Introduction
Prostate cancer (PCa) is second only to lung cancer
as the most frequent type of cancer in men worldwide. In terms of
mortality, PCa ranks sixth internationally as a cause of
cancer-related mortality in men. Each year, PCa is responsible for
~250,000 mortalities in men worldwide (1,2). PCa is a
heterogeneous disease that exhibits a range of clinical behaviors,
from indolent, slow-growing tumors to aggressive, fast-growing
tumors with lethal progression. Accurate discrimination between
cases of aggressive and non-aggressive PCa, using parameters such
as clinical stage, prostate-specific antigen level and Gleason
score, are not adequate to diagnose the risk category (3). In addition, chemotherapy resistance is a
major problem in the treatment of PCa, particularly at advanced
stages (3,4).
Cancer stem cells (CSCs) have specific functional
features, including self-renewal capacity, long-term repopulation
potential and differentiation ability in order to give rise to a
heterogeneous phenotype of tumor cells. Therefore, CSCs differ from
the bulk tumor cells, and are known to be involved in metastasis,
recurrence and therapy resistance in various cancer types. The
genetic evolution and epigenetic plasticity (i.e., genetic
heterogeneity) of CSCs promote the emergence of tumor adaptations,
consequently leading to therapeutic failure (5–7).
Midkine (MK) is a heparin binding small protein (13
kDa) with growth factor and cytokine actions. Together with
pleiotrophin/heparin-binding growth-associated molecule, MK
comprises a family of heparin binding growth factors. MK is highly
expressed at the mid-gestation period, however, its expression is
decreased and/or lost in adults. MK exhibits its activity through
several receptors, including anaplastic lymphoma kinase, proteine
tyrosine phosphatase ζ, Notch 2, low density lipoprotein
receptor-related protein (LRP), proteoglycans and integrins. MK has
significant roles in inflammation immunity, blood pressure,
development, tissue protection/renewal/repair, cancer [including
tumor growth, chemoresistance, transformation, anti-apoptosis,
epithelial-mesenchymal transition (EMT)] and determination of cell
fate (from autophagy to cell resistance/survival or autophagic cell
death/apoptosis) (8–12). High MK expression has been observed in
several cancer types, including PCa, in preclinical and clinical
studies (9,13,14).
Lithium chloride (LiCl) is a Food and Drug
Administration-approved drug for bipolar psychiatric disorder. LiCl
has been a well-known drug with anti-manic properties for 60 years.
It has cancerogenic and anti-cancerogenic properties, meaning that
it can inhibit or stimulate the growth of normal and cancer cells
[i.e., it has a biphasic effect). LiCl acts through inhibition of
the glycogen synthase kinase-3β (GSK-3β) pathway (an agonist known
to activate the Wnt/β-catenin signaling], the phosphoinositide
signaling pathway and adenylate cyclase (15–19).
Cancer and/or the cancer treatment itself may lead to psychiatric
disorders causing a reduction in life quality and a desire to
overcome the illness (20).
Therefore, the safety profile of the anti-manic drug LiCl needs to
be re-evaluated.
CSCs have also been identified in PCa (21). Advances in research have shown that,
similar to normal tissue, certain types of cancer have a
hierarchical organization where tumorigenic CSCs differentiate into
non-tumorigenic progenies (22).
CSCs, LiCl with biphasic effects and the differentiation growth
factor MK appear to be part of the Russian Roulette of cancer
therapy. Therefore, the aims of the present study were to
investigate the effect of different concentrations of LiCl on PCa
stem cells (whether a shift from tumorigenic to non-tumorigenic
situation occurs or not) and to determine if these results can be
explained through MK levels.
Materials and methods
Cell culture and reagents
The DU145 human PCa cell line (HTB-81) was supplied
by the American Type Culture Collection (Manassas, VA, USA) and was
grown in a monolayer culture in Dulbecco's modified Eagle's
medium-F12 (DMEM-F12; Biological Industries Israel Beit-Haemek
Ltd., Kibbutz Beit-Haemek, Israel) supplemented with 10%
heat-inactivated fetal calf serum (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 100 U/ml penicillin and 100 µg/ml
streptomycin (Sigma-Aldrich, St. Louis, MO, USA) at 37°C in a
humidified atmosphere of 5% CO2. Cells in semi-confluent
flasks (70% of confluency) were harvested using 0.05% trypsin
(Sigma-Aldrich). Harvested cells were added to DMEM-F12 for trypsin
inactivation and centrifuged (NF200; Nüve Laboratory and
Sterilization Technology, Ankara, Turkey) at 500 × g for 3 min at
22°C and then resuspended in culture medium (23).
Stem cell sorting
For fluorescence-activated cell sorting (FACS),
cells were detached using non-enzymatic cell dissociation solution
(Sigma-Aldrich) and ~5×104 cells were incubated with
cluster of differentiation CD(133) and CD44 antibodies diluted to
1:100 in FACS wash (0.5% bovine serum albumin, 2 mM NaN3
and 5 mM ethylenediaminetetraacetic acid; Miltenyi Biotec, Bisley,
UK) for 15 min at 4°C: An isotype and concentration-matched
phycoerythrin (PE) (monoclonal mouse IgG1; clone, IS5-21F5;
dilution, 1:100; catalog no., 130-092-212; Miltenyi Biotec) and
fluorescein isothiocyanate (FITC)-labeled (monoclonal mouse IgG1;
1:100; clone, IS5-21F5; catalog no., 130-092-213; Miltenyi Biotec)
control antibody were used, and the tested samples were PE-labeled
CD133/1 (monoclonal mouse; clone, AC133; dilution, 1:100; catalog
no., 130-080-801; Miltenyi Biotec) and FITC-labeled CD44
(monoclonal mouse; clone, G44-26; dilution, 1:100; catalog no.,
555478; BD Pharmingen, San Diego, CA, USA). After 3–5 min, the
cells were washed with FACS wash and resuspended with FACS wash.
