Introduction
The prostate is the biggest subsidiary gonad organ
in the male urogenital system, which is located in the male pelvic
cavity and generally has a cone, chestnut-like shape (1). The prostate is situated between the
bladder neck and the urogenital diaphragm, being its specific
location below the pubic bone, under the bladder, on the upper
urogenital diaphragm and before the rectum (2). The normal size of the prostate is as
follows: The upper transverse diameter measures ~4 cm, the vertical
diameter measures ~3 cm and the antero-posterior diameter measures
~2 cm (3). Its surface comprises a
thin layer of fiber muscular tissue called the prostate capsule,
which is the outer sheath of the prostate (3). Morphologically, the prostate can be
divided into three zones: The central zone, the peripheral zone and
the transition zone (4). The
production of prostate fluid is the main physiological function of
the prostate, and a variety of enzymes and small molecular
components in the prostate fluid are necessary to maintain normal
sperm activity (4). The transitional
zone is usually associated with hyperplasia of the prostate, while
prostate cancer (PCa), the most popular malignant disease of the
prostate, most commonly occurs in the peripheral zone (5). PCa is one of the most common tumors of
the genitourinary system, and causes serious damage to men's health
(2).
PCa is the most common malignant tumor in the male
reproductive system (1). At present,
PCa is acknowledged as one of the most important medical problems
affecting old men (1). PCa is one of
the most common solid tumors in Europe and the USA, and the number
of cases of PCa has exceeded the number of lung and colorectal
cancer cases, thus becoming the first type of tumor affecting men's
health (3). According to the World
Health Organization International Cancer Research Center GLOBOCAN
2008 estimates (6), there were
~900,000 new global PCa cases in 2008, with PCa incidence ranking
in second place, behind lung cancer (7). The number of newly diagnosed PCa
patients increased by 186,320 cases in the USA in 2008, and~290,000
patients succumbed to PCa (8). In
Europe, the PCa rates increased ≤214/10 (males) in 2008, with 2.6
million new cases being diagnosed each year (9). In China, the incidence of PCa is
currently far lower than that in Europe, USA and other Western
countries, and its morbidity and mortality rates are also far lower
than the world average rates, being the global standardized
incidence and the global standardized mortality rate 27.9/1,000,000
and 7.4/1,000,000, respectively, vs. 83.8/100,000 and 9.7/100,000
reported in the USA and other developed countries (8). However, with the increase in life
expectancy, the westernization of the diet and the improvement in
diagnostic technology, the morbidity and mortality rates of PCa are
in clearly rising in China, particularly in patients with clinical
stage T1 and T2, for whom a significant increase in detection rate
has been reported (7,10).
Deoxypodophyllotoxin (DPPT) is extracted and
separated from citrus-related plants, including Podophyllum (P.)
peltatum, P. pleianthum, P. emodi (also known as P.
hexandrum) and Diphylleia grayi with good antitumor
activity (11). Due to the advantages
of DPPT in terms of its small molecular weight and high activity,
DPPT is expected to become a new type of high-efficient antitumor
drug, whose research and development may provide a new road for
antineoplastic drugs (12). The
present study aimed to investigate the anticancer effect of DPPT on
the induction of apoptosis in human PCa cells, and to further
characterize its mechanism.
Materials and methods
Materials
The RPMI-1640 medium and fetal bovine serum (FBS)
were obtained from Gibco (Thermo Fisher Scientific, Inc., Waltham,
MA, USA), while
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was obtained from Sigma-Aldrich (St. Louis, MO, USA). DPPT was
purchased from the Medicinal Chemical Institute of China
Pharmaceutical University (Nanjing, China). Dimethyl sulfoxide
(DMSO) was purchased from Invitrogen (Thermo Fisher Scientific,
Inc.). Annexin V-FITC/PI Apoptosis Detection kit was purchased from
BestBio. Co. Ltd. (Shanghai, China).
Cell culture
The human PCa cell line DU-145 was obtained from the
American Type Culture Collection (Manassas, VA, USA), and was
cultured in RPMI-1640 and 10% FBS, supplemented with 100 U/ml
penicillin and 100 µg/ml streptomycin, at 37°C and 5%
CO2 in a humidified atmosphere.
Cell proliferation analysis
The anticancer effectiveness of DPPT on the cell
viability of PCa cells was measured by MTT assay. DU-145 cells were
plated into 96-well plates at a density of 1.5–3×103 cells/well and
then treated with DPPT (0, 50, 75 and 100 nM) for 12, 24 and 48 h.
Next, 20 µl MTT (5 mg/ml) was added into each well, and incubated
for 4 h at 37°C and 5% CO2 in a humidified atmosphere.
After treatment for 4 h, the medium was removed, and 150 µl DMSO
was added to each well and agitated for 20 min. The optical density
was measured at 490 nm using a microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA).
Apoptosis detection
The anticancer effectiveness of DPPT on the
apoptosis of PCa cells was measured by Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) assay according to the
manufacturer's protocol (BestBio. Co. Ltd.). DU-145 cells were
plated into 6-well plates at a density of 1–2×106 cells/well and
then treated with DPPT (0, 50, 75 and 100 nM) for 24 h. Flow
cytometry (FACSCalibur™; BD Biosciences, Franklin Lakes, NJ, USA)
was used to measure cell apoptosis of DU-145 cells using CellQuest
Pro software (BD Biosciences).
Caspase-3 activity assay
The anticancer effectiveness of DPPT on the
apoptosis of PCa cells was measured by Annexin V-FITC/PI assay
according to the manufacturer's protocol (BestBio. Co. Ltd.).
DU-145 cells were plated into 6-well plates at a density of 1–2×106
cells/well and then treated with DPPT (0, 50, 75 and 100 nM) for 24
h. The proteins were extracted from cellular lysates dissolved with
radioimmunoprecipitation assay buffer, and the protein
concentrations were determined with a Protein Assay kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Equivalent amounts of
proteins (10–20 µg) were incubated with
N-acetyl-Asp-Glu-Val-Asp-p-nitroaniline for 12 h and measured at
405 nm with a microplate reader (Safire2™; Tecan; Thermo Fisher
Scientific, Inc.).
Western blot analysis
DU-145 cells were plated into 6-well plates at a
density of 1–2×106 cells/well and then treated with DPPT (0, 50, 75
and 100 nM) for 24 h. The proteins were extracted from cellular
lysates dissolved with buffer, and the protein concentrations were
determined with a Protein Assay kit (Bio-Rad Laboratories, Inc.).
Equivalent amounts of proteins (10–20 µg) were separated by 8–12%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred to nitrocellulose membranes (EMD Millipore, Billerica,
MA, USA). The blots were incubated with the following antibodies:
Anti-phosphorylated (p)-Akt (1:2,000; catalog no. sc-135650; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), anti-p53 (1:2,000;
catalog no. sc-1311-R; Santa Cruz Biotechnology, Inc.), anti-B-cell
lymphoma 2 (Bcl-2) associated X protein (Bax) (1:1,000; catalog no.
sc-783; Santa Cruz Biotechnology, Inc.), anti-phosphatase and
tensin homolog (PTEN) (1:1,500; catalog no. sc-9145; Santa Cruz
Biotechnology, Inc.) and anti-β-actin (1:1,000; catalog no.
sc-130656; Santa Cruz Biotechnology, Inc.) at 4°C overnight. Then,
the blots were washed with Tris-buffered saline with Tween-20
(TBST) and incubated with the appropriate secondary antibody
(1:5,000; catalog no. A0208; Beyotime Institute of Biotechnology,
Haimen, China) at room temperature for 2 h, prior to be developed
with enhanced chemiluminescence western blot detection reagents
(catalog no. P0018A; Beyotime Institute of Biotechnology).
Statistical analysis
All data are expressed as the mean ± standard
deviation. SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) was
used for statistical analyses, including one-way analysis of
variance and Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Anticancer effect of DPPT reduces cell
viability of human PCa cells
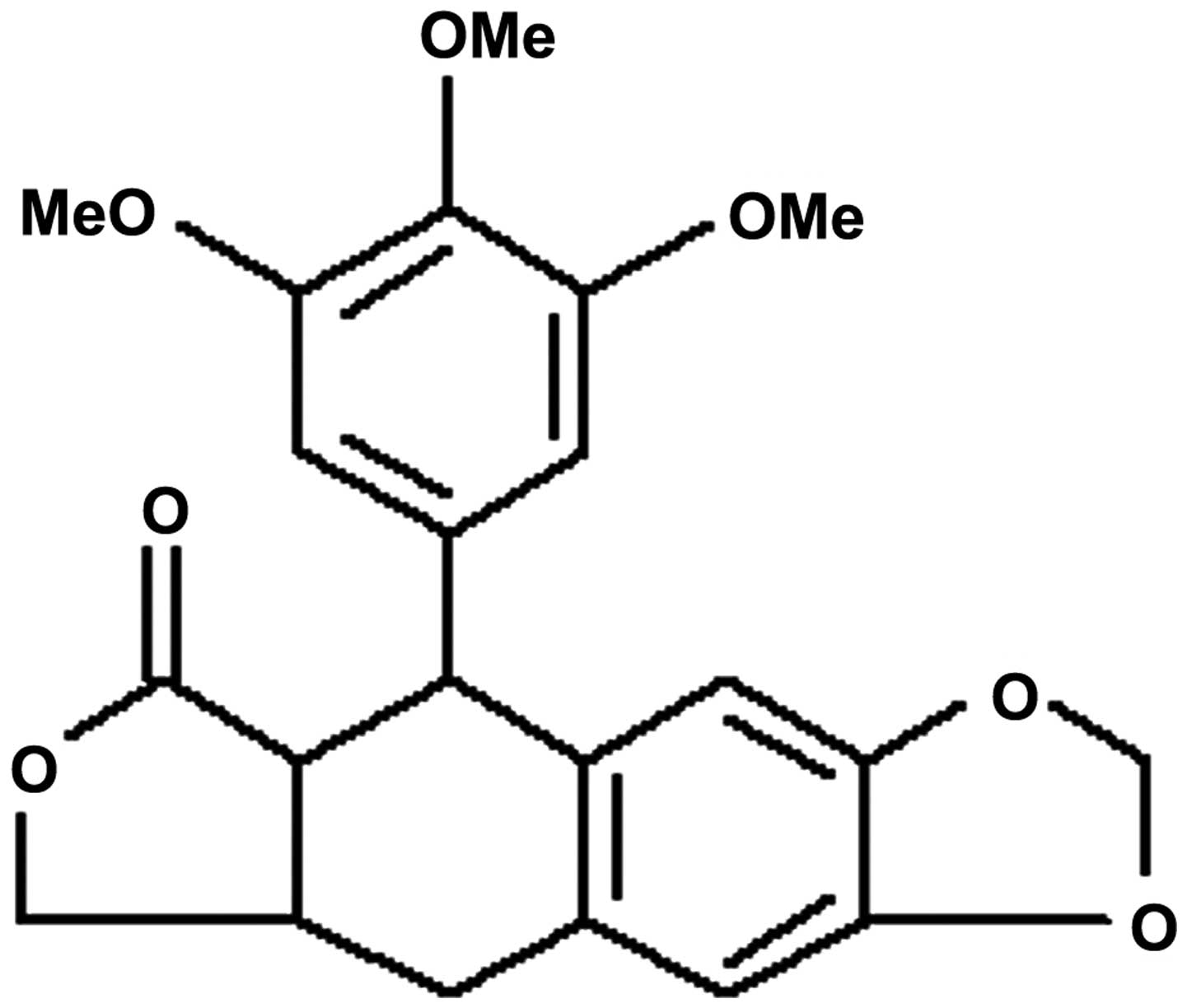
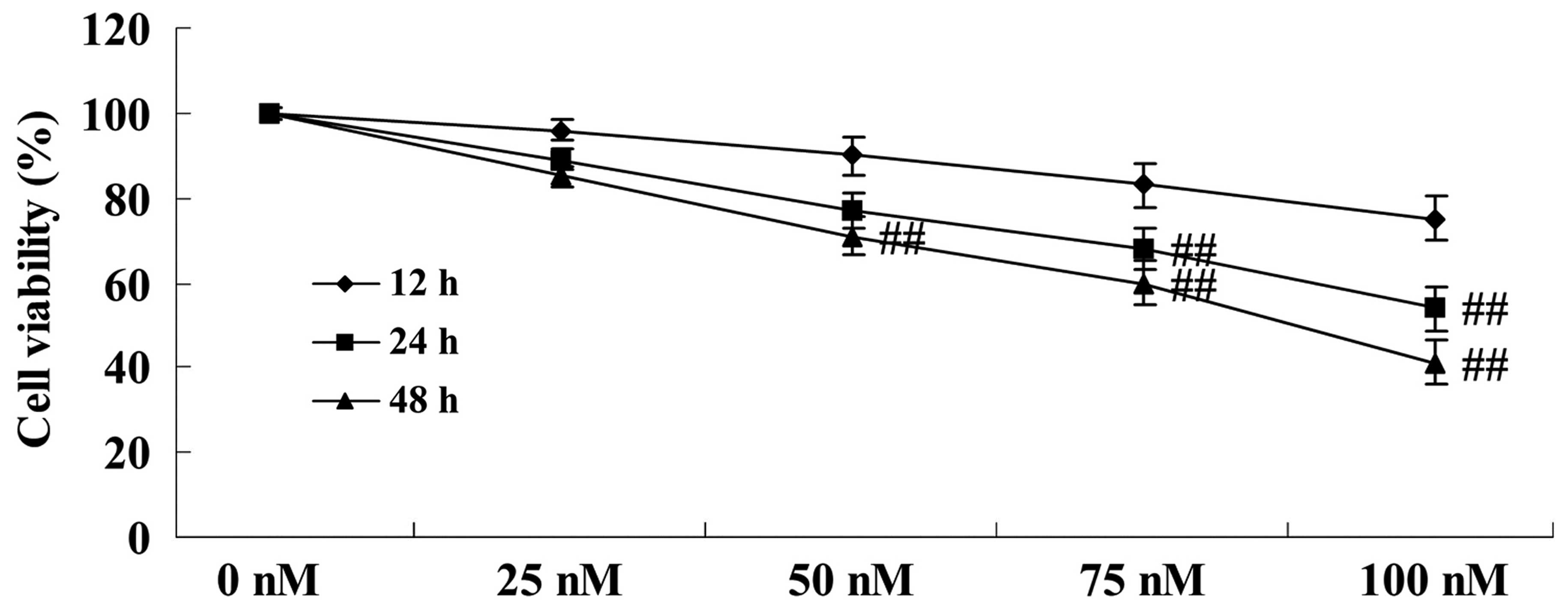
The chemical structure of DPPT is shown in Fig. 1. To determine the anticancer effect of
DPPT in terms of reducing the cell viability of human PCa cells,
DU-145 cells were treated with DPPT (0, 50, 75 and 100 nM) for 12,
24 and 48 h, and cell proliferation was determined using MTT assay.
Cell proliferation of DU-145 cells was effectively inhibited by
treatment with DPPT in a dose- and time-dependent manner (Fig. 2).
Anticancer effect of DPPT induces cell
apoptosis of human PCa cells
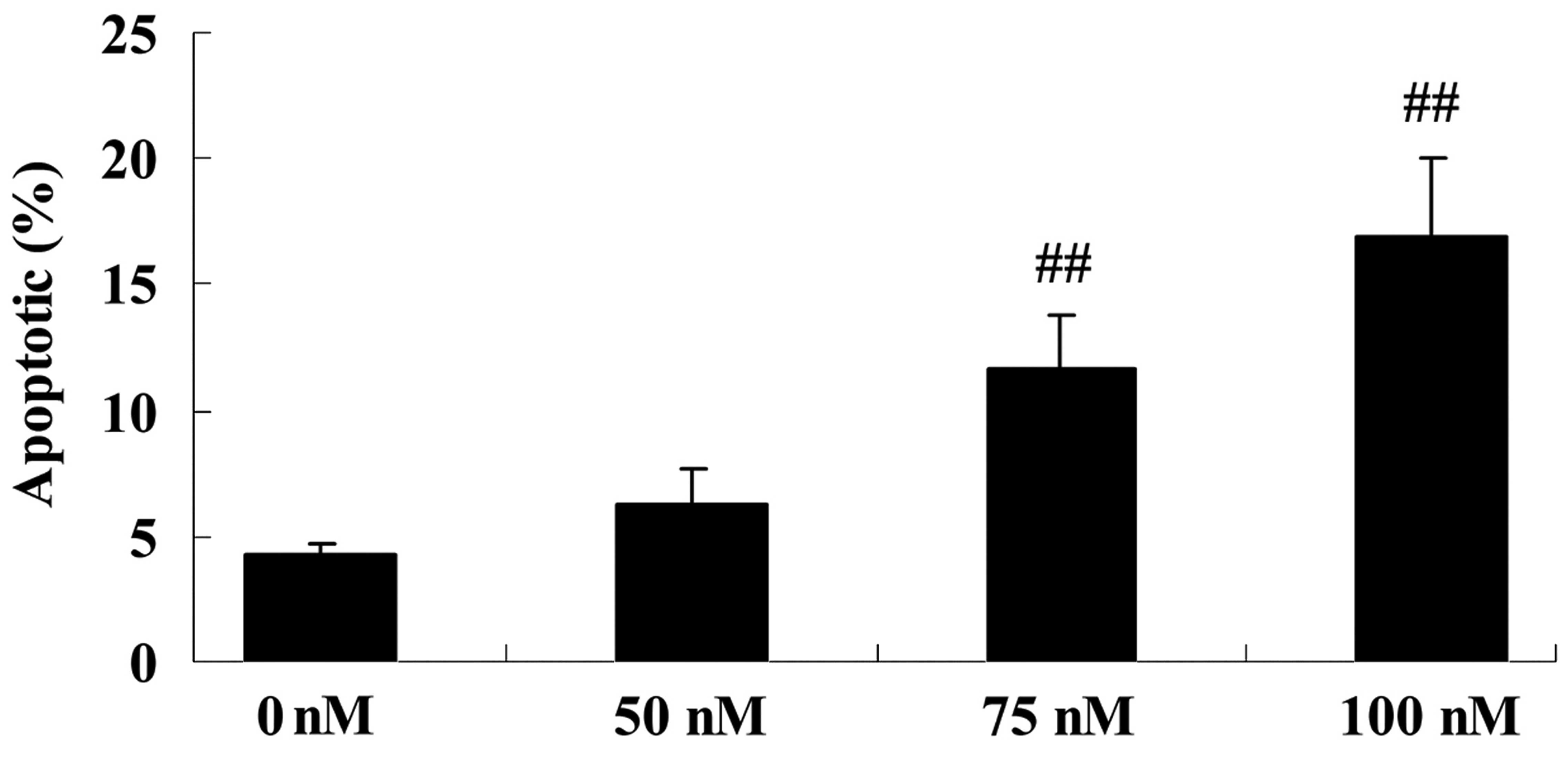
To explore the anticancer effect of DPPT in terms of
inducing cell apoptosis of human PCa cells, DU-145 cells were
treated with DPPT (0, 50, 75 and 100 nM) for 12, 24 and 48 h, and
cell proliferation was determined by Annexin V-FITC/PI assay. As
shown in Fig. 3, the apoptosis of
DU-145 cells was induced by DPPT treatment in a dose-dependent
manner (Fig. 3).
Anticancer effect of DPPT induces
caspase-3 activity of human PCa cells
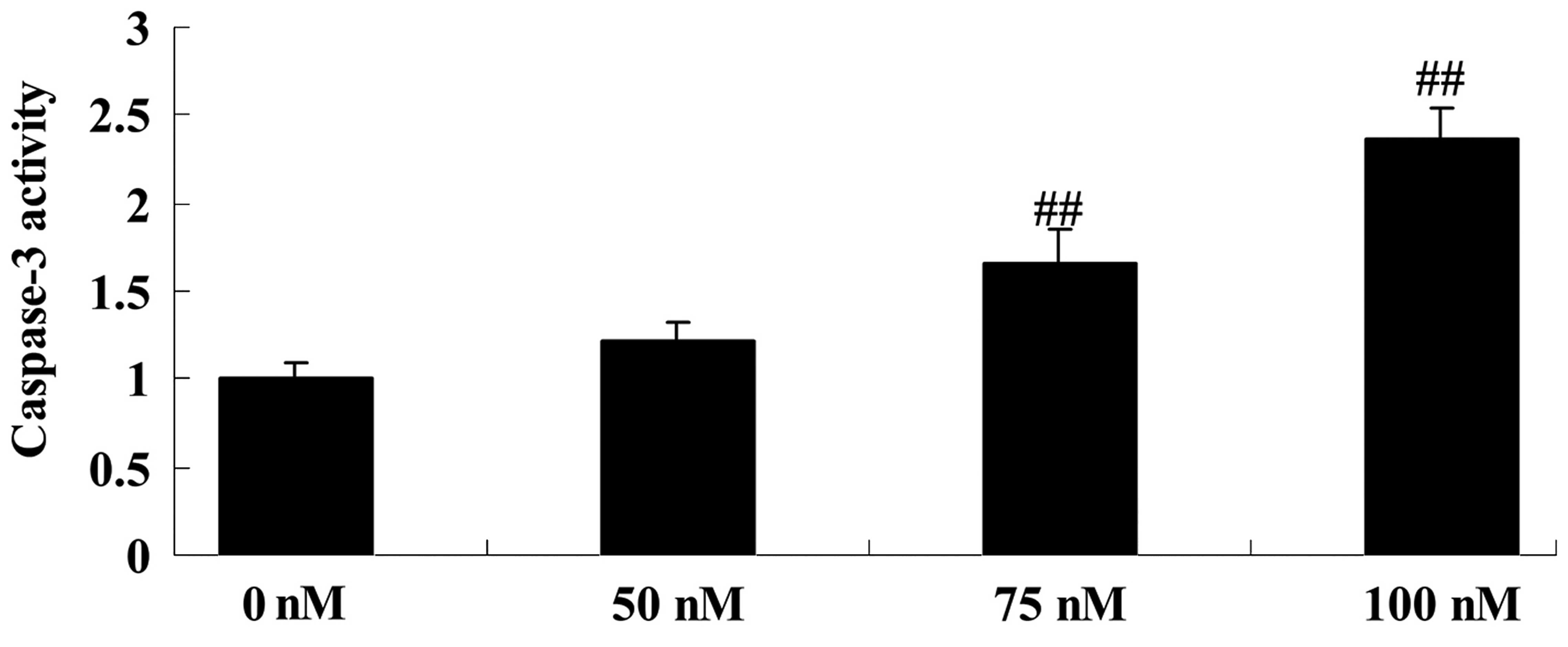
To explore the mechanism of DPPT by which it induces
cell apoptosis in human PCa cells, the caspase-3 activity of DU-145
cells was evaluated following DPPT treatment. As expected,
DPPT-treated cells exhibited a significant increase in caspase-3
activity compared with untreated cells in a dose-dependent manner
(Fig. 4).
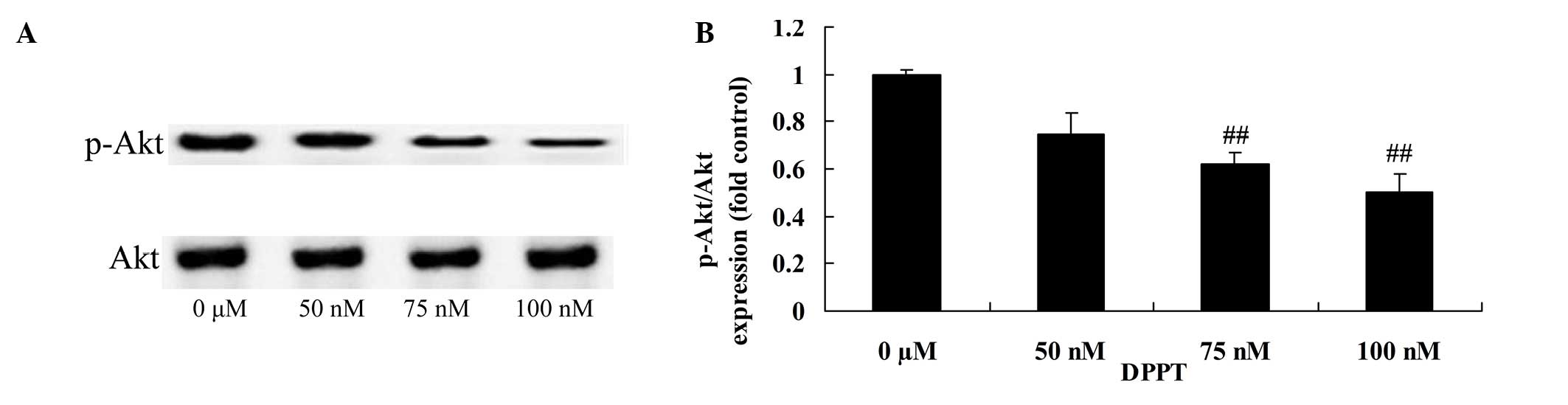
Anticancer effect of DPPT reduces
p-Akt protein expression in human PCa cells
Compared with the blank control, the p-Akt/Akt rate
was significantly inhibited in DU-145 cells treated with DPPT in a
dose-dependent manner (Fig. 5).
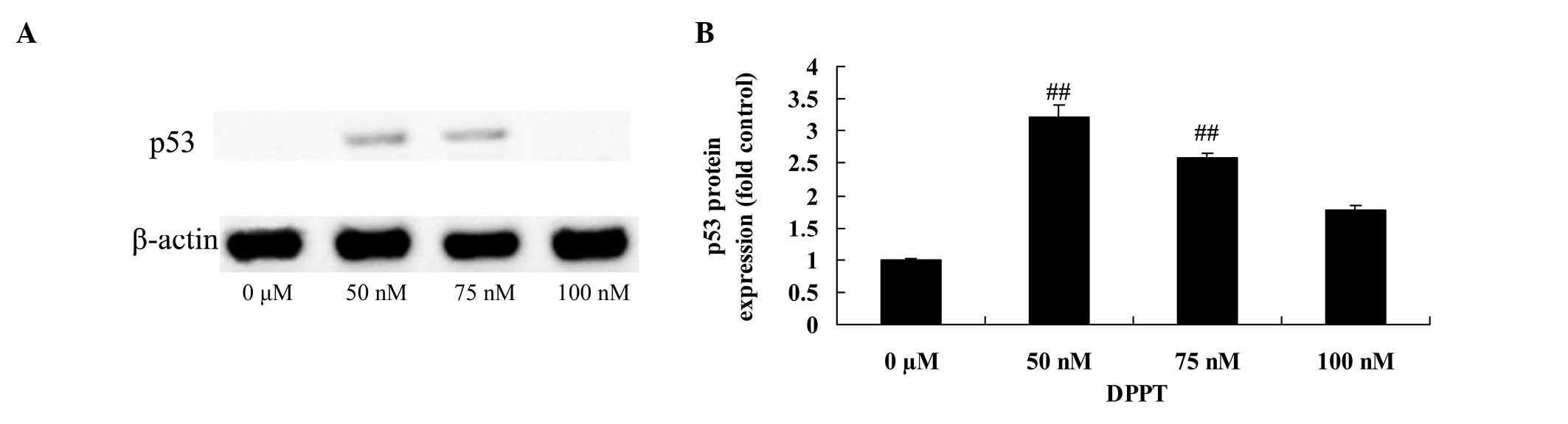
Anticancer effect of DPPT induces p53
protein expression in human PCa cells
Treatment with DPPT (50 or 100 nM) for 24 h resulted
in an increase in p53 protein expression in DU-145 cells compared
with untreated cells (Fig. 6). This
effect was also dose-dependent (Fig.
5).
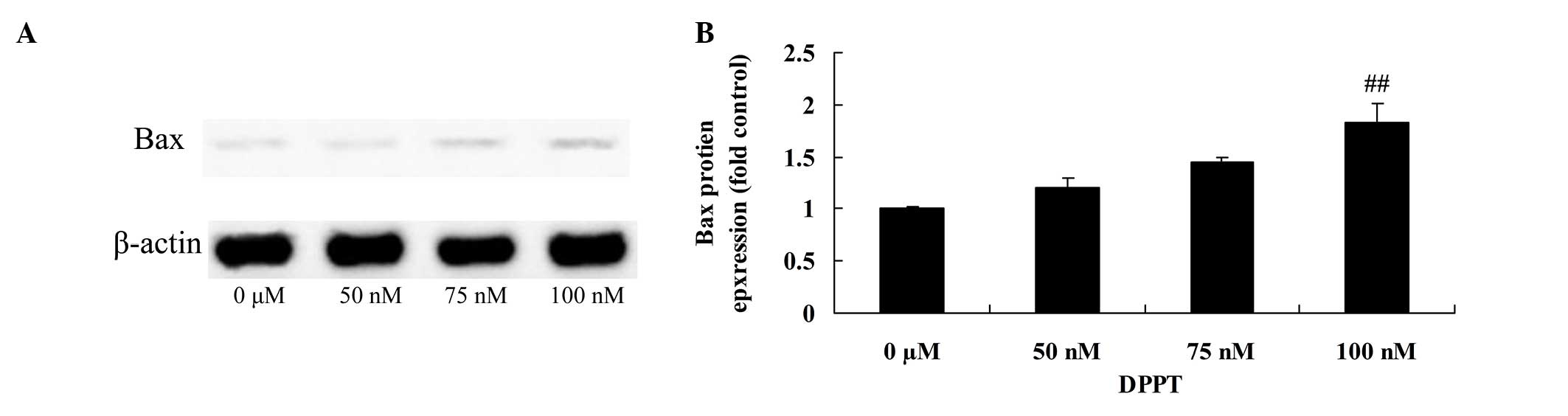
Anticancer effect of DPPT induces Bax
protein expression in human PCa cells
In order to characterize the anticancer mechanism of
DPPT against DU-145 cells, the effects of DPPT on Bax protein
expression were examined. Bax protein expression was markedly
induced in DPPT-treated DU-145 cells compared with the blank
control (Fig. 7).
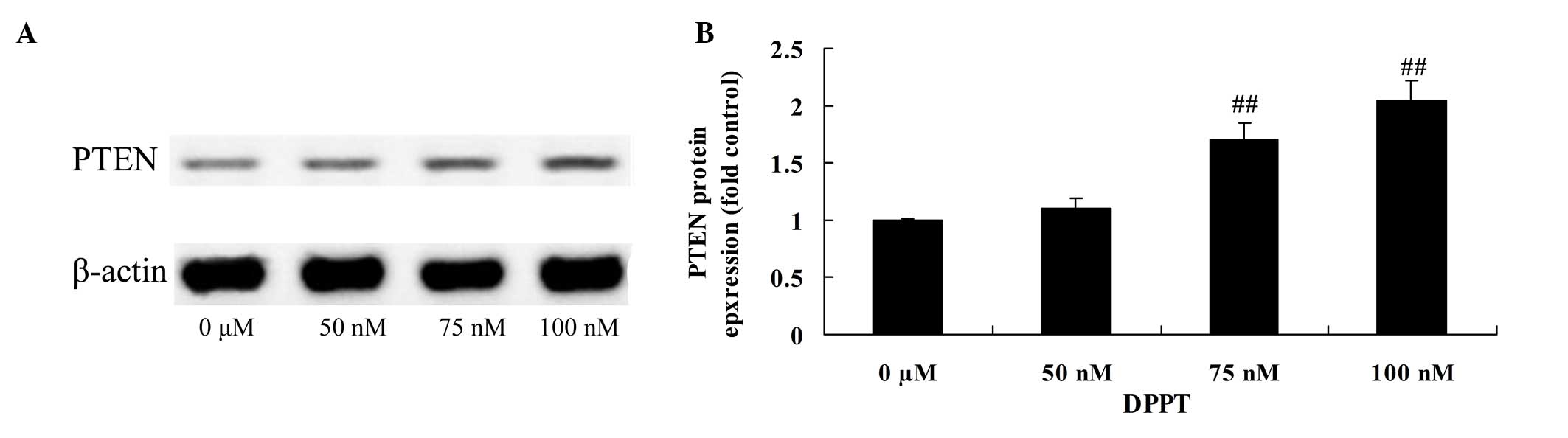
Anticancer effect of DPPT reduces PTEN
protein expression in human PCa cells
To further elucidate the mechanism involved in
DPPT-mediated apoptosis, the effects of DPPT on PTEN protein
expression were examined. Treatment with DPPT resulted in a
dose-dependent increase in PTEN protein expression, compared with
untreated control cells (Fig. 8).
Discussion
In 2008, ~34,000 new PCa cases were reported in
China (10). The standardized
incidence of PCa is 4.3/100,000 of the world population, of which,
140,000 cases succumbed to disease, thus leading to a standardized
mortality rate of 1.8/100,000 individuals (10). In Shanghai, the incidence of PCa
increased from 12.6/100,000 (males) to 21.8/100,000 during
2000–2005, while in Beijing, the incidence of PCa increased 2.3
times during 1985–1995 (10). In
2004, the incidence of PCa in the Chinese Taiwan and Singapore
areas increased 4.8 and 8.5 times, respectively, making the
morbidity of PCa to be considered dangerous (10). Over the next 20 years, the incidence
of PCa and the number of associated mortalities in China is
expected to increase (13). It is
estimated that by 2030, the average number of PCa cases and
associated mortalities per year will increase from 106.4 to 111.4%,
and from 4.8 to 5.1%, respectively (10,13). In
the present study, DPPT significantly inhibited cell proliferation
and cell apoptosis of DU-145 cells in a dose- and time-dependent
manner. Several studies indicated that DPPT significantly induced
cell apoptosis of HeLa cells (14),
breast cancer cells (15) and
non-small cell lung cancer cells (16). These results indicated that DPPT may
be considered as a drug to target PCa.
The activation of the caspase family plays a key
role in the process of cell apoptosis (17). Under normal conditions, caspases are
synthesized and stored in the form of inactive precursors, and
apoptosis signaling can initiate the caspase cascade (18). Caspase-3 is the main executing factor
in the process of apoptosis, since the activation of caspase-3 has
a strong effect on the induction of tumor cell apoptosis. Based on
this, caspases inducing caspase-3/8/9 activation participate in the
apoptosis program and cause cancer cell apoptosis (17,19). In
the present study, significant caspase-3 activity was observed in
DU-145 cells upon treatment with DPPT. Sang et al (20) reported that DPPT inhibited microtubule
formation, and induced caspase-3, Bax and p53 expression. Yong
et al (21) indicated that
DPPT inhibits tubulin polymerization and activates caspases-3/7 in
HeLa human cervix carcinoma cells. The present results demonstrated
that DPPT induces caspase-3 activity, and this pathway appears to
be involved in the mechanism of DPPT-induced cell death.
In recent years, studies on molecular biology have
demonstrated that cell proliferation and apoptosis are regulated by
different genes; therefore, further studies on the regulation of
genes at a molecular level will help to increase the understanding
of the occurrence and development of PCa (22). Bcl-2, Bax and p53 affect the
proliferation, apoptosis, occurrence and development of PCa
(22,23). Among the genes involved in the
regulation of cell apoptosis, Bcl-2 is currently recognized as an
anti-apoptotic gene (24), which
participates in the occurrence of tumors by inhibiting cell
apoptosis and prolonging cell survival (25). The present results revealed that
treatment with DPPT significantly reduced p-Akt protein expression,
and increased p53 and Bax protein expression in DU-145 cells. Shin
et al (14) suggested that
DPPT induces G2/M cell-cycle arrest through inhibition of Akt and
upregulation of the p53 and Bax signaling pathway in HeLa cells.
Sang et al (20) demonstrated
that DPPT induces cell apoptosis of non-small cell lung cancer A549
cells through p53/cell division cycle 2/Bax signaling.
Previous studies indicated that, in certain tumors
such as lung cancer, malignant lymphoma and cancer of the liver,
the expression of PTEN in cancerous tissues is significantly higher
than that in the corresponding noncancerous tissues. High PTEN
expression may be due to the cancer cell proliferation and DNA
double chain fractures (26,27). PTEN is one of the key regulation
factors in the process of DNA repair and the expression is then
increased in order for it to participate in the process of cancer
development (26,27). In previous studies, PTEN was tested in
PCa and hyperplastic prostate tissues, and it was observed to be
mainly expressed in PCa tissues and in the cell nuclei of
hyperplastic prostate tissues (28,29). The
expression of PTEN in PCa was significantly higher than that in
prostate hyperplasia, which indicated that increased PTEN
expression levels may be associated with the incidence of PCa
(30,31). In the present study, treatment with
DPPT significantly promoted PTEN protein expression in DU-145
cells. Shin et al (14)
suggested that DPPT induces G2/M cell-cycle arrest through
inhibition of Akt and activation of the PTEN signaling pathway in
HeLa cells. Consequently, the present results provide the initial
evidence that DPPT induces cell apoptosis via the PTEN signaling
pathway.
Taken together, the results of the present study
indicated that the anticancer effect of DPPT inhibited cell
proliferation and induced cell apoptosis in human PCa cells through
the Akt/p53/Bax/PTEN signaling pathway. Thus, the present study may
provide experimental data for the use of DPPT in the treatment of
human PCa.
References
|
1
|
Bashir MN and Malik MA: Case-control study
of diet and prostate cancer in a rural population of Faisalabad,
Pakistan. Asian Pac J Cancer Prev. 16:2375–2378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bettuzzi S, Brausi M, Rizzi F, Castagnetti
G, Peracchia G and Corti A: Chemoprevention of human prostate
cancer by oral administration of green tea catechins in volunteers
with high-grade prostate intraepithelial neoplasia: A preliminary
report from a one-year proof-of-principle study. Cancer Res.
66:1234–1240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jannini EA, Gravina GL, Morgentaler A,
Morales A, Incrocci L and Hellstrom WJ: Is testosterone a friend or
a foe of the prostate? J Sex Med. 8:946–955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loch T: Prostate cancer diagnostics:
Innovative imaging in case of multiple negative biopsies. World J
Urol. 29:607–614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saunders EJ, Dadaev T, Leongamornlert DA,
JugurnauthLittle S, Tymrakiewicz M, Wiklund F, Al Olama AA,
Benlloch S, Neal DE, Hamdy FC, et al: Fine-mapping the HOXB region
detects common variants tagging a rare coding allele: Evidence for
synthetic association in prostate cancer. PLoS Genet.
10:e10041292014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sverrisson EF, Zens MS, Fei DL, Andrews A,
Schned A, Robbins D, Kelsey KT, Li H, DiRenzo J, Karagas MR and
Seigne JD: Clinicopathological correlates of Gli1 expression in a
population-based cohort of patients with newly diagnosed bladder
cancer. Urol Oncol. 32:539–545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cullen J, Elsamanoudi S, Brassell SA, Chen
Y, Colombo M, Srivastava A and McLeod DG: The burden of prostate
cancer in Asian nations. J Carcinog. 11:72012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Center MM, Jemal A, LortetTieulent J, Ward
E, Ferlay J, Brawley O and Bray F: International variation in
prostate cancer incidence and mortality rates. Eur Urol.
61:1079–1092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsilidis KK, Papadimitriou N, Capothanassi
D, Bamia C, Benetou V, Jenab M, Freisling H, Kee F, Nelen A,
O'Doherty MG, et al: Burden of cancer in a large consortium of
prospective cohorts in Europe. J Natl Cancer Inst. 108:djw1272016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the Human
development index (2008-2030): A population-based study. Lancet
Oncol. 13:790–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bianchi E, Caldwell ME and Cole JR:
Antitumor agents from Bursera microphylla (Burseraceae) I.
Isolation and characterization of deoxypodophyllotoxin. J Pharm
Sci. 57:696–697. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khaled M, Jiang ZZ and Zhang LY:
Deoxypodophyllotoxin: A promising therapeutic agent from herbal
medicine. J Ethnopharmacol. 149:24–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu TT and Huang JK: The clinical
usefulness of prostate-specific antigen (PSA) level and
age-specific PSA reference ranges for detecting prostate cancer in
Chinese. Urol Int. 72:208–211. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shin SY, Yong Y, Kim CG, Lee YH and Lim Y:
Deoxypodophyllotoxin induces G2/M cell cycle arrest and apoptosis
in HeLa cells. Cancer Lett. 287:231–239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Benzina S, Harquail J, Jean S, Beauregard
AP, Colquhoun CD, Carroll M, Bos A, Gray CA and Robichaud GA:
Deoxypodophyllotoxin isolated from Juniperus communis induces
apoptosis in breast cancer cells. Anticancer Agents Med Chem.
15:79–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu M, Jiang Z, Duan H, Sun L, Zhang S,
Chen M, Wang Y, Gao Q, Song Y, Zhu X and Zhang L:
Deoxypodophyllotoxin triggers necroptosis in human non-small cell
lung cancer NCI-H460 cells. Biomed Pharmacother. 67:701–706. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan JH, Feng GG, Huang L, Tang GD, Jiang
HX and Xu J: Naofen promotes TNF-α-mediated apoptosis of
hepatocytes by activating caspase-3 in lipopolysaccharide-treated
rats. World J Gastroenterol. 20:4963–4971. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamamoto Y, Koma H, Hiramatsu H, Abe M,
Murakami K, Ohya A and Yagami T: Treatment of etoposide combined
with 15-deoxy-Δ12,14-prostaglandin J2 exerted synergistic antitumor
effects against renal cell carcinoma via peroxisome
proliferator-activated receptor-γ-independent pathways. Mol Clin
Oncol. 2:292–296. 2014.PubMed/NCBI
|
|
19
|
Carrillo García C, Riedt T, Li J, Dotten
M, Brossart P and Janzen V: Simultaneous deletion of p21Cip1/Waf1
and caspase-3 accelerates proliferation and partially rescues the
differentiation defects of caspase-3 deficient hematopoietic stem
cells. PLoS One. 9:e1092662014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sang CY, Xu XH, Qin WW, Liu JF, Hui L and
Chen SW: DPMA, a deoxypodophyllotoxin derivative, induces apoptosis
and anti-angiogenesis in non-small cell lung cancer A549 cells.
Bioorg Med Chem Lett. 23:6650–6655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yong Y, Shin SY, Lee YH and Lim Y:
Antitumor activity of deoxypodophyllotoxin isolated from Anthriscus
sylvestris: Induction of G2/M cell cycle arrest and
caspase-dependent apoptosis. Bioorg Med Chem Lett. 19:4367–4371.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee JH, Cho HD, Jeong IY, Lee MK and Seo
KI: Sensitization of tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL)-resistant primary prostate cancer
cells by isoegomaketone from Perilla frutescens. J Nat Prod.
77:2438–2443. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith B, Randle D, Mezencev R, Thomas L,
Hinton C and Odero-Marah V: Camalexin-induced apoptosis in prostate
cancer cells involves alterations of expression and activity of
lysosomal protease cathepsin D. Molecules. 19:3988–4005. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wakabayashi K, Saito H, Ebinuma H, Saito
Y, Takagi T, Nakamura M, Umezawa A, Hata J and Ishii H: Bcl-2
related proteins are dramatically induced at the early stage of
differentiation in human liver cancer cells by a histone
deacetylase inhibitor projecting an anti-apoptotic role during this
period. Oncol Rep. 7:285–288. 2000.PubMed/NCBI
|
|
25
|
Yeh YC, Liu TJ and Lai HC: Shikonin
induces apoptosis, necrosis, and premature senescence of human A549
lung cancer cells through Upregulation of p53 Expression. Evid
Based Complement Alternat Med. 2015:6203832015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nishimura R, Arima N, Toyoshima S, Ohi Y,
Anan K, Sagara Y, Mitsuyama S and Tamura K: Evaluation of PTEN loss
and PIK3CA mutations and their correlation with efficacy of
trastuzumab treatment in HER2-positive metastatic breast cancer: A
retrospective study (KBC-SG 1001). Mol Clin Oncol. 1:47–52.
2013.PubMed/NCBI
|
|
27
|
Jung S, Li C, Jeong D, Lee S, Ohk J, Park
M, Han S, Duan J, Kim C, Yang Y, et al: Oncogenic function of
p34SEI-1 via NEDD4-1-mediated PTEN ubiquitination/degradation and
activation of the PI3K/AKT pathway. Int J Oncol. 43:1587–1595.
2013.PubMed/NCBI
|
|
28
|
Hancox U, Cosulich S, Hanson L, Trigwell
C, Lenaghan C, Ellston R, Dry H, Crafter C, Barlaam B, Fitzek M, et
al: Inhibition of PI3Kβ signaling with AZD8186 inhibits growth of
PTEN-deficient breast and prostate tumors alone and in combination
with docetaxel. Mol Cancer Ther. 14:48–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ghalali A, Wiklund F, Zheng H, Stenius U
and Högberg J: Atorvastatin prevents ATP-driven invasiveness via
P2X7 and EHBP1 signaling in PTEN-expressing prostate cancer cells.
Carcinogenesis. 35:1547–1555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu Z, McRoberts KS and Theodorescu D: The
role of PTEN in prostate cancer cell tropism to the bone
micro-environment. Carcinogenesis. 28:1393–1400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schmitz M, Grignard G, Margue C, Dippel W,
Capesius C, Mossong J, Nathan M, Giacchi S, Scheiden R and Kieffer
N: Complete loss of PTEN expression as a possible early prognostic
marker for prostate cancer metastasis. Int J Cancer. 120:1284–1292.
2007. View Article : Google Scholar : PubMed/NCBI
|