltransferase (MGMT), Ras
association domain family member 1A (RASSF1A), retinoic acid
receptor β (3–5), mutL homolog 1 (6), fragile histidine triad, death associated
protein kinase, runt related transcription factor 3, TIMP
metallopeptidase inhibitor 3 (4) or
adenomatous polyposis coli (5).
Certain studies even report promoter methylation in the bronchial
lavage (7) or blood samples (8) of lung cancer patients, whereas others
elucidate the increasing risk of developing this tumor with the
accumulation of this epigenetic change in sputum (9). In future, the knowledge of the
methylation status of tumor related genes could be helpful for the
identification of persons at risk, the early detection of lung
cancer and for the prediction of prognosis and therapeutic success.
Certain studies suspect an association between promoter methylation
and smoking habits (10) or exposure
to environmental and industrial factors, such as smoky coal
emission or chromate exposure (6,11).
To identify potential marker genes in lung cancer
and in possible precursor lesions, the methylation status of
MGMT, RASSF1A, Ras protein activator like 1
(RASAL1), programmed cell death 4 (PDCD4), metastasis
suppressor 1 (MTSS1) and tumor suppressor candidate 3
(TUSC3) in lung tumors and corresponding non-malignant
bronchus and lung tissue were quantitatively assessed using
methylation-quantification of endonuclease-resistant DNA
(MethyQESD). MethyQESD is a reliable bisulfite-conversion
independent quantitative methylation sensitive polymerase chain
reaction (qPCR) method. These six genes were chosen as they all
appear to be relevant in tumor development and progression.
The inactivation and, therefore, loss of expression
of MGMT and RASSF1A in lung cancer has been described
previously (3–6,8,12–17). The
reduced expression of RASAL1 was observed in several
malignant tumors, including brain, head and neck, bladder, breast,
colorectal, hepatocellular and thyroid carcinoma (18–21), and
appears to be associated with neoplastic progression (19). In addition, certain studies provide
evidence of an association between promoter RASAL1
methylation and loss of expression (18–21), while
treatment with the DNA methyltransferase inhibitor 5′-azacitidine
restored the expression of RASAL1 (18). PDCD4 is also suspected to act
as tumor suppressor gene (22).
Reduction or loss of expression has also been observed in lung
cancer (23,24), and may be associated with tumor
progression and a poorer prognosis (23). The impact of MTSS1 on malignant
tumors is not yet well defined. Certain studies provide evidence
that MTSS1 promotes tumor initiation and progression in
early stages (25), whereas
MTSS1 appears to have a tumor suppressive effect in advanced
stages and in metastasis (25–27).
Furthermore, the reduction and loss of expression of MTSS1
has been observed in malignancies (26,27). Other
studies contradict this finding, and found increased levels of
MTSS1 in aggressive and metastatic tumors and tumor cell
lines (28). However, in lung cancer
cell lines, MTSS1 was downregulated compared with benign
human bronchial epithelial cell lines (29). Finally, in a number of studies,
genetic and epigenetic changes and a loss of expression of
TUSC3 have been detected in several malignancies, including
prostate, ovarian, colorectal, larynx and pharynx carcinoma and
acute lymphoblastic leukemia (30–34). In
addition, TUSC3 involvement has already been observed in
non-small cell lung cancer (NSCLC) downregulation (24) and promoter methylation (35). Therefore, it is likely that
TUSC3 functions as a tumor suppressor gene.
Materials and methods
Sample collection
In total, 42 patients who had been diagnosed with
primary lung cancer at the University Hospital of Regensburg
(Regensburg, Germany) were selected for the present study. In
particular, 42 primary lung tumors (17 adenocarcinomas, 20 squamous
carcinomas, 3 large cell carcinomas and 2 neuroendocrine
carcinomas) as well as corresponding normal lung tissue (n=42) and
non-malignant bronchus tissues (n=28 for MGMT and
TUSC3, n=29 for RASSF1A, n=27 for RASAL1 and
PDCD4 and n=24 for MTSS1) were retrospectively
analyzed. Archival tissue samples were obtained from the Institute
of Pathology at the University Hospital of Regensburg, of which the
Institutional Review Board approved the study in January 1997. All
lung cancer patients underwent surgical resection between January
2000 and November 2002 at the Department of Thoracic Surgery,
University Hospital of Regensburg and all histological data were
provided by the Department of Pathology. Tumor staging was
performed according to the 7th edition of the TNM Classification of
Malignant Tumours. The age of the patients at diagnosis ranged
between 38 and 77 years (mean, 59 years). In total, 33 patients
were males (78.6%) and 9 were females (21.4%). Two patients
received neoadjuvant therapy. Clinical and histopathological data
are given in Table I.
 | Table I.Clinicopathological characteristics
of 42 non-small cell lung cancer patients. |
Table I.
Clinicopathological characteristics
of 42 non-small cell lung cancer patients.
|
Characteristics | No. of patients
(%) |
|---|
| Total | 42
(100.0) |
| Gender |
|
|
Male | 33 (78.6) |
|
Female | 9
(21.4) |
| Age at diagnosis,
years |
|
|
≤60 | 23 (54.8) |
|
>60 | 19 (45.2) |
|
Survival |
|
|
Yes | 13 (31.0) |
| No | 28 (66.7) |
|
Unknown | 1 (2.4) |
| Smoking status |
|
|
Smoker | 37 (88.1) |
|
Non-smoker | 5
(11.9) |
| Histology |
|
|
Adenocarcinoma | 17 (40.5) |
|
Squamous carcinoma | 20 (47.6) |
| Large
cell carcinoma | 3 (7.1) |
|
Neuroendocrine carcinoma | 2 (4.8) |
| T category |
|
|
T1a | 8
(19.0) |
|
T1b | 2 (4.8) |
|
T2a | 16 (38.1) |
|
T2b | 8
(19.0) |
| T3 | 3 (7.1) |
| T4 | 5
(11.9) |
| N category |
|
| N0 | 28 (66.7) |
| N1 | 11 (26.2) |
| N2 | 3 (7.1) |
| M category |
|
| M0 | 38 (90.5) |
| M1 | 3 (7.1) |
|
Unknown | 1 (2.4) |
| Grading |
|
| G2 | 24 (57.1) |
| G3 | 18 (42.9) |
| Resection
boundaries |
|
| R0 | 38 (90.5) |
| R1 | 3 (7.1) |
| R2 | 1 (2.4) |
| Stage |
|
| I | 19 (45.2) |
| II | 10 (23.8) |
|
III | 9
(21.4) |
| IV | 3 (7.1) |
|
Unknown | 1 (2.4) |
DNA extraction
Formalin-fixed paraffin-embedded 4-µm thick slides
were deparaffinized after incubation at 70°C for 30 min in xylene,
and then rehydrated in ethanol (graded series, 100, 96 and 70%) and
deionized water. Subsequently, the slides were stained with 0.01%
methylene blue. Microdissections of the bronchus, lung and tumor
tissues were performed with a stereo microscope (magnification,
×40). DNA was isolated using the MagNA Pure LC DNA-Isolation Kit II
(Roche Diagnostics, Mannheim, Germany), according to the
manufacturer's recommendations, and quantified photometrically.
Samples with low DNA content were concentrated with
Amicon® Ultra 0.5 ml Centrifugal Filters (Merck
Millipore, Darmstadt, Germany).
Quantitative methylation analysis
Methylation analysis was performed using MethyQESD
(36), a combination of
methylation-sensitive digestion and qPCR. A methylation specific
quantification digestion (MQD) containing 40 units (U) of the
methylation-sensitive endonuclease, Hin6I (Fermentas; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), and a calibrator digestion
(CalD) containing the methylation-independent endonucleases, XBaI
(20 U) and DraI (20 U) (Fermentas; Thermo Fisher Scientific, Inc.),
were set up for each sample. Each digestion, with a total reaction
volume of 20 µl, was performed using 5 µl DNA (minimum
concentration, 20 ng/µl) and 2 µl of 10x Buffer Tango™ (Fermentas;
Thermo Fisher Scientific, Inc.). Cell-line DNA served as a positive
control (SW48 for MGMT, RASSF1A, MTSS1 and
TUSC3; HT29 for RASAL1; none for PDCD4)
whereas pooled blood DNA from healthy individuals and a sample
containing no DNA were used as negative controls. Following
incubation at 37°C overnight, the reaction was stopped at 70°C for
20 min. qPCR was performed using the LightCycler 1.0 (Roche
Diagnostics) for MGMT, RASSF1A, RASAL1,
PDCD4 and MTSS1 and the LightCycler® 480
(Roche Diagnostics) for TUSC3. The total reaction volume
contained 10 µl QuantiTect® SYBR® Green PCR
Kit (Qiagen GmbH, Hilden, Germany), 0.5 µM Primer and 3 µl digested
DNA. The primers sequences were as follows: MGMT forward:
5′-CCCGGATATGCTGGGACAG-3′; MGMT reverse:
5′-CCCAGACACTCACCAAGTCG-3′; RASSF1A forward:
5′-GCTGGGCGCGCTGGGAAG-3′; RASSF1A reverse:
5′-CAGGGACCAGCTGCCGTGT-3′; RASAL1 forward:
5′-CTCCAGACGCCTCGGCAAGAG-3′; RASAL1 reverse:
5′-AGCGCCCGTCCGGACTCTAC-3′; PDCD4 forward:
5′-CCAGTCCCAGGAGCCACAT-3′; PDCD4 reverse:
5′-GAGGAAAAGGGAGAGGAGTGA-3′; MTSS1 forward:
5′-GAGCCCAGCCAGAGCGAGC-3′; MTSS1 reverse:
5′-CGGCGTCCGGATCTGTTGCT-3′; TUSC3 forward:
5′-TACCGCGCGTGGAGGAGACA-3′; TUSC3 reverse:
5′-GTGGGCAGGTACCGCAGCC-3′. After an initial denaturation of 15 min
at 95°C 45 cycles of amplification followed: Denaturation at 95°C
for 10 sec (MGMT, RASSF1A, PDCD4) or 15 sec
(RASAL1, MTSS1, TUSC3) and annealing at 60 °C
(MGMT, PDCD4, TUSC3), 65 °C (RASSF1A) and 66 °C (RASAL1, MTSS1) for
17 sec (MGMT, RASSF1A, PDCD4), 20 sec (MTSS1) and 34 sec (TUSC3).
For RASAL1 a two-step PCR was performed at 66°C for 20 sec.
For the melting point analysis, PCR products were heated from 55 to
98°C with an increase of 0.2°C/sec (MGMT, RASSF1A,
RASAL1, PDCD4, MTSS1) or 0.11°C/sec
(TUSC3). Fluorescence was measured continuously. Methylation
was quantified according to the formula: Methylation (%) =
E(CtCalD -
CtMQD)x100. (Ct =
Ct value; E = PCR efficiency). For calculation of the
Ct-Value LightCycler Software Version 3.5 (Roche Diagnostics)
(MGMT, RASSF1A, RASAL1, PDCD4 and MTSS1) and
LightCycler® 480 Software 1.5.0 (Roche Diagnostics) (TUSC3) were
used. PCR-efficiency was obtained by standard curves for MQD and
CalD with the dilution levels 1:4, 1:16, 1:64, 1:256, 1:1024,
1:4096 and 1:16384, respectively. LightCycler Software ver. 3.5 and
LightCycler® 480 Software ver. 1.5.0 was used to calculate PCR
efficiencies, which were 1.97 for MGMT, RASSF1A,
RASAL1, PDCD4 and MTSS1 and 1.94 for
TUSC3. The cut-off value for positive methylation was
>4%.
Statistical analysis
The association between two variables was analyzed
using Fisher's exact test (two-sided). Survival was estimated
according to Kaplan-Meier, and comparisons between differences in
survival were performed with the log-rank test. P<0.05 was
assumed to indicate a statistically significant difference.
Results
Methylation frequencies
The promoter methylation frequencies of MGMT,
RASSF1A, RASAL1, PDCD4, MTSS1 and
TUSC3 in lung tumor and corresponding non-malignant bronchus
and lung tissues were quantitatively assessed, with a cut-off value
of >4 per cent for positive methylation applied (Table II). No methylation was identified for
PDCD4 and MTSS1 in any tissue types, and only
sporadically for RASAL1 [bronchus, 0.0% (0/27); lung, 2.4%
(1/42); tumor, 4.8% (2/42)]. MGMT showed methylation in 7.1%
of benign bronchus (2/28) and tumor (3/42) samples, as well as in
2.4% (1/42) of non-malignant lung tissue. Simultaneous methylation
in two tissue types occurred only in one case (tumor and lung).
RASSF1A was scarcely methylated in bronchial tissue (3.4%;
1/29), not at all in normal lung (0.0%; 0/42) and in 26.2% of tumor
tissues (11/42). However, in the one case of bronchus methylation,
no other tissue type was affected. The highest methylation
frequencies were detected for TUSC3 in all three tissue
types: Bronchus, 67.9% (19/28); lung tissue, 31.0% (13/42); and
tumor tissue, 59.5% (25/42). Notably, 5 cases demonstrated
TUSC3 methylation in all three tissue types, 8 cases
demonstrated TUSC3 methylation in the bronchus and tumor
tissues, 3 cases in the lung and tumor tissues, and 9 cases
demonstrated TUSC3 methylation in the tumor tissues only. Of
the 3 cases with methylation in the lung and tumor and from 6/9
cases with tumor methylation, bronchial material was not available
for examination. In addition, 12 patients showed methylation of
>1 gene in the three tissue types. MGMT, RASSF1A
and TUSC3 were methylated more often in the bronchus
compared with in the lung tissue: MGMT, 7.1% (2/28) vs. 2.4%
(1/42); RASSF1A, 3.4% (1/29) vs. 0.0% (0/42); and
TUSC3, 67.9% (10/28) vs. 31.0% (13/42). No methylation of
any gene was detected in pooled blood DNA of healthy
individuals.
 | Table II.Frequencies of methylation in lung
cancer and corresponding non-malignant lung tissues from the same
patients. |
Table II.
Frequencies of methylation in lung
cancer and corresponding non-malignant lung tissues from the same
patients.
|
| Bronchus
tissue | Lung tissue | Tumor tissue |
|---|
|
|
|
|
|
|---|
| Analyzed gene | % patients |
n/ntotal | % patients |
n/ntotal | % patients |
n/ntotal |
|---|
| MGMT |
7.1 |
2/28 |
2.4 |
1/42 | 7.1 |
3/42 |
| RASSF1A |
3.4 |
1/29 |
0.0 |
0/42 | 26.2 | 11/42 |
| RASAL1 |
0.0 |
0/27 |
2.4 |
1/42 | 4.8 |
2/42 |
| PDCD4 |
0.0 |
0/27 |
0.0 |
0/42 | 0.0 |
0/42 |
| MTSS1 |
0.0 |
0/24 |
0.0 |
0/42 | 0.0 |
0/42 |
| TUSC3 | 67.9 | 19/28 | 31.0 | 13/42 | 59.5 | 25/42 |
Survival and clinicopathological
parameters
There was no significant association between
survival time and gender (P=0.864), smoking habits (P=0.322), tumor
histology (P=0.788) and grading (P=0.301). However, patients
diagnosed with lung cancer that were older than 60 years of age
lived significantly longer (P=0.034) compared with patients that
were younger than 60 years of age at the time of diagnosis.
Furthermore, the association between survival time and detailed
T-(tumor; detailed: Stadium subdivided in a and b), N (node)-, and
M (metastasis)-stadium (P=0.019; P=0.000; P=0.000), tumor stage
(P=0.000) and R-classification (P=0.017) reached statistical
significance.
Promoter methylation and
clinicopathological parameters
No statistically significant associations between
promoter methylation and gender, age at diagnosis, smoking habits,
lymph node and distant metastasis, grading, R-classification and
tumor histology were observed for MGMT, RASSF1A, RASAL1, PDCD4,
MTSS1 and TUSC3 and bronchus, lung and tumor tissue.
However, tumor promoter methylation of TUSC3 significantly
associated with R0-resection (R0 vs. R1 and R2, P=0.021) and
smaller tumor size (T1a, T1b and T2a vs. T2b, T3 and T4; P=0.008;
Table III). The latter effect,
however, disappeared when dismissing the subdivision of T-Stadiums
in a and b (T1 and T2 vs. T3 and T4, P=0.235). TUSC3 was also
significantly more often methylated in bronchus tissues in lower
tumor stages (stage I and II vs stage III and IV, P=0.035). In
addition, a slight tendency for higher methylation frequencies of
TUSC3 in bronchus (10 of 17 vs.9 of 11 patients) and lesser
in tumor (13 of 23 vs. 12 of 19 patients) can be observed in
patients >60 years at time of diagnosis.
 | Table III.Number of patients with positive
methylation status and clinicopathological data. |
Table III.
Number of patients with positive
methylation status and clinicopathological data.
|
| MGMT | RASSF1A | RASAL1 | TUSC3 |
|---|
|
|
|
|
|
|
|---|
| Tissue | B | L | T | B | L | T | B | L | T | B | L | T |
|---|
| Gender |
|
|
Female |
2/22 |
1/33 |
3/33 |
1/23 |
0/33 |
8/33 |
0/21 |
1/33 |
2/33 | 17/22 | 12/33 | 20/33 |
|
Male | 0/6 | 0/9 | 0/9 | 0/6 | 0/9 | 0/9 | 0/6 | 0/9 | 0/9 | 2/6 | 1/9 | 5/9 |
| Age |
|
|
≤60 |
2/18 |
1/23 |
2/23 |
0/18 |
0/23 |
6/23 |
0/17 |
1/23 |
2/23 | 10/17 |
7/23 | 13/23 |
|
>60 |
0/10 |
0/19 |
1/19 |
1/11 |
0/19 |
5/19 |
0/10 |
0/19 |
0/19 |
9/11 |
6/19 | 12/19 |
| Survival |
|
|
Yes | 1/8 |
0/13 |
1/13 | 0/9 |
0/13 |
2/13 | 0/8 |
0/13 |
0/13 | 7/9 |
6/13 |
11/13a |
| No |
1/20 |
1/28 |
2/28 |
1/20 |
0/28 |
9/28 |
0/19 |
1/28 |
2/28 | 12/19 |
7/28 |
13/28a |
| Smoking |
|
|
Yes |
2/26 |
1/37 |
3/37 |
1/27 |
0/37 |
9/37 |
0/27 |
1/37 |
2/37 | 19/26 | 11/37 | 22/37 |
| No | 0/2 | 0/5 | 0/5 | 0/2 | 0/5 | 2/5 | 0/2 | 0/5 | 0/5 | 0/2 | 2/5 | 3/5 |
| T category |
|
|
T1a | 0/2 | 1/8 | 1/8 | 0/3 | 0/8 | 2/8 | 0/2 | 1/8 | 1/8 | 3/3 | 2/8 |
6/8b |
|
T1b | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 1/2 | 0/2 | 0/2 | 0/2 | 0/2 | 2/2 |
0/2b |
|
T2a |
1/10 |
0/16 |
1/16 |
1/10 |
0/16 |
3/16 |
0/10 |
0/16 |
0/16 |
8/10 |
5/16 |
14/16b |
|
T2b | 0/7 | 0/8 | 1/8 | 0/7 | 0/8 | 4/8 | 0/6 | 0/8 | 1/8 | 5/6 | 3/8 |
2/8b |
| T3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 1/3 | 0/3 | 0/3 | 0/3 | 1/3 | 0/3 |
1/3b |
| T4 | 1/4 | 0/5 | 0/5 | 0/4 | 0/5 | 0/5 | 0/4 | 0/5 | 0/5 | 2/4 | 1/5 |
2/5b |
| N category |
|
| N0 |
2/18 |
0/28 |
1/28 |
0/19 |
0/28 |
7/28 |
0/17 |
0/28 |
1/28 | 14/18 |
9/28 | 18/28 |
| N1 | 0/8 |
1/11 |
2/11 | 1/8 |
0/11 |
3/11 | 0/8 |
1/11 |
1/11 | 4/8 |
2/11 |
5/11 |
| N2 | 0/2 | 0/3 | 0/3 | 0/2 | 0/3 | 1/3 | 0/2 | 0/3 | 0/3 | 1/2 | 2/3 | 2/3 |
| M category |
|
| M0 |
2/26 |
1/38 |
3/38 |
1/27 |
0/38 | 10/38 |
0/25 |
1/38 |
2/38 | 19/26 | 11/38 | 23/38 |
| M1 | 0/2 | 0/3 | 0/3 | 0/2 | 0/3 | 1/3 | 0/2 | 0/3 | 0/3 | 0/2 | 2/3 | 1/3 |
| Grading |
|
| G1 |
1/15 |
1/24 |
1/24 |
0/16 |
0/24 |
6/24 |
0/15 |
1/24 |
2/24 | 11/16 | 7/24 | 15/24 |
| G2 |
1/13 |
0/18 |
2/18 |
1/13 |
0/18 |
5/18 |
0/12 |
0/18 |
0/18 |
8/12 | 6/18 | 10/18 |
| Resection
boundaries |
|
| R0 | 2/26 | 1/38 | 2/38 | 1/26 | 0/38 | 11/38 | 0/25 | 1/38 | 2/38 | 18/26 | 11/38 |
25/38c |
| R1 | 0/1 | 0/3 | 1/3 | 0/1 | 0/3 | 0/3 | 0/1 | 0/3 | 0/3 | 1/1 | 1/3 |
0/3c |
| R2 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 1/1 |
0/1c |
| Histology |
|
| AC | 1/11 | 0/17 | 0/17 | 0/11 | 0/17 | 6/17 | 0/11 | 0/17 | 0/17 | 6/11 | 3/17 | 12/17 |
|
SCC | 0/13 | 1/20 | 2/20 | 1/14 | 0/20 | 4/20 | 0/12 | 1/20 | 2/20 | 10/13 | 9/20 | 11/20 |
|
LCA | 1/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 2/3 | 1/3 | 1/3 |
|
Carc | 0/1 | 0/2 | 1/2 | 0/1 | 0/2 | 1/2 | 0/1 | 0/2 | 0/2 | 1/1 | 0/2 | 1/2 |
| Stage |
|
| I | 1/11 | 0/19 | 1/19 | 0/12 | 0/19 | 4/19 | 0/11 | 0/19 | 0/19 |
9/12d | 6/19 | 15/19 |
| II | 0/7 | 1/10 | 2/10 | 1/7 | 0/10 | 4/10 | 0/6 | 1/10 | 2/10 |
6/6d | 3/10 | 4/10 |
|
III | 1/8 | 0/9 | 0/9 | 0/8 | 0/9 | 2/9 | 0/8 | 0/9 | 0/9 |
4/8d | 2/9 | 4/9 |
| IV | 0/2 | 0/3 | 0/3 | 0/2 | 0/3 | 1/3 | 0/2 | 0/3 | 0/3 |
0/2d | 2/3 | 1/3 |
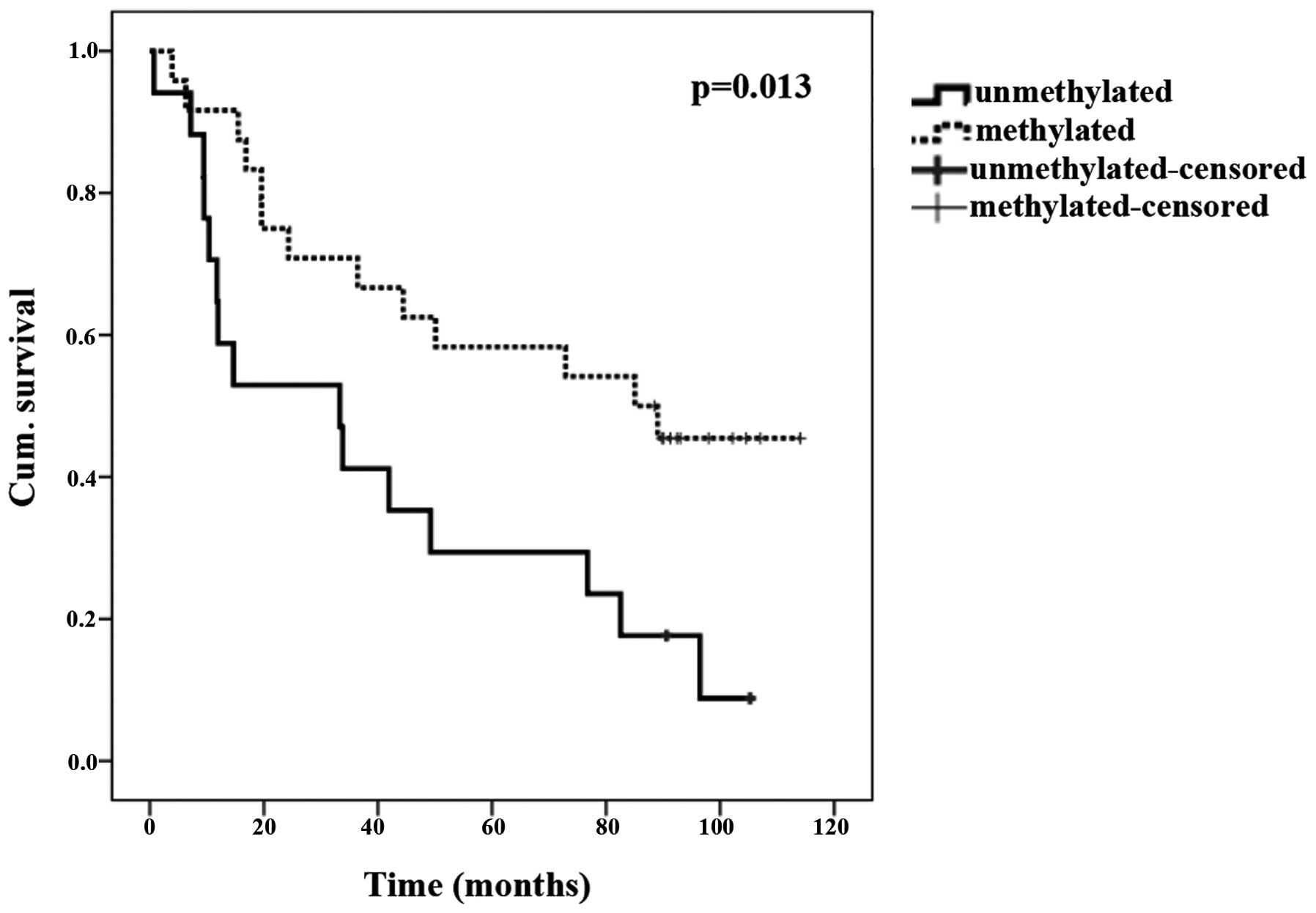
Promoter methylation and survival
Patients with promoter methylation of TUSC3
in tumor tissues lived significantly longer compared with those
without this epigenetic modification (P=0.039; Fisher's exact
test). The Kaplan-Meier curve is shown in Fig. 1 (P=0.013; log-rank test).
Discussion
In the present study, the promoter methylation
status of MGMT, RASSF1A, RASAL1, PDCD4,
MTSS1 and TUSC3 was quantitatively examined using a
highly reliable methylation-sensitive qPCR, avoiding
bisulfite-conversion.
In the patients studied, MGMT methylation was
less frequently observed in lung tumor tissues compared with other
studies, which describe methylation frequencies of 10.0–77.8%
(3,4,6,8,12,16,17). In
contrast to the present study, the majority of other studies did
not analyze normal tissue; therefore, statements concerning the use
of MGMT methylation in the early detection of lung cancer by
analyzing non-malignant samples cannot be reasonably made. However,
two reports (3,12) with normal tissues had greater
methylation frequencies (~20%) compared with the 7.1% MGMT
methylation found in bronchial or 2.4% found in lung specimens in
the present study, which are similar to the 3% methylation reported
by Safar et al (16).
The findings of low RASSF1A promoter
methylation levels are in accordance with other reports, which
describe methylation frequencies of 0.0–6.0% in normal tissues
(5,13,16,37,38),
12.8% methylation in benign lung tissues (3) and 15.0–45.0% in NSCLC (3–5,7,8,13–16,37,38).
Differences in MGMT and RASSF1A
methylation frequencies between the results of the present study
and the results of previous studies may be due to different
analysis methods. In contrast to the MethyQESD technique (36), previous studies used methods such as
bisulfite-conversion, which bears the risk of incomplete conversion
of unmethylated cytosines (39),
leading to an overestimation of methylation. Furthermore, the use
of methylation specific primers without enough discrimination
capacity between methylated and unmethylated DNA can also result in
false positive methylation results (39). In addition, cut off values are not
mentioned (12), or set at 0%
(8), or methylation is analyzed only
qualitatively (3,4,6,14,15,17).
Certain studies employed a nested PCR (3,6), which
improves sensitivity but can lower specificity and reproducibility
(40). This effect is further
demonstrated by the experiments of Lee et al (38), which show that MGMT methylation
differed depending on whether methylation specific PCR (qualitative
analysis) or pyrosequencing (quantitative analysis) was used.
Therefore, the comparability of methylation studies is limited.
In the present study, only low levels or no
methylation of RASAL1 (tumor tissue, 4.8%; lung, 2.4%;
bronchus, 0.0%) were observed, and consequently no association with
clinicopathological parameters was found. To the best of our
knowledge, only two other studies have dealt with methylation of
RASAL1 in lung carcinoma: Jin et al (18) observed methylation in 2/4 lung cancer
cell lines; whereas Calvisi et al (20) reported a methylation frequency of
16.7% (5/30) in primary tumors, but did not describe the
characteristics of the patient population nor the histology of the
tumors analyzed. This is especially important as RASAL1
shows tumor and tissue-specific expression (19) with increased expression levels in
endocrine organs (19), which raises
the question of whether RASAL1, in general, is a cancer
relevant gene in lung adenocarcinoma.
In the present study, no methylation of PDCD4
or MTSS1 was found in any of the 3 tissue types. Promoter
methylation of PDCD4 and MTSS1 genes has been
previously examined in other tumors (41,42), but
not yet in lung cancer. According to the current results, factors
other than methylation could be involved in the regulation of the
expression of MTSS1, such as binding of DNA
(cytosine-5-)-methyltransferase 3β to the MTSS1 5′ region
(43,44), and PDCD4, such as transcription
growth factor β (22), zinc finger
protein 148 and histone modifications (44).
In contrast to the other analyzed genes,
TUSC3 showed frequent methylation in all three tissue types:
Bronchus, 67.9% (19/28); lung, 31.0% (13/42); and tumor, 59.5%,
(25/42). Thus, TUSC3 promoter methylation could be an early
event during bronchial tumor development, and the detection of
TUSC3 methylation could be beneficial for the early
detection of lung cancer. Regarding prognostic aspects, the present
study shows that patients with TUSC3 methylation in tumor
tissues lived significantly longer compared with patients without
this epigenetic modification (Fig.
1). In addition, patients that were diagnosed with lung cancer
when they were older than 60 years of age lived significantly
longer (P=0.034) compared with patients diagnosed when they were
younger than 60 years of age, and showed slightly more methylation
in bronchus and tumor tissues, although without statistical
significance. This finding could be due to an association between
longer survival and TUSC3 methylation. Tumor methylation was
significantly associated with R0-resection and smaller tumor size
as T-stadiums were subdivided into a and b. Although longer
survival is an evident result, the potential causality between
TUSC3 methylation and longer survival remains to be
elucidated.
In certain aspects, the present study contrasts
with previous studies, which found an association between
TUSC3 methylation and a poorer prognosis or advanced tumor
stage in other tumor types (31,33).
Furthermore, the first evidence of a loss of TUSC3
expression was found in metastatic prostate cancer (30), which implies that the loss of
TUSC3 expression may be associated with progressed disease
in this tumor type. Contrarily, TUSC3 can be reasonably
assumed to have a non-oncogenic function due to a defect in
TUSC3 that was previously described to cause non-syndromic
autosomal mental retardation without tumor formation in affected
patients (45).
Finally, the methylation of TUSC3 may
potentially occur due to collateral damage along with other gene
methylation events in the 8p22 chromosome region (46). Consequently, its loss could be without
direct consequence for tumorigenesis. Previous studies showed that
TUSC3 methylation was not beneficial for tumor growth in one
cell culture experiment (32),
whereas in another experiment, it was (31).
In conclusion, the present study identified little
or no promoter methylation of MGMT, RASSF1A,
RASAL1, PDCD4 and MTSS1 in bronchial, lung and
lung cancer tissues, but the relatively frequent methylation of
TUSC3 in the same tissues. The fact that TUSC3
methylation was found to be associated with a longer survival time
contradicts the hypothesis that TUSC3 has a tumor suppressor
function and underlines that TUSC3 methylation has a
prognostic value in lung cancer patients. In addition, the
methylation of TUSC3, particularly in combination with other
markers, may be useful for the early detection of lung cancer, as
TUSC3 was frequently observed in the tumor and benign
bronchus and lung tissues of lung cancer patients, but not in
pooled blood DNA of healthy individuals. Additional studies are
required to clarify the functional role of TUSC3 methylation
in lung cancer. Prospective studies are recommended to be
undertaken to further evaluate TUSC3 methylation as a
prognostic biomarker and its usefulness for the early detection of
disease in lung cancer patients.
Acknowledgements
The present study was supported by the
Wilhelm-Sander-Foundation (grant no., 2000.127.1). The authors
would like to thank Mrs Irene Schardt, Mrs Beate Reil, Mrs Sigi
Appel and Mrs Jutta Förster for excellent technical assistance and
Dr Corinna Lang-Schwarz for tissue sampling.
References
|
1
|
Adamietz IA and Niederle N: Lung cancer.
Onkologe. 16:615–628. 2010.(In German). View Article : Google Scholar
|
|
2
|
Herman JG and Baylin SB: Gene silencing in
cancer in association with promoter hypermethylation. N Engl J Med.
349:2042–2054. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Licchesi JD, Westra WH, Hooker CM and
Herman JG: Promoter hypermethylation of hallmark cancer genes in
atypical adenomatous hyperplasia of the lung. Clin Cancer Res.
14:2570–2578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yanagawa N, Tamura G, Oizumi H, Kanauchi
N, Endoh M, Sadahiro M and Motoyama T: Promoter hypermethylation of
RASSF1A and RUNX3 genes as an independent prognostic prediction
marker in surgically resected non-small cell lung cancers. Lung
Cancer. 58:131–138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feng Q, Hawes SE, Stern JE, Wiens L, Lu H,
Dong ZM, Jordan CD, Kiviat NB and Vesselle H: DNA methylation in
tumor and matched normal tissues from non-small cell lung cancer
patients. Cancer Epidemiol Biomarkers Prev. 17:645–654. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ali AH, Kondo K, Namura T, Senba Y,
Takizawa H, Nakagawa Y, Toba H, Kenzaki K, Sakiyama S and Tangoku
A: Aberrant DNA methylation of some tumor suppressor genes in lung
cancers from workers with chromate exposure. Mol Carcinog.
50:89–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim H, Kwon YM, Kim JS, Lee H, Park JH,
Shim YM, Han J, Park J and Kim DH: Tumor-specific methylation in
bronchial lavage for the early detection of non-small-cell lung
cancer. J Clin Oncol. 22:2363–2370. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Begum S, Brait M, Dasgupta S, Ostrow KL,
Zahurak M, Carvalho AL, Califano JA, Goodman SN, Westra WH, Hoque
MO and Sidransky D: An epigenetic marker panel for detection of
lung cancer using cell-free serum DNA. Clin Cancer Res.
17:4494–4503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leng S, Do K, Yingling CM, Picchi MA, Wolf
HJ, Kennedy TC, Feser WJ, Baron AE, Franklin WA, Brock MV, et al:
Defining a gene promoter methylation signature in sputum for lung
cancer risk assessment. Clin Cancer Res. 18:3387–3395. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang J, Shen Y, Liu B and Tong Y: Promoter
methylation of BRMS1 correlates with smoking history and poor
survival in non-small cell lung cancer patients. Lung Cancer.
74:305–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Lan Q, Shen M, Jin J, Mumford J,
Ren D and Keohavong P: Aberrant gene promoter methylation in sputum
from individuals exposed to smoky coal emissions. Anticancer Res.
28:2061–2066. 2008.PubMed/NCBI
|
|
12
|
Brabender J, Usadel H, Metzger R,
Schneider PM, Park J, Salonga D, TsaoWei DD, Groshen S, Lord RV,
Takebe N, et al: Quantitative O(6)-methylguanine DNA
methyltransferase methylation analysis in curatively resected
non-small cell lung cancer: Associations with clinical outcome.
Clin Cancer Res. 9:223–227. 2003.PubMed/NCBI
|
|
13
|
DeJong WK, Verpooten GF, Kramer H,
Louwagie J and Groen HJ: Promoter methylation primarily occurs in
tumor cells of patients with non-small cell lung cancer. Anticancer
Res. 29:363–369. 2009.PubMed/NCBI
|
|
14
|
Lin Q, Geng J, Ma K, Yu J, Sun J, Shen Z,
Bao G, Chen Y, Zhang H, He Y, et al: RASSF1A, APC, ESR1, ABCB1 and
HOXC9, but not p16INK4A, DAPK1, PTEN and MT1G genes were frequently
methylated in the stage I non-small cell lung cancer in China. J
Cancer Res Clin Oncol. 135:1675–1684. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maruyama R, Sugio K, Yoshino I, Maehara Y
and Gazdar AF: Hypermethylation of FHIT as a prognostic marker in
nonsmall cell lung carcinoma. Cancer. 100:1472–1477. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Safar AM, Spencer H III, Su X, Coffey M,
Cooney CA, Ratnasinghe LD, Hutchins LF and Fan CY: Methylation
profiling of archived non-small cell lung cancer: A promising
prognostic system. Clin Cancer Res. 11:4400–4405. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Toyooka S, Maruyama R, Toyooka KO,
McLerran D, Feng Z, Fukuyama Y, Virmani AK, ZochbauerMuller S,
Tsukuda K, Sugio K, et al: Smoke exposure, histologic type and
geography-related differences in the methylation profiles of
non-small cell lung cancer. Int J Cancer. 103:153–160. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin H, Wang X, Ying J, Wong AH, Cui Y,
Srivastava G, Shen ZY, Li EM, Zhang Q, Jin J, et al: Epigenetic
silencing of a Ca(2+)-regulated Ras GTPase-activating protein RASAL
defines a new mechanism of Ras activation in human cancers. Proc
Natl Acad Sci USA. 104:12353–12358. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ohta M, Seto M, Ijichi H, Miyabayashi K,
Kudo Y, Mohri D, Asaoka Y, Tada M, Tanaka Y, Ikenoue T, et al:
Decreased expression of the RAS-GTPase activating protein RASAL1 is
associated with colorectal tumor progression. Gastroenterology.
136:206–216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Calvisi DF, Ladu S, Conner EA, Seo D,
Hsieh JT, Factor VM and Thorgeirsson SS: Inactivation of Ras
GTPase-activating proteins promotes unrestrained activity of
wild-type Ras in human liver cancer. J Hepatol. 54:311–319. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seto M, Ohta M, Ikenoue T, Sugimoto T,
Asaoka Y, Tada M, Mohri D, Kudo Y, Ijichi H, Tateishi K, et al:
Reduced expression of RAS protein activator like-1 in gastric
cancer. Int J Cancer. 128:1293–1302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang H, Ozaki I, Mizuta T, Hamajima H,
Yasutake T, Eguchi Y, Ideguchi H, Yamamoto K and Matsuhashi S:
Involvement of programmed cell death 4 in transforming growth
factor-betal-induced apoptosis in human hepatocellular carcinoma.
Oncogene. 25:6101–6112. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Y, Knösel T, Kristiansen G, Pietas A,
Garber ME, Matsuhashi S, Ozaki I and Petersen I: Loss of PDCD4
expression in human lung cancer correlates with tumour progression
and prognosis. J Pathol. 200:640–646. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Woenckhaus M, KleinHitpass L, Grepmeier U,
Merk J, Pfeifer M, Wild P, Bettstetter M, Wuensch P, Blaszyk H,
Hartmann A, et al: Smoking and cancer-related gene expression in
bronchial epithelium an non-small-cell lung cancers. J Pathol.
210:192–204. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dawson JC, Timpson P, Kalna G and Machesky
LM: Mtss1 regulates epidermal growth factor signaling in head and
neck squamous carcinoma cells. Oncogene. 31:1781–1793. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu K, Wang G, Ding H, Chen Y, Yu G and
Wang J: Downregulation of metastasis suppressor 1 (MTSS1) is
associated with nodal metastasis and poor outcome in Chinese
patients with gastric cancer. BMC Cancer. 10:4282010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie F, Ye L, Chen J, Wu N, Zhang Z, Yang
Y, Zhang L and Jiang WG: The impact of metastasis suppressor-1,
MTSS1, on oesophageal squamous cell carcinoma and its clinical
significance. J Transl Med. 9:952011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jahid S, Sun J, Edwards RA, Dizon D,
Panarelli NC, Milsom JW, Sikandar SS, Gümüs ZH and Lipkin SM:
miR-23a promotes the transition from indolent to invasive
colorectal cancer. Cancer Discov. 2:540–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lam DC, Girard L, Suen WS, Chung L, Tin
VP, Lam WK, Minna JD and Wong MP: Establishment and expression
profiling of new lung cancer cell lines from Chinese smokers and
lifetime never-smokers. J Thorac Oncol. 1:932–942. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bova GS, MacGrogan D, Levy A, Pin SS,
Bookstein R and Isaacs WB: Physical mapping of chromosome 8p22
markers and their homozygous deletion in a metastatic prostate
cancer. Genomics. 35:46–54. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pils D, Horak P, Vanhara P, Anees M, Petz
M, Alfanz A, Gugerell A, Wittinger M, Gleiss A, Auner V, et al:
Methylation status of TUSC3 is a prognostic factor in ovarian
cancer. Cancer. 119:946–954. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ahuja N, Li Q, Mohan AL, Baylin SB and
Issa JP: Aging and DNA methylation in colorectal mucosa and cancer.
Cancer Res. 58:5489–5494. 1998.PubMed/NCBI
|
|
33
|
Guervòs MA, Marcos CA, Hermsen M, Nuño AS,
Suárez C and Llorente JL: Deletions of N33, STK11 and TP53 are
involved in the development of lymph node metastasis in larynx and
pharynx carcinomas. Cell Oncol. 29:327–334. 2007.PubMed/NCBI
|
|
34
|
Scholz C, Nimmrich I, Burger M, Becker E,
Dörken B, Ludwig WD and Maier S: Distinction of acute lymphoblastic
leukemia from acute myeloid leukemia through microarray-based DNA
methylation analysis. Ann Hematol. 84:236–244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zemlyakova VV, Zhevlova AI, Zborovskaya
IB, Strelnikov VV, Laktionov KK, Zaletaev DV and Nemtsova MV:
Methylation profile of several tumor suppressor genes in
non-small-cell lung cancer. Mol Biol. 37:836–840. 2003. View Article : Google Scholar
|
|
36
|
Bettstetter M, Dechant S, Ruemmele P,
Vogel C, Kurz K, Morak M, Keller G, HolinskiFeder E, Hofstaedter F
and Dietmaier W: MethyQESD, a robust and fast method for
quantitative methylation analyses in HNPCC diagnostics using
formalin-fixed and paraffin-embedded tissue samples. Lab Invest.
88:1367–1375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kubo T, Yamamoto H, Ichimura K, Jida M,
Hayashi T, Otani H, Tsukuda K, Sano Y, Kiura K and Toyooka S: DNA
methylation in small lung adenocarcinoma with bronchioloalveolar
carcinoma components. Lung Cancer. 65:328–332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee SM, Lee WK, Kim DS and Park JY:
Quantitative promoter hypermethylation analysis of RASSF1A in lung
cancer: Comparison with methylation-specific PCR technique and
clinical significance. Mol Med Rep. 5:239–244. 2012.PubMed/NCBI
|
|
39
|
Kristensen LS, Mikeska T, Krypuy M and
Dobrovic A: Sensitive melting analysis after real time-methylation
specific PCR (SMART-MSP): High-throughput and probe-free
quantitative DNA methylation detection. Nucleic Acids Res.
36:e422008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Grote HJ, Schmiemann V, Kazimirek M and
Böcking A: Quantitative methylation-specific PCR for the diagnosis
of lung cancer. Pathologe. 28:377–383. 2007.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cao Z, Yoon JH, Nam SW, Lee JY and Park
WS: PDCD4 expression inversely correlated with miR-21 levels in
gastric cancers. J Cancer Res Clin Oncol. 138:611–619. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Utikal J, Gratchev A, MullerMolinet I,
Oerther S, Kzhyshkowska J, Arens N, Grobholz R, Kannookadan S and
Goerdt S: The expression of metastasis suppressor MIM/MTSS1 is
regulated by DNA methylation. Int J Cancer. 119:2287–2293. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fan H, Chen L, Zhang F, Quan Y, Su X, Qiu
X, Zhao Z, Kong KL, Dong S, Song Y, et al: MTSS1, a novel target of
DNA methyltransferase 3B, functions as a tumor suppressor in
hepatocellular carcinoma. Oncogene. 31:2298–2308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Leupold JH, Asangani IA, Mudduluru G and
Allgayer H: Promoter cloning and characterization of the human
programmed cell death protein 4 (pdcd4) gene: Evidence for ZBP-89
and Sp-binding motifs as essential Pdcd4 regulators. Biosci Rep.
32:281–297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Garshasbi M, Hadavi V, Habibi H, Kahrizi
K, Kariminejad R, Behjati F, Tzschach A, Najmabadi H, Ropers HH and
Kuss AW: A defekt in the TUSC3 gene is associated with autosomal
recessive mental retardation. Am J Hum Genet. 82:1158–1164. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Levy A, Dang UC and Bookstein R:
High-density screen of human tumor cell lines for homozygous
deletions of loci on chromosome arm 8p. Genes Chromosomes Cancer.
24:42–47. 1999. View Article : Google Scholar : PubMed/NCBI
|