The cells were sorted into CD133high/CD44high
(CSC) and non-CSC subpopulations using a FACSAria cell sorter (BD
Biosciences), and termed DU145(+) and DU145(−), respectively. The
two subpopulations were cultured in two different settings:
Monolayer culture or multicellular tumor spheroids (23,24).
Experimental design
Stem cells and non-stem cells were incubated with
low (1 and 10 µM) and high (100, 500 µM) concentrations of LiCl for
72 h at 37°C in a humidified atmosphere of 5% CO2. The
four drug groups and an untreated control group were evaluated for
total cell numbers, apoptotic index [flow cytometric Annexin
V-FITC/propidium iodide (PI) staining], MK levels (enzyme-linked
immunosorbent assay; ELISA) of monolayer cultures and
ultrastructure of spheroid cultures [transmission electron
microscopy (TEM)].
Cell proliferation index analysis
A starter kit (catalog no., M1293-0020; ChemoMetec
A/S, Allerod, Denmark) and software (NucleoView Software, version
1.0; ChemoMetec A/S) compatible with an automated cell counter
(NucleoCounter; ChemoMetec A/S) were used to determine the total
cell number. The kit included lysis buffer, stabilization buffer
and nucleocasettes. Briefly, cells were harvested every 24 h for 72
h. Cells were pre-treated with lysis and stabilization buffers to
dissolve cell aggregates and lyse cell membranes for 5–10 min.
Pre-treated cells were loaded to nucleocasettes coated with PI dye
and their nuclei were stained with PI. Nucleocasettes were placed
into the cell counter for 30–35 sec to measure PI fluorescence.
Subsequently, cell counts were analyzed using the software and
recorded (24).
MK concentration level analysis
MK protein concentration levels were detected using
an ELISA kit (catalog no., CDYELISA; Cellmid Limited, Sydney,
Australia), according to the manufacturers' instructions with minor
modifications. Briefly, 100 µl/well of test samples (the
supernatants of lysed stem cells and non-stem cells), positive
controls (one supplied in the ELISA kit and one a high concentrated
MK from T98 glioblastoma cells) and standards (concentrations of 0,
25, 50, 100, 250, 500, 750 and 1,000 pg/ml were obtained by
diluting MK master standard in a concentration of 107
pg/ml) were all incubated at room temperature for 2 h with
continuous shaking. Standards, controls and samples were run in
triplicate. After every step except for the application of stop
reagent, four washing applications with washing buffer supplied by
the kit were performed for stem cells and non-stem cells. Detector
antibody (100 µl/well) was added and incubated at room temperature
for 1 h with continuous shaking. Then, samples were incubated with
100 µl/well streptavidin-peroxidase solution for 20 min and 100
µl/well Substrate Solution for 15 min in the dark at room
temperature with continuous shaking. After 15 min, 100 µl/well stop
solution was added in order to inactivate the enzyme and detect
blue color formation. Then, results were measured at a wavelength
of 450 nm using an ELISA microplate reader within 3 min of stopping
(24).
Apoptotic index analysis
One of the manifestations of apoptosis is the
translocation of phosphotidylserine (PS) from the inner membrane to
the outer side of the plasma membrane. Externalization of PS was
studied by the Annexin V binding assay. The apoptotic index was
evaluated using flow cytometric Annexin-V-FITC/PI staining (BD
Biosciences), according to the manufacturer's instructions.
Briefly, cells were washed twice with phosphate-buffered saline,
then resuspended in binding buffer containing 0.01 M HEPES, 0.14 mM
NaCl and 2.5 mM CaCl2. A cell suspension
(1×105 cells in 100 µl binding buffer) was incubated
with 5 µl FITC-labeled Annexin V dye and PI (BD Biosciences) for 15
min in the dark at room temperature. Following incubation, PI
fluorescence and Annexin V were measured simultaneously in a
FACSCalibur machine and analyzed with the instrument's operating
software (CellQuest; BD Biosciences). Data acquisition and analysis
were undertaken with CellQuest version 5.1 and Windows Multiple
Document Interface for Flow Cytometry version 2.8 programs
(24). The programs calculated 4
types of situations (viability, early apoptosis, late apoptosis and
death) and presented the data through panels with 4 quadrants: The
lower left quadrant shows density plots for the number of viable
cells (Annexin V−, PI−); the lower right
quadrant shows the number of earlier stages of apoptotic cells
(Annexin V+, PI−); the upper right quadrant
shows the number of late stages of apoptotic cells (Annexin
V+, PI+); and the upper left quadrant shows
the number of dead cells. The program provided the percentage using
the formula: Cells in quadrant/total cell number. In the graph of
results, only the percentage of apoptotic cells (the sum of lower
right and upper right) is presented.
Multicellular spheroid model
For spheroid cultures, the tumor cells grown as a
monolayer were resuspended with trypsin and the clonogenic
potential of various phenotypic populations was analyzed in a 3D
non-adherent culture condition (plates coated with 3% Noble Agar;
Gibco; Thermo Fisher Scientific, Inc.). The cells were counted,
resuspended and plated at 1×106 cells per well in a
6-well plate. The plates were inspected for colony (sphere) growth
1 week after initiation. The number of colonies within each well
was counted under a microscope (Olympus BX-51; Olympus, Hamburg,
Germany) and images of representative fields were captured
(24).
Ultrastructure analysis
Harvested spheroids were fixed with 2.5%
glutaraldehyde in 0.1 M sodium cacodylate buffer for 1 h at 4°C and
post-fixed in 1% osmium tetraoxide in 0.1 M sodium cacodylate
buffer for 20–30 min at 4°C. Spheroids were incubated in 1% uranyl
acetate for 1 h at 4°C, dehydrated in a graded ethanol series and
embedded in in Epon 812 substitute as Epoxy Embedding Medium
(Sigma-Aldrich). Samples were cut using a rotating blade microtome
(Leica Biosystems, Heerbrugg, Switzerland) to a thickness of 70 nm
and sections were mounted on copper grids. Sections were
subsequently stained with 5% uranyl acetate for 30 min and
counterstained with Reynold's lead citrate for 3–5 min at 22°C.
Sections were examined with a JEM-1011 TEM (JEOL, Inc., Peabody,
MA, USA). Photographs of stem cell and non-stem cell spheroids
applied with the lowest (1 µM) and highest (500 µM) concentrations
of LiCl were captured at several different magnifications (24).
Statistical analysis
SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) and
Microsoft Excel 2010 (Microsoft, Inc., Redmond, WA, USA) were used
for the statistical analysis. Results were statistically analyzed
using one-way analysis of variance and relevant differences were
analyzed by post-hoc comparisons using the Bonferroni method. Data
are represented as mean ± standard error of the mean (n=6).
Experiments were repeated three times, each experiment was
triplicated. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cell proliferation index analysis
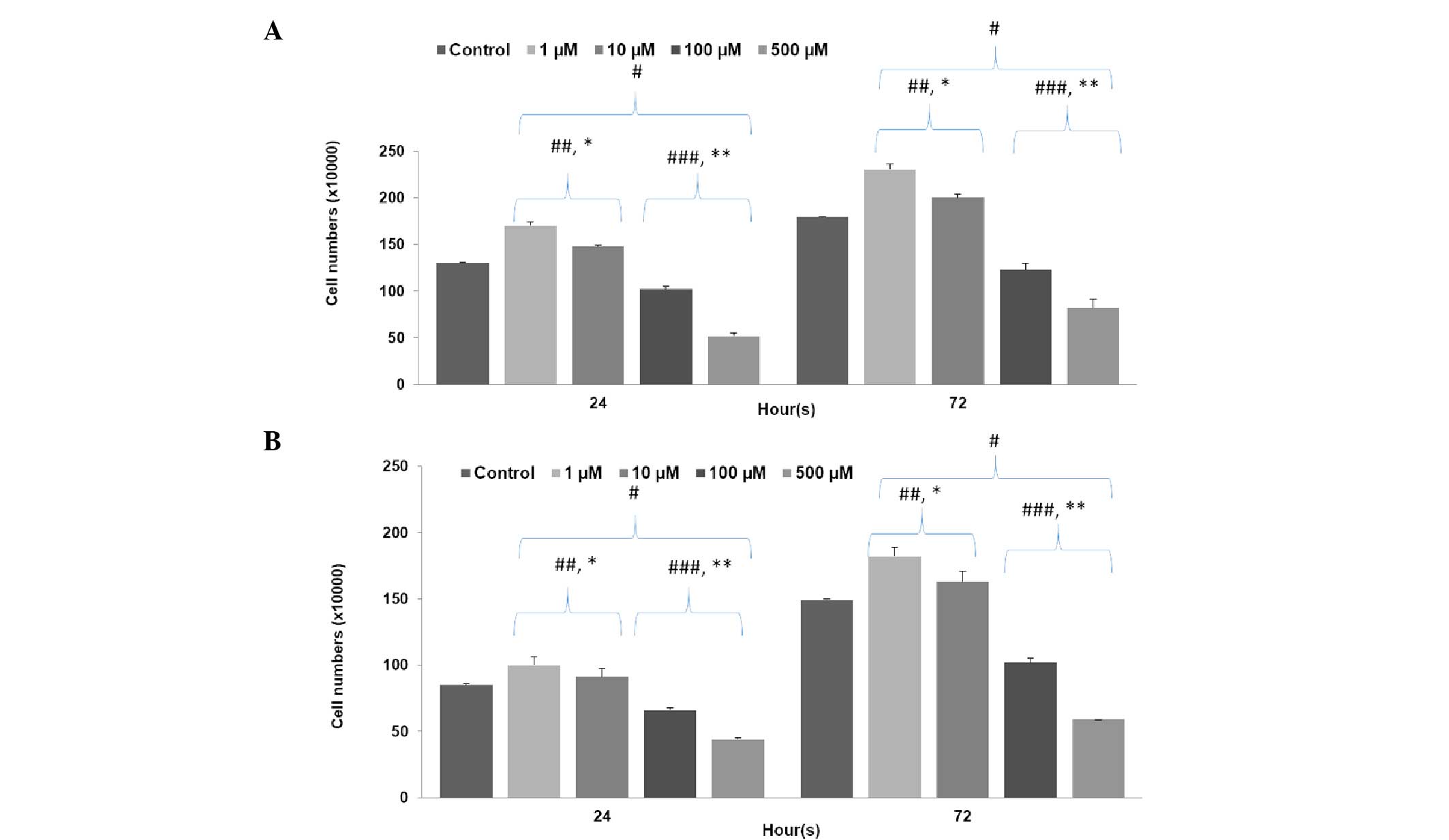
Figure 1 clearly shows
that low concentrations of LiCl significantly increased cell
number, while high concentrations of LiCl significantly decreased
cell number in both stem (P500=0.0001;
P100=0.001; P10=0.001; P1=0.0001)
and non-stem cells (P500=0.0001; P100=0.001;
P10=0.0001; P1=0.0001) in comparison with the
control group (P<0.001). The inhibition rate observed with high
concentrations of LiCl in the non-stem cell group was higher than
that observed for the stem cell group (P500=0.02;
P100=0.04); however, the increase in proliferation
observed with low concentrations of LiCl was much lower than in the
stem cell group (P10=0.02; P1=0.03).
Furthermore, treatment with 500 µM LiCl decreased cell numbers
significantly more than in the 100 µM group and 1 µM LiCl increased
cell numbers significantly more than in the than 10 µM group for
both stem (P500=0.001; P1=0.001) and non-stem
cell groups (P500=0.001; P1=0.0001).
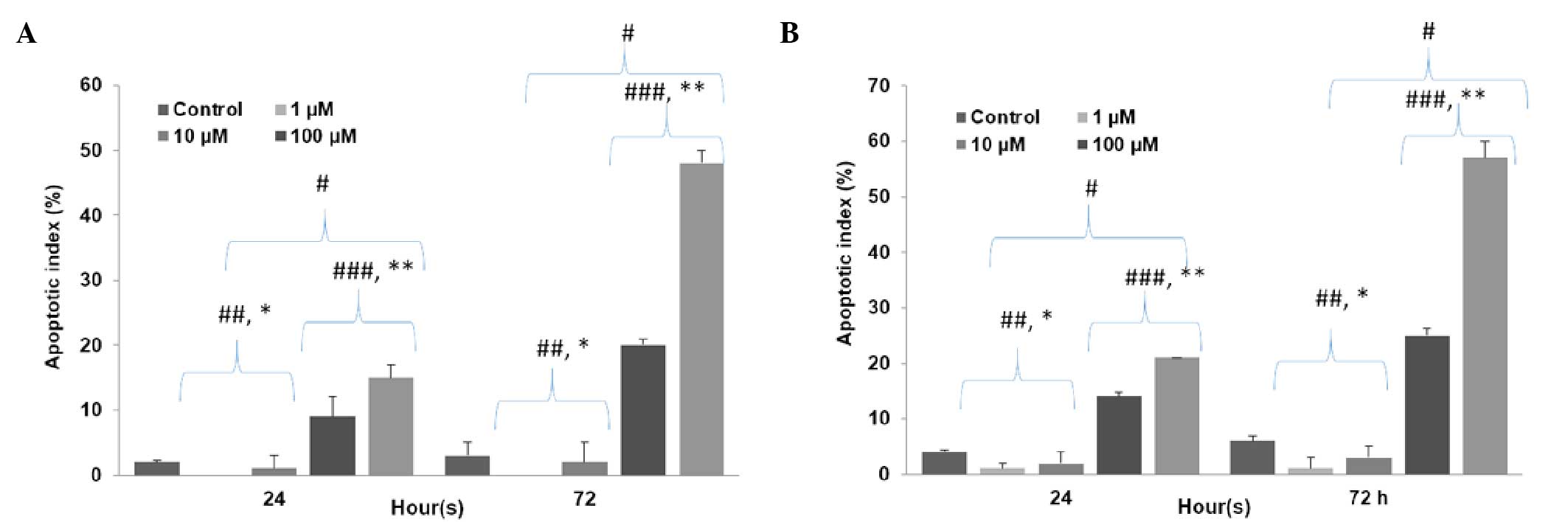
Apoptotic index analysis
It was determined that high concentrations of LiCl
significantly increased the apoptotic index, while low
concentrations significantly decreased the apoptotic index in both
stem cells (P500=0.00001; P100=0.00001;
P10=0.04; P1=0.02) and non-stem cells
(P500=0.00001; P100=0.00001;
P10=0.03; P1=0.01) in comparison with the
control group. High concentrations of LiCl increased the apoptotic
index in non-stem cells significantly more than in stem cells
(P500=0.002; P100=0.004) and low
concentrations of LiCl decreased the apoptotic index in non-stem
cells significantly more than in stem cells (P10=0.04;
P1=0.02). In addition, the apoptotic index was lowest at
1 µM LiCl and highest at 500 µM LiCl for both stem cell group
(P500 vs. 100=0.0001; P1 vs. 10=0.03) and
non-stem cell group (P500 vs. 100=0.002; P1 vs.
10=0.01) (Fig. 2).
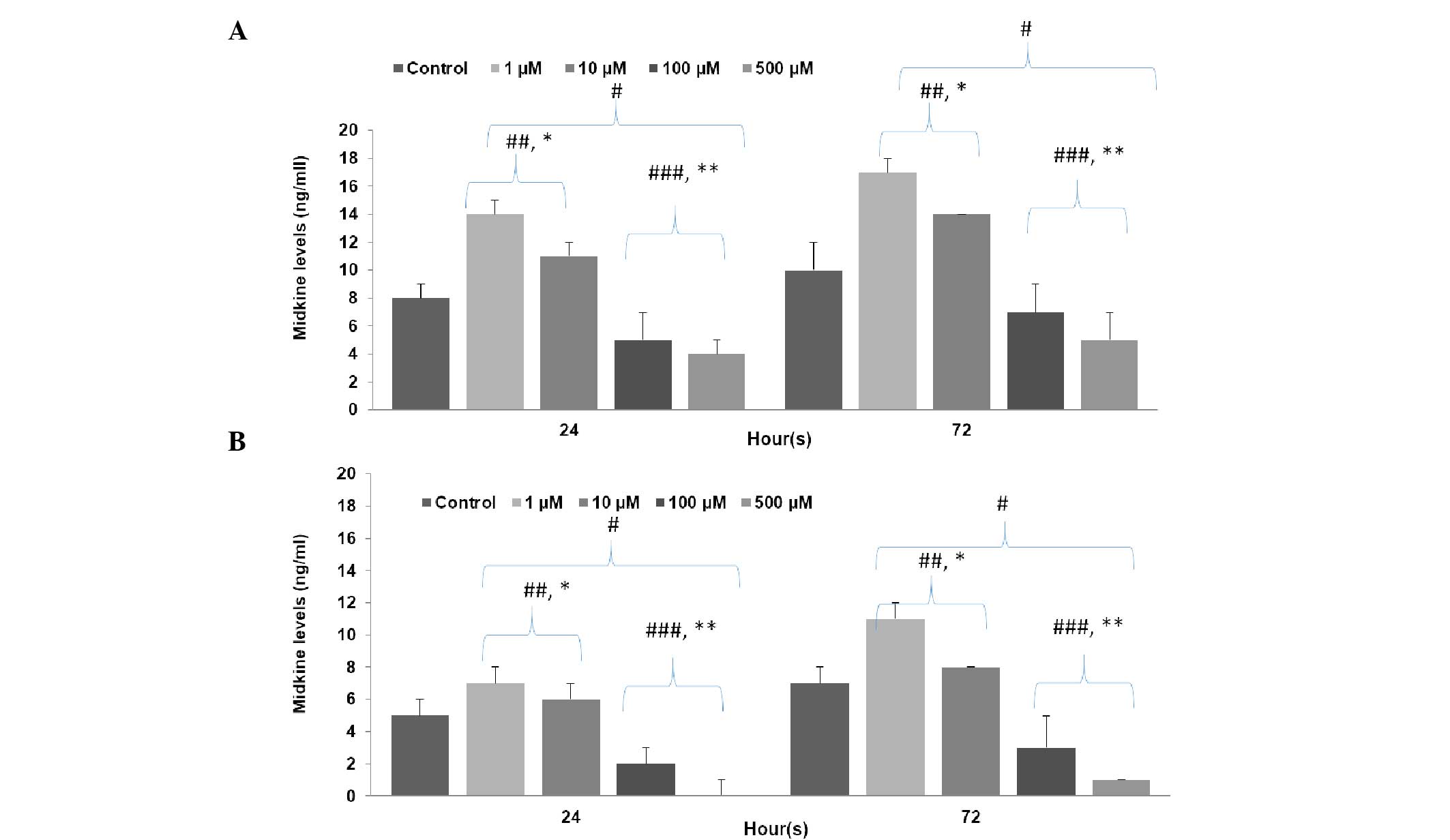
MK concentration level analysis
As shown in Fig. 3,
low concentrations of LiCl significantly increased MK levels, while
high concentrations significantly decreased MK levels in both stem
cells (P500=0.0001; P100=0.0001;
P10=0.01; P1=0.001) and non-stem cells
(P500=0.0001; P100=0.001;
P10=0.03; P1=0.001) in comparison with the
control group. The inhibition rates caused by high concentrations
of LiCl in the non-stem cell group were significantly higher than
in the stem cell group (P500=0.01;
P100=0.01); however, the proliferation stimulation rates
of cells MK levels treated with low concentrations of LiCl was
significantly lower in the non-stem cell group than the stem cell
group (P10=0.0001; P1=0.001). Application of
500 µM LiCl decreased MK levels significantly more than 100 µM and
1 µM LiCl increased MK levels significantly more than 10 µM for
both stem group (P500 vs. 100=0.01; P1 vs.
10=0.01) and non-stem cell group (P500 vs.
100=0.01; P1 vs. 10=0.02).
Ultrastructure analysis
In the stem cell group, spheroids treated with 1 µM
LiCl had the same structure as the control group, displaying intact
spheroid integrity (i.e., strong cell-to-cell adhesions),
heterochromatic nucleus, healthy mitochondria and prominent
endoplasmic reticulum. One or two autophagic vacuole-like
structures and numerous lipid droplets were observed in the stem
cell group, however, no membrane breakdown in the plasma and
nucleus membranes, and no vacuoles were observed (Fig. 4A and B). Treatment of stem cells with
the highest concentration of LiCl (500 µM) resulted in 3 various
cell ultrastructures: i) Apoptotic cells; ii) necrotic cells; and
most frequently, iii) healthy cells, some of which had 3–5
autophagic vacuoles (Fig. 4C). The
control group of non-stem cells showed a healthy structure, as did
the control group of stem cells. The non-stem cell group treated
with 1 µM LiCl had a similar structure to the control group with
certain exceptions, such as the loss of spheroid integrity (i.e.
weak and/or lost cell-to-cell adhesions) in certain spheroids and a
few damaged mitochondria in certain cells (Fig. 4D and E). By contrast, destructive
images were frequently observed in non-stem cells treated with 500
µM LiCl, including no spheroid structure only apoptotic bodies, the
presence of single cells with apoptotic appearance, and cell
remnants (lytic cells). No healthy cells were observed (Fig. 4F).
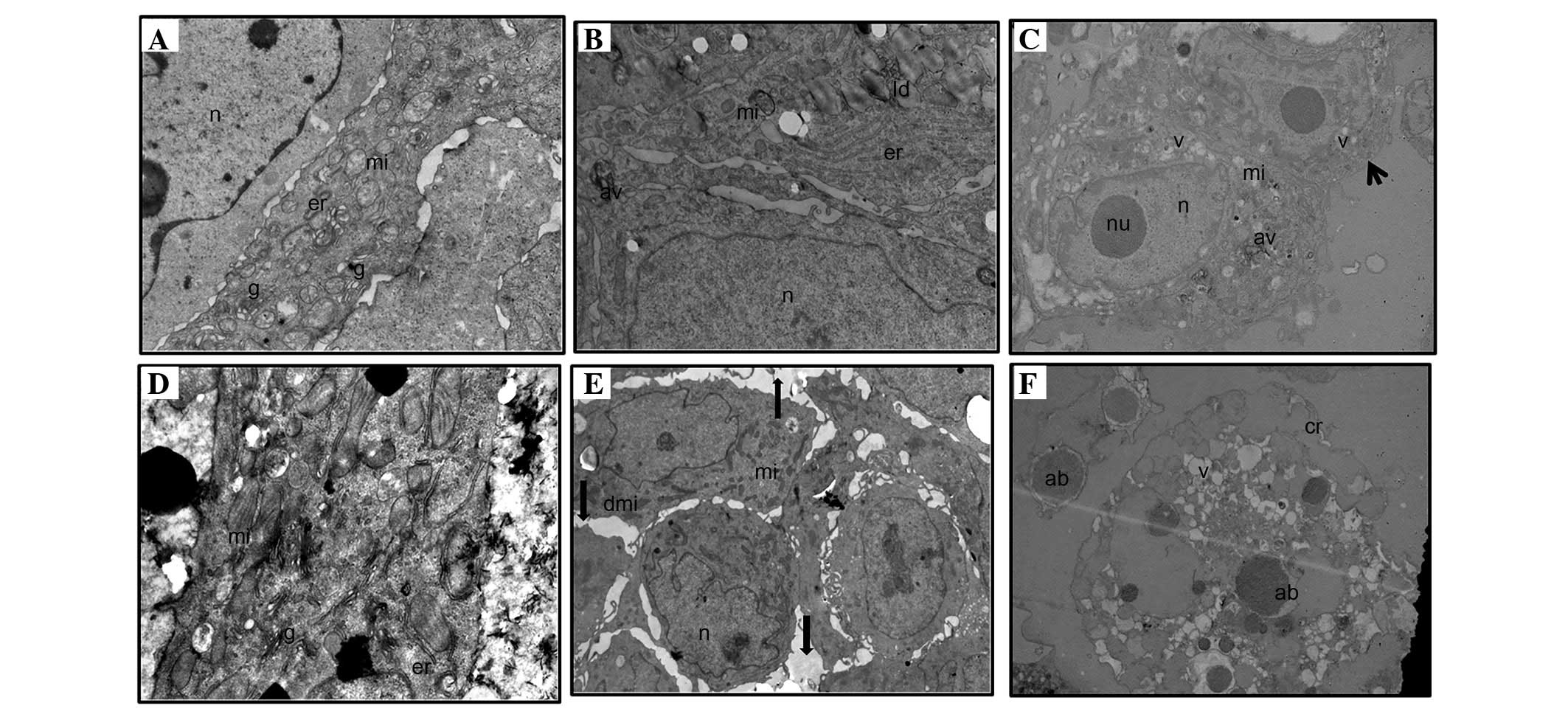 | Figure 4.Ultrastructure analysis of spheroids
of (A-C) stem cells and (D-F) non-stem cells. (A) Control group
(magnification, ×12,000), (B) 1 µM LiCl group (magnification,
×10,000), (C) 500 µM LiCl group (magnification, ×7,500; arrow
indicates a healthy cell with some autophagic vacuoles), (D)
control group (magnification, ×12,000), (E) 1 µM LiCl group
(magnification, ×6,000; arrows indicate gaps between cells in the
spheroid), (F) 500 µM LiCl group (magnification, ×7,500). n,
nucleus; mi, mitochondria; er, endoplasmic reticulum; g, golgi
apparatus; ld, lipid droplets; nu, nucleolus; av, autophagic
vacuoles; dmi, damaged mitochondria; cr, cell remnants (lytic
cells); ab, apoptotic bodies. |
Discussion
To the best of our knowledge, the present study
showed for the first time that the effects of LiCl on PCa stem
cells are also concentration-dependent/biphasic, as in normal
prostate cells, and MK levels are changed directly proportional to
this effect in PCa stem cells in vitro. Hossein et al
(25) previously evaluated LiCl with
various concentrations (2.5, 10 and 25 mM) in the
androgen-independent human prostate cell line, DU145. In the study,
the viability of DU145 cells in the presence or absence of LiCl
(2.5–25 mM) was assessed as a percentage of viable cells compared
with the control (absence of LiCl). After 48 h, DU145 cells showed
a 32 and 53% reduced cell viability with 10 and 25 mM LiCl,
respectively, and a significantly decreased cell viability of 13%
was observed with low and high doses of LiCl after 72 h (25). In addition, LiCl [half maximal
inhibitory concentration (IC50), 20 nM] was combined
with IC50 concentrations and low concentrations of other
well-known anti-neoplastic drugs, such as doxorubicin (Dox),
etoposide (Eto) or vinblastine (Vin) (25). The study determined the synergistic
effect of LiCl with these drugs and concluded that the
IC50 concentrations of all three drugs combined with
LiCl demonstrated a decreased cell percentage in the G1 phase and
increased p53 levels compared with the control or LiCl alone
(25).
A different study performed by Azimian-Zavareh et
al (26) used androgen-dependent
PCa LNCap cells and the same drug treatments (Dox, Eto and Vin).
The results showed that LiCl increases apoptosis of these cells in
the presence of Eto, which is S and G2 phase-specific drug.
Suganthi et al (15) treated
human breast cancer cells (MCF-7) with low (1, 5 and 10 mM) and
high (50 and 100 mM) concentrations of LiCl. The results were
similar to those of Hossein et al (25) and Azimian-Zavareh et al
(26), and indicated that LiCl
induces cell survival by inhibiting apoptosis through regulation of
GSK-3β, caspase-2, Bcl2-associated X protein and cleaved caspase-7,
and by activation anti-apoptotic proteins (Akt, β-catenin, B cell
lymphoma-2, and cyclinD1). However high concentrations induced
apoptosis by reversing these effects. Considering the results of
the aforementioned studies, it can be concluded that LiCl exhibits
a cytotoxic effect in a dose- and time-dependent manner.
The etiology of cancer is complex and appears to
include several mechanisms: i) Normal cells with mutations or
epigenetic changes can become cancer cells; ii) normal stem cells
can transform into CSCs via specific mechanisms; iii) CSCs can
originate from cancer cells that are hierarchically downstream of
CSCs but have not differentiated and have acquired the capacity for
self-renewal; and iv) cancer cells can be derived from progenitor
cells or from more differentiated cells via a dedifferentiation
process (EMT) (6,27–32). EMT
appears to have an important role by endowing cells with some of
the characteristics and behaviors of CSCs (33). It is unclear which characteristic is
responsible for cancer progession or metastasis. However, it is
clear that only one injured cell or one cell that manages to escape
from therapy, regardless of whether it is a stem cell or non-stem
cell, can lead to the progression and recurrence of cancer,
consequently causing therapy to collapse (6,29).
Furthermore, it is well-known that CSCs in the bulk of the tumor
are responsible for poor prognosis and therapy resistance,
particularly in chemotherapy. Preclinical and clinical trials
targeted to eradicate this population have improved prognosis
(29,34). Wang et al (32) used afatinib, a small-molecule
inhibitor of the epidermal growth factor receptor and erb-b2
receptor tyrosine kinase 2 and 4 tyrosine kinases, in order to
prefentially eliminate side population cells with CSC characters in
cell lines and patient-derived leukemia cells, by decreasing ATP
binding cassette subfamily G member 2 expression. In these cells,
afatinib also acted in parallel to suppress self-renewal capacity
and tumorigenicity. CSC self-renewal is one approach to cancer
therapy, amongst others.
The Wnt/β-catenin signaling pathway, which is one of
the major targets of LiCl, has a significant role in several
developmental processes and the maintenance of adult tissue
homeostasis by regulating cell proliferation, differentiation,
migration, genetic stability and apoptosis, and maintaining adult
stem cells in a pluripotent state (35). Silva et al showed that the
application of 20 and 40 mM LiCl increased the number of stem-like
cells in retinoblastoma through the Wnt/β-catenin pathway,
maintaining stem cell renewal and leading to tumor formation
(36). Concomitant with the study by
Silva et al (36), Cai and Zhu
(37) showed that LiCl improved the
self-renewal of human gastric cancer stem-like cells by using 10 mM
LiCl. Another study by Teng et al (38) stimulated the Wnt/β-catenin signaling
pathway of lung cancer cells via treatment with 10 mM LiCl. This
resulted in enhanced proliferation, clone formation and drug
resistance abilities of the cells through an increase in the number
of stem cells, as determined by upregulation of the stem cell
marker, OCT-4.
MK is highly correlated with cancer resistance and
EMT (9,10,14). Wnt
ligands bind to Frizzled-LRP5/6 receptor complexes and activate the
cytoplasmic scaffold protein, Dishevelled, resulting in inhibition
of β-catenin phosphorylation and degradation (38). One of the candidate receptors of MK is
LRP, therefore, MK and Wnt signaling pathways have a common
receptor (14).
In contrast to the studies by Silva et al
(36), Cai and Zhu (37), and Teng et al (38), the present study used micromolar
concentrations of LiCl following initial optimization experiments.
Similar to studies by Hossein et al (25) and Azimian-Zavareh et al
(26), which used the same cell lines
as the present study, low concentrations of LiCl were found to
increase stem cell proliferation as well as non-stem cell
proliferation in the current study; however, opposite results were
obtained in the high LiCl concentration groups. In addition, in the
low LiCl concentration groups, the apoptotic index was very low and
MK levels were very high. Opposite results were obtained in the
high LiCl concentration group. Furthermore, the stem cell group
with the lowest apoptotic index and highest MK levels increased
their cell numbers significantly more than non-stem cells during
low LiCl concentration stimulation. The stem cell group was only
marginally effected by the application of high LiCl concentrations,
exhibiting a lower apoptotic index and higher MK levels than
non-stem cells.
Ultrastructure evaluation of stem cell and non-stem
cell spheroids treated with the lowest (1 µM) and highest (500 µM)
concentrations of LiCl showed the biphasic effect more prominently.
Ultrastructure evaluation showed that stem cells treated with the
lowest concentration of LiCl (1 µM) had similar ultrastructure to
the control group. By contrast, stem cells treated with 500 µM LiCl
showed apoptotic and necrotic cells, as well as numerous healthy
cells. That means healthy spheroids are common scheme. The
ultrastructure of non-stem cells at 1 µM LiCl was also similar to
the control group; a low degree of damage was rarely observed,
including loss of cell-to-cell interactions and spheroid integrity.
LiCl (500 µM) damaged non-stem cells severely, as indicated by
their apoptotic appearance, cell remnants (lytic cells), apoptotic
bodies and the loss of spheroid integrity (numerous single damaged
cells). Comparison of the electron micrographs of the stem and
non-stem cell control groups (prior to treatment) revealed no
differences.
EMT is characterized by the loss of polarity of
epithelial cells and decreased adhesion with surrounding cells,
resulting in single, motile cells (i.e., metastatic ability).
Therefore, the inhibition of cellular adhesive ability is
associated with EMT (33,39). According to this data, no loss of
spheroid integrity and no single cell motility was observed in the
group treated with the lowest LiCl concentration, where high levels
of MK were determined. This indicates that MK may not exhibit its
mechanism of action through EMT. MK levels in the stem cell groups
treated with high concentrations of LiCl were higher than the
non-stem cell group, however, MK levels did not appear to be
involved in the PCa stem cell fate resistance pathway. A previous
study revealed the co-existence of multiple genetically diverse
clones within the same tumor (40).
Upon treatment, this intratumoral diversity, which is associated
with distinct treatment sensitivity due to genetic heterogeneity,
caused different clones to exhibit distinct mechanisms of
resistance within the same tumor. Similar to normal tissue, certain
types of cancer have hierarchical organization where tumorigenic
CSCs differentiate into non-tumorigenic progenies (29,40). We
propose that this hierarchy may explain why MK levels in PCa stem
cells were lower than expected. Hierarchy may have occurred
spontaneously or after LiCl application. Thus, MK levels are not
involved in the biphasic effect of LiCl, however, they are affected
proportionally.
In conlusion, the present study showed that LiCl
acted through cancer supression or promotion through stem cells in
an in vitro prostate cancer model in a concentration
dependent manner. In addition, the underlying mechanism was
evaluated through MK, which is a neglected biomarker in numerous
studies. The present study concluded that MK had no effect on the
prostate cancer stem cell resistance mechanism during LiCl
administration. The current findings elucidate the important
pathophysiological process that drives PCa progression toward
resistance/lethality during chemotherapy with LiCl.
Acknowledgements
The authors thank the technical laboratory staff
from the Department of Histology and Embryology (İstanbul Faculty
of Medicine, İstanbul University, İstanbul, Turkey). The abstract
was presented at the 25th National Biochemistry Congress Sept 3–6
2013 in İzmir, Turkey [Turk J Biochem 38 (S1): abstract no. S-001,
2013] and the Third Midkine Symposium Apr 21–23 2014 in Kyoto,
Japan (Third Midkine Symposium Abstract Book, pp17-17, Kyoto,
Japan, 2014). The authors also thank Dr Kewın Jeffrey Pavlak
(Assistant Professor, Department of Physiology, Faculty of
Medicine, Zirve University, Gaziantep, Turkey) for editing the
English language and Dr Mehmet Karadağ (Department of Biostatistics
and Medical Informatics, Faculty of Medicine, Zirve University) for
statistical evaluation.
Glossary
Abbreviations
Abbreviations:
|
PCa
|
prostate cancer
|
|
MK
|
midkine
|
|
EMT
|
epithelial-mesenchymal transition
|
|
LiCl
|
lithium chloride
|
|
CSCs
|
cancer stem cells
|
|
GSK-3β
|
glycogen synthase kinase-3β
|
|
DMEM-F12
|
Dulbecco's modified Eagle's
medium-F12
|
|
LRP
|
low density lipoprotein
receptor-related protein
|
|
PE
|
phycoerythrin
|
|
FITC
|
fluorescein isothiocyanate
|
|
TEM
|
transmission electron microscopy
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
PI
|
propidium iodide
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
Dox
|
doxorubicin
|
|
Eto
|
etoposide
|
|
Vin
|
vinblastine
|
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mavroudi M, Zarogoulidis P, Porpodis K,
Kioumis I, Lampaki S, Yarmus L, Malecki R, Zarogoulidis K and
Malecki M: Stem cells' guided gene therapy of cancer: New frontier
in personalized and targeted therapy. J Cancer Res Ther (Manch).
2:22–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boorjian SA, Eastham JA, Graefen M,
Guillonneau B, Karnes RJ, Moul JW, Schaeffer EM, Stief C and Zorn
KC: A critical analysis of the long-term impact of radical
prostatectomy on cancer control and function outcomes. Eur Urol.
61:664–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thompson I, Thrasher JB, Aus G, Burnett
AL, CanbyHagino ED, Cookson MS, D'Amico AV, Dmochowski RR, Eton DT,
Forman JD, et al: Guideline for the management of clinically
localized prostate cancer: 2007 update. J Urol. 177:2106–2131.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peitzsch C, Kurth I, KunzSchughart L,
Baumann M and Dubrovska A: Discovery of the cancer stem cell
related determinants of radioresistance. Radiother Oncol.
108:378–387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kreso A and Dick JE: Evolution of the
cancer stem cell model. Cell Stem Cell. 14:275–291. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kreso A, O'Brien CA, van Galen P, Gan OI,
Notta F, Brown AM, Ng K, Ma J, Wienholds E, Dunant C, et al:
Variable clonal repopulation dynamics influence chemotherapy
response in colorectal cancer. Science. 339:543–548. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kadomatsu K, Kishida S and Tsubota S: The
heparin-binding growth factor midkine: The biological activities
and candidate receptors. J Biochem. 153:511–521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muramatsu T and Kadomatsu K: Midkine: An
emerging target of drug development for treatment of multiple
diseases. Br J Pharmacol. 171:811–813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Erguven M, Bilir A, Yazihan N, Ermis E,
Sabanci A, Aktas E, Aras Y and Alpman V: Decreased therapeutic
effects of noscapine combined with imatinib mesylate on human
glioblastