Introduction
Keloid disease (KD), alternatively known as keloid
scarring, is a type of fibrous tissue proliferation that commonly
occurs following skin trauma or plastic surgery (1). Previous studies (2) have demonstrated that the pathogenesis of
KD is associated with the functional activity of multiple signaling
transduction pathways, genes and cytokines. Fibroblasts are the
primary effector cells of KD tissue, the typical pathological
features of which include excessive proliferation, disordered
apoptosis and secretion of large amounts of extracellular matrix
(3).
Long non-coding RNAs (lncRNAs) are a group of RNA
molecules, which range in length from 200 to 100,000 nucleotides
and do not encode any proteins (4).
Numerous studies (5) have
demonstrated that lncRNAs participate in various important
regulatory processes, including transcriptional activation,
transcriptional interference and intranuclear transport. Aberrant
expression of the lncRNA H19 has been observed in various tumor
types, suggesting potential oncogenic activity (6). Conversely, deletion of H19 reduces the
proliferation and invasiveness of tumor cells (7). Given that H19 has a unique role in gene
expression and tumorigenesis, the present study aimed to
investigate the hypothesis that H19 may also be associated with KD.
The expression of H19 RNA in KD tissue from patients was initially
measured using reverse transcription-polymerase chain reaction
(RT-PCR), and the association between H19 and proliferation of KD
fibroblasts was subsequently investigated using small interfering
(si)RNA transfection and pathway analysis. The results define the
function of H19 in KD and suggest a signaling mechanism that may be
associated with the proliferation of keloid fibroblasts.
Materials and methods
Study participants and sample
collection
Tissue specimens were collected from 8 KD patients
who received treatment at The First Affiliated Hospital of Nanchang
University (Nanchang, China) between May 2006 and June 2013. None
of the patients had received any medical treatment prior to
surgery. Informed consent was obtained from all patients prior to
surgery, and the research protocol was approved by the Ethics
Committee of The First Affiliated Hospital of Nanchang University
(approval no., 2014D0307) prior to sample collection. The diagnosis
of KD was confirmed by postoperative pathology, according to scar
management practical guidelines (8,9). As a
control, an additional 8 cases of normal skin and 8 cases of normal
mature scars were collected from the chest and back, which was
similar to those of the KD cases.
Isolation and culture of
fibroblasts
Freshly resected specimens were washed with normal
saline three times. Following removal of the epithelial tissue, the
specimens were cut into 2×2-mm tissue blocks and spread evenly
across sterile flasks. Following the addition of 0.5 ml Dulbecco's
modified Eagle's medium (DMEM; InvivoGen, San Diego, CA, USA), the
tissues were cultured at 37°C in an atmosphere of 5% CO2
in an incubator for 4 h. Subsequently, an additional 5 ml DMEM and
a small amount of fetal bovine serum (FBS; InvivoGen) were added,
the flasks were rotated and culture was continued. The medium was
replaced every 3–4 days. When the cultured fibroblasts grew to
confluence, they were washed with phosphate-buffered saline,
digested with 0.25% trypsin (InvivoGen) and passaged in fresh
medium. Passage-three cells were used for subsequent
experiments.
Liposome-mediated siRNA
interference
In vitro-cultured fibroblasts were left
untransfected (blank control group), or were transfected with H19
siRNA (H19 siRNA group) or a non-homologous siRNA (negative control
group). The H19 siRNA was composed of a mixture of three siRNAs:
H19-siRNA1, 5′-CCAACAUCAAAGACACCA UdTdT-3′; H19-siRNA2,
5′-GCAGGACAUGACAUGGUC CdTdT-3′; and H19-siRNA3,
5′-UAAGUCAUUUGCACUGGU UdTdT-3′ (10).
When cells grew to 60–80% confluence, transfection was performed
with Attractene Transfection Reagent (Qiagen, Inc., Valencia, CA,
USA) according to the manufacturer's protocol (8 wells/group).
Following 5 h of transfection, DMEM containing 20% FBS was added,
and cells were cultured at 37°C for 24 h. Subsequently, the medium
was replaced with fresh DMEM containing 10% FBS, and cell culture
was continued for 24–48 h prior to harvesting.
Detection of lncRNA H19 expression by
RT-PCR
Total RNA was isolated from fresh tissue specimens
(100 mg) from each patient, or from cultured transfected cells
harvested 1 day subsequent to fibroblast transfection. RNA was
extracted using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. Reverse transcription was performed with
the SuperScript® II Reverse Transcriptase kit
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Subsequently, PCR was performed in order
to assess the expression of lncRNA H19 using the
GeneAmp® PCR System 9700 (Applied Biosystems; Thermo
Fisher Scientific, Inc.) using PrimeSTAR HS DNA Polymerase with GC
Buffer (Takara Bio, Dalian, China). Glyceraldehyde-3 phosphate
dehydrogenase (GAPDH) was used as the internal loading control. The
primers were designed using Primer Premier 5.0 software (Premier
Biosoft International, Palo Alto, CA, USA) and synthesized by
Sangon Biotech Co. Ltd. (Shanghai, China) as follows: H19, forward
5′-TACAACCACTGCACTACCTG-3′ and reverse 5′-TGGAATGCTTGAAGGCTGCT-3′;
and GAPDH, forward 5′-GGGAGCCAAAAGGGTCAT-3′ and reverse
5′-GAGTCCTTCCACGATACCAA-3′. The reaction conditions were 95°C for
10 min, followed by 40 cycles of 95°C for 30 sec and 60°C for 1
min. The specificity of amplification products was confirmed using
a negative control (no cDNA) and a RT-minus control (no RT). The
relative expression of H19 in each group was quantified by agarose
gel electrophoresis with ethidium bromide [Tiangen Biotech
(Beijing) Co., Ltd., Beijing, China], followed by measurement of
the optical density (OD) of the electrophoretic bands (NanoDrop
1000 Spectrophotometer; Thermo Fisher Scientific Inc., Wilmington,
DE, USA). The DNA ladder was also obtained from Tiangen Biotech
(Beijing) Co., Ltd.. Each specimen was tested 8 times.
Assessment of proliferation of KD
fibroblasts by 3-(4,5-
dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The transfected fibroblasts were digested with
trypsin and seeded as cell suspensions at a concentration of
1×104 cells/ml into 96-well plates, with 100 µl
suspension per well. A total of 20 µl MTT (5 mg/ml) was added to
each of 8 wells per group, and culture was continued for 12, 24 or
48 h. Following the end of this incubation period, the supernatants
were discarded and 150 µl dimethyl sulfoxide was added to each
well. Plates were vibrated to dissolve the crystals thoroughly, and
OD was measured at 490 nm (NanoDrop 1000 Spectrophotometer).
Detection of vascular endothelial
growth factor (VEGF) and mammalian target of rapamycin (mTOR)
expression in transfected fibroblasts by western blotting
Cells were harvested 48 h subsequent to
transfection, and lysates were prepared using RIPA cell lysis
reagent (Beyotime Institute of Biotechnology, Beijing, China)
according to the manufacturer's protocol. Protein concentrations in
lysates were quantified by the Bradford method (11), and equal amounts of protein were
loaded onto sodium dodecyl sulfate-polyacrylamide gels. Following
electrophoresis, the proteins were transferred to membranes using a
wet transfer method, blocked with 1% bovine serum albumin in
Tris-buffered saline containing 0.1% Tween 20 (TBST; Sangon Biotech
Co. Ltd.) and incubated with primary antibodies (dilution, 1:500)
overnight. The polyclonal goat anti-rabbit anti-VEGF (catalog no.,
sc-507), polyclonal goat anti-rabbit anti-mTOR (catalog no.,
sc-8319) and polyclonal goat anti-rabbit anti-caspase-3 (catalog
no., sc-7148) antibodies were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The internal reference, a
polyclonal goat anti-rabbit anti-GAPDH antibody (dilution, 1:5,000;
catalog no., G9545) was from Sigma-Aldrich (St. Louis, MO, USA).
The membranes were washed in TBST and incubated with goat
anti-rabbit horseradish peroxidase-labeled secondary antibody
(dilution, 1:5,000; catalog no., CW0103S; ComWin Biotech Co., Ltd.,
Beijing, China) at room temperature for 1 h. Protein bands were
detected using Immoblion Western Chemiluminescent HRP Substrate
(EMD Millipore, Billerica, MA, USA), and the intensity of the bands
was analyzed using ImageJ software (National Institute of Health,
Bethesda, MD, USA).
Statistical analysis
Data were analyzed by SPSS version 19.0 (IBM SPSS,
Armonk, NY, USA) and are expressed as the mean ± standard
deviation. Comparisons were performed using analysis of variance
and the Student's t-test. Paired comparisons were performed
by Fisher's least significant difference test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of lncRNA H19 is elevated
in KD tissues
To determine whether H19 expression is associated
with KD, tissue samples were collected from 24 study participants,
including 8 with keloids, 8 with normal scars and 8 normal skin
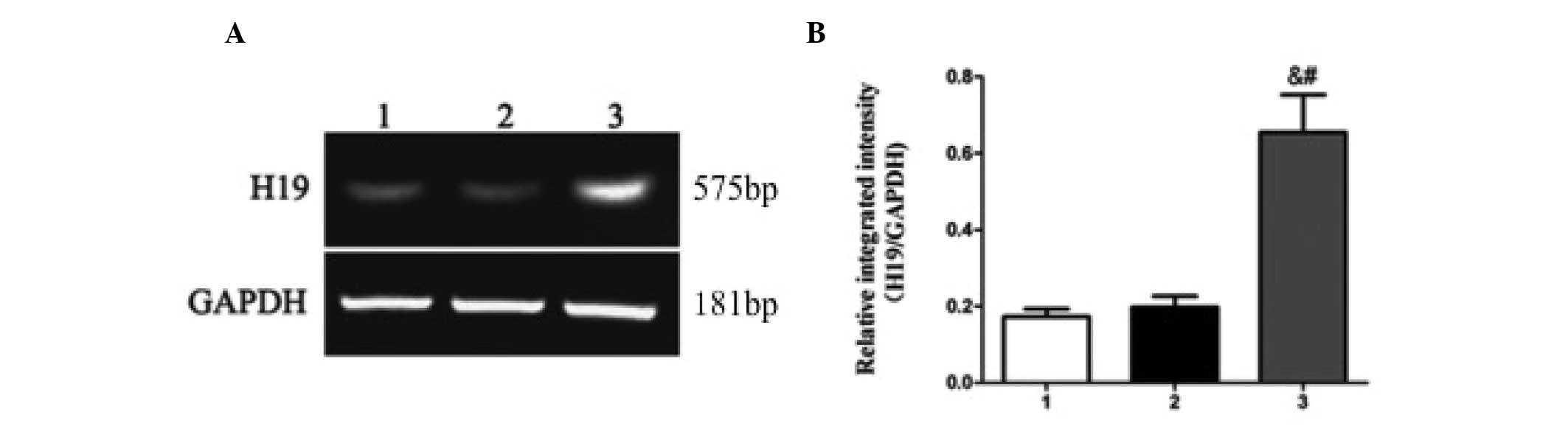
controls. The expression of lncRNA H19 was significantly increased
in KD tissues (0.6550±0.2767) compared with normal (0.1725±0.0568)
and mature (0.1975±0.0808) fibrous tissues (P=0.017; Fig. 1). The results indicated that elevated
H19 may be associated with KD. As keloid fibroblasts proliferate
more rapidly compared with normal fibroblasts (12), it is possible that H19 may regulate
proliferation in KD tissues.
H19 regulates the proliferation of KD
fibroblasts
In order to directly assess whether H19 is involved
in the proliferation of keloid fibroblasts, RNA interference
technology was used to silence the expression of H19 in cultured
keloid fibroblasts. Reduced H19 RNA expression was confirmed at 24
h following transfection of specific H19 siRNA (si-H19;
0.0350±0.0.0293) as compared with the blank (0.6280±0.1880) or
negative control siRNA (si-scramble; 0.5825±0.2440) groups
(P=0.017; Fig. 2A and B).
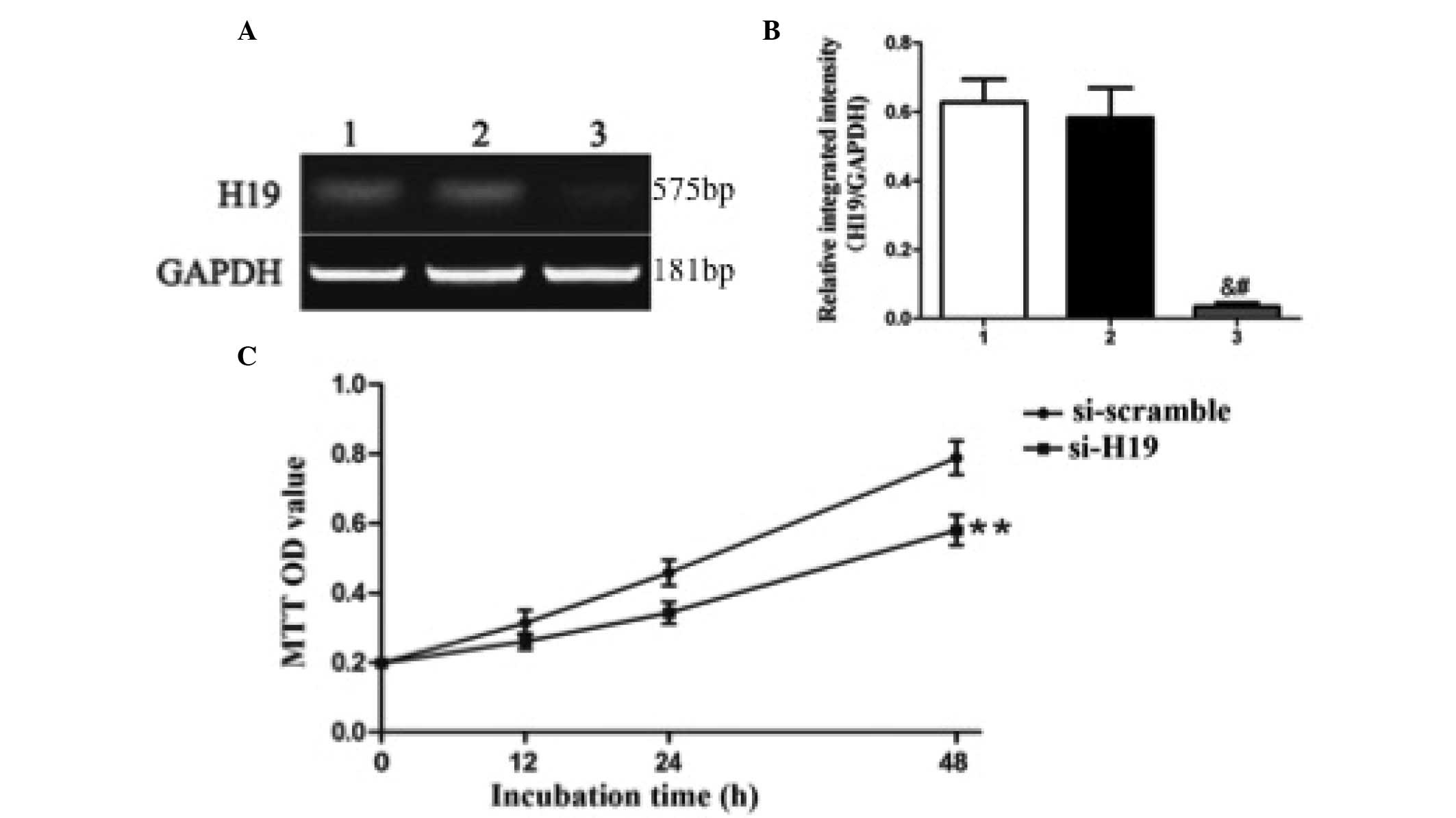 | Figure 2.Knockdown of H19 expression suppresses
the proliferation of keloid fibroblasts. (A and B) Keloid
fibroblasts were transfected with blank control (lane 1), negative
control siRNA (si-scramble; lane 2) or H19 siRNA (si-H19; lane 3),
and the levels of H19 RNA expression relative to GAPDH mRNA
expression were assessed by reverse transcription-polymerase chain
reaction, gel electrophoresis and densitometry. (A) A
representative gel is shown, in addition to (B) the semi-quantified
results, presented as the mean ± standard deviation of 8
experimental replicates. &P<0.05 compared with
the blank control group; #P<0.05 compared with the
negative control siRNA group. (C) Proliferation of keloid
fibroblasts was assessed by MTT assay 12, 24 or 48 h subsequent to
transfection. Results represent the mean ± standard deviation of 8
experimental replicates. **P<0.01 compared with negative control
(si-scramble). siRNA, small interfering RNA; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; OD,
optical density. |
Furthermore, compared with si-scramble, fibroblast
proliferation was significantly inhibited by si-H19 at 12, 24 and
48 h following transfection (Fig.
2C): Si-scramble vs. si-H19; 12 h, 0.1982±0.0279 vs.
0.1421±0.0153 (P<0.01); 24 h, 0.2563±0.05127 vs. 0.2015±0.0281
(P<0.01); and 48 h, 0.3143±0.0984 vs. 0.2312±0.0315 (P<0.05).
These findings demonstrated that H19 may mediate proliferation in
keloid fibroblasts.
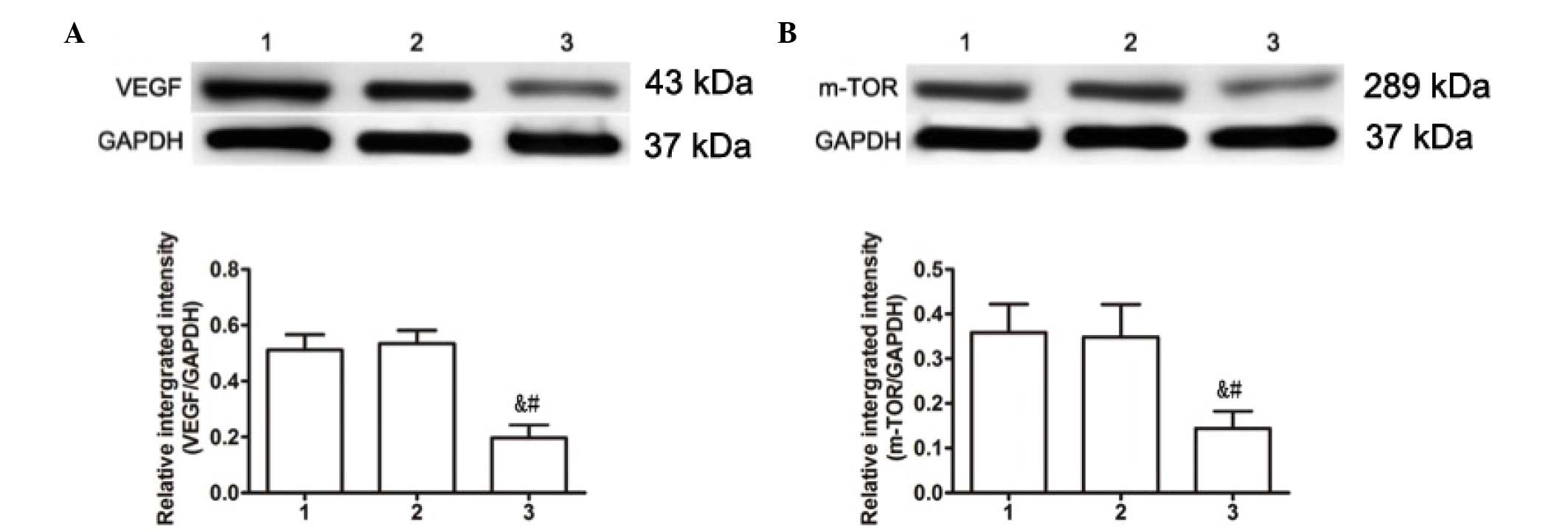
H19 mediates the expression of VEGF
and mTOR
In order to identify proliferative signaling
pathways that may be associated with H19 expression in keloid
fibroblasts, western blotting was performed on two signaling
molecules associated with keloid cell proliferation, VEGF and mTOR.
Compared with the blank (VEGF, 0.950±0.1007; mTOR, 0.610±0.0972)
and negative (VEGF, 0.934±0.1119; mTOR, 0.634±0.0939) control
groups, the expression levels of VEGF and mTOR were significantly
decreased in H19 siRNA-transfected cells (0.356±0.0808, P=0.013 and
0.184±0.06914, P=0.009, respectively; Fig. 3). These findings suggest a signaling
pathway that may explain the effects of H19 on proliferation in
KD.
Discussion
To the best of our knowledge, the present study is
the first to demonstrate that the expression of the lncRNA H19 is
abnormally increased in KD tissues. Additionally, using
RNA-mediated silencing, it was directly demonstrated that H19
expression regulates the proliferation of keloid fibroblasts.
Furthermore, the functions of several proteins that may serve as
downstream regulators of H19 were investigated. The results
demonstrate that knockdown of H19 is able to reduce the expression
of mTOR and VEGF, which may explain the functional effects of H19
on keloid cell proliferation.
Reduced mTOR levels following H19 knockdown are
consistent with its effects on proliferation. The mTOR/P70S6k
signaling pathway is known to participate significantly in the
pathogenesis of KD, and inhibition of mTOR activity may inhibit the
proliferation of KD fibroblasts (13). mTOR is the target protein of rapamycin
in mammals. Inhibition of mTOR activity may block the
phosphorylation of P70S6K and eukaryotic translation initiation
factor 4E (EIF4E)-binding protein 1, and prevent the release and
transcription of EIF4E, thus inhibiting cell growth and
proliferation (13). P70S6K is the
ribosome 40s small subunit of the protein kinase S6K.
Phosphorylation of S6K leads to translational initiation of
5′-terminal oligopyrimidine mRNA, the product of which controls the
majority of the components of translation; these translational
components have a significant role in cell growth and proliferation
induced by mitogen stimulation (14).
Therefore, the observation in the present study that downregulation
of H19 RNA may markedly reduce the amount of mTOR expression
suggested that H19 is able to control the proliferation of KD
fibroblasts via the mTOR signaling pathway.
The effects of H19 knockdown on VEGF may
additionally contribute to the control of proliferation. VEGF
overexpression is a significant factor in the formation and
progression of KD (15,16) and, consequently, inhibiting the
production of VEGF in KD may reduce the number of newly formed
vessels to inhibit KD growth (16).
VEGF is a vascular endothelial cell-stimulating factor important in
the formation of new vessels (17).
It is expressed widely in the normal human body (18). Under normal physiological conditions,
the expression of VEGF is relatively low; however, its expression
is increased under certain pathological conditions, including in
tumor tissues (19–21). In addition to promoting vascular
formation, VEGF increases the blood supply and repair of damaged
tissues and vascular endothelial cells (17). Previous studies have demonstrated that
full-length VEGF is expressed in KD tissues, primarily in
keratinocytes and fibroblasts (22,23). In
the present study, it was demonstrated that a reduction in H19
expression inhibited the formation of VEGF, thus inhibiting the
proliferation of KD fibroblasts. These findings indicate that H19
is a significant factor regulating the generation of VEGF from
fibroblasts.
Previous studies have revealed that the occurrence
of pathological KD may be associated with abnormalities in
additional signaling transduction pathways, including c-Myc and E2F
transcription factor 1 (E2F1) (24,25). The
expression of the proto-oncogene c-Myc is markedly elevated in KD
tissue (25). c-Myc is thought to be
closely associated with cell proliferation and differentiation,
primarily via a mechanism involving the activation of growth
factors, receptors and intracellular signaling molecules (26). A previous study reported that c-Myc
was able to induce the expression of H19 lncRNA (27). Therefore, H19 expression may be
regulated by c-Myc in keloid fibroblasts. The expression of E2F1
protein in KD tissues is also elevated compared with normal skin
tissue, indicating that E2F1 has a significant role in fibroblast
differentiation, proliferation or phenotype transformation and
collagen synthesis (28). In
addition, E2F1 is capable of inducing the expression of H19
(29). Thus, we hypothesize that H19
expression in KD fibroblasts may be regulated via c-Myc and E2F1,
and the activation of H19 may have a significant role in the
proliferation of fibroblasts in KD, via downstream effects on mTOR
and VEGF.
In conclusion, the current study demonstrated that
H19 is overexpressed in KD, and that this overexpression is closely
associated with the proliferation of fibroblasts in KD.
Downregulation of H19 expression was able to inhibit the generation
of mTOR and VEGF, thus inhibiting the proliferation of KD
fibroblasts. These findings indicate that H19 may serve as a novel
target for the treatment of KD.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81460295) and
Jiangxi Provincial Health and Family Planning Commission Science
and Technology Project (grant no. 20155201).
References
|
1
|
Alster TS and Tanzi EL: Hypertrophic scars
and keloids: Etiology and management. Am J Clin Dermatol.
4:235–243. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wolfram D, Tzankov A, Pülzl P and
Piza-Katzer H: Hypertrophic scars and keloids - a review of their
pathophysiology, risk factors, and therapeutic management. Dermatol
Surg. 35:171–181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mofikoya BO, Adeyemo WL and Abdus-salam
AA: Keloid and hypertrophic scars: A review of recent developments
in pathogenesis and management. Nig Q J Hosp Med. 17:134–139.
2007.PubMed/NCBI
|
|
4
|
Gibb EA, Brown CJ and Lam WL: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10:382011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prensner JR and Chinnaiyan AM: The
emergence of lncRNAs in cancer biology. Cancer Discov. 1:391–407.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matouk IJ, DeGroot N, Mezan S, Ayesh S,
Abulail R, Hochberg A and Galun E: The H19 non-coding RNA is
essential for human tumor growth. PLoS One. 2:e8452007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matouk I, Raveh E, Ohana P, Lail RA,
Gershtain E, Gilon M, De Groot N, Czerniak A and Hochberg A: The
increasing complexity of the oncofetal h19 gene locus: Functional
dissection and therapeutic intervention. Int J Mol Sci.
14:4298–4316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee JY, Yang CC, Chao SC and Wong TW:
Histopathological differential diagnosis of keloid and hypertrophic
scar. Am J Dermatopathol. 26:379–384. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Monstrey S, Middelkoop E, Vranckx JJ,
Bassetto F, Ziegler UE, Meaume S and Téot L: Updated scar
management practical guidelines: Non-invasive and invasive
measures. J Plast Reconstr Aesthet Surg. 67:1017–1025. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang F, Bi J, Xue X, Zheng L, Zhi K, Hua J
and Fang G: Upregulated long non-coding RNA H19 contributes to
proliferation of gastric cancer cells. FEBS J. 279:3159–3165. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kruger NJ: The Bradford method for protein
quantitation. Methods Mol Biol. 32:9–15. 1994.PubMed/NCBI
|
|
12
|
Calderon M, Lawrence WT and Banes AJ:
Increased proliferation in keloid fibroblasts wounded in vitro. J
Surg Res. 61:343–347. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ong CT, Khoo YT, Mukhopadhyay A, Do DV,
Lim IJ, Aalami O and Phan TT: mTOR as a potential therapeutic
target for treatment of keloids and excessive scars. Exp Dermatol.
16:394–404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clark JA, Leung KS, Cheng JC and Leung PC:
The hypertrophic scar and microcirculation properties. Burns.
22:447–450. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu WS, Wang FS, Yang KD, Huang CC and Kuo
YR: Dexamethasone induction of keloid regression through effective
suppression of VEGF expression and keloid fibroblast proliferation.
J Invest Dermatol. 126:1264–1271. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Crafts TD, Jensen AR, BlocherSmith EC and
Markel TA: Vascular endothelial growth factor: Therapeutic
possibilities and challenges for the treatment of ischemia.
Cytokine. 71:385–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Otrock ZK, Makarem JA and Shamseddine AI:
Vascular endothelial growth factor family of ligands and receptors:
Review. Blood Cells Mol Dis. 38:258–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Otrock ZK, Mahfouz RA, Makarem JA and
Shamseddine AI: Understanding the biology of angiogenesis: Review
of the most important molecular mechanisms. Blood Cells Mol Dis.
39:212–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Farhat FS, Tfayli A, Fakhruddin N, Mahfouz
R, Otrock ZK, Alameddine RS, Awada AH and Shamseddine A:
Expression, prognostic and predictive impact of VEGF and bFGF in
non-small cell lung cancer. Crit Rev Oncol Hematol. 84:149–160.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Trompezinski S, Denis A, Vinche A, Schmitt
D and Viac J: IL-4 and interferon-gamma differentially modulate
vascular endothelial growth factor release from normal human
keratinocytes and fibroblasts. Exp Dermatol. 11:224–231. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Trompezinski S, BerthierVergnes O, Denis
A, Schmitt D and Viac J: Comparative expression of vascular
endothelial growth factor family members, VEGF-B, -C and -D, by
normal human keratinocytes and fibroblasts. Exp Dermatol.
13:98–105. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
DeFelice B, Garbi C, Wilson RR,
Santoriello M and Nacca M: Effect of selenocystine on gene
expression profiles in human keloid fibroblasts. Genomics.
97:265–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu Z, Lou L and Luo S: Experimental study
of the expression of c-myc, c-fos and proto-oncogenes on
hypertrophic and scars. Zhonghua Zheng Xing Wai Ke Za Zhi.
18:165–167. 2002.(In Chinese). PubMed/NCBI
|
|
26
|
Bretones G, Delgado MD and León J: Myc and
cell cycle control. Biochim Biophys Acta. 1849:506–516. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
BarsyteLovejoy D, Lau SK, Boutros PC,
Khosravi F, Jurisica I, Andrulis IL, Tsao MS and Penn LZ: The c-Myc
oncogene directly induces the H19 noncoding RNA by allele-specific
binding to potentiate tumorigenesis. Cancer Res. 66:5330–5337.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo X, Pan Q, Liu L and Chegini N: Genomic
and proteomic profiling II: Comparative assessment of gene
expression profiles in leiomyomas, keloids, and surgically-induced
scars. Reprod Biol Endocrinol. 5:352007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Berteaux N, Lottin S, Monté D, Pinte S,
Quatannens B, Coll J, Hondermarck H, Curgy JJ, Dugimont T and
Adriaenssens E: H19 mRNA-like noncoding RNA promotes breast cancer
cell proliferation through positive control by E2F1. J Biol Chem.
280:29625–29636. 2005. View Article : Google Scholar : PubMed/NCBI
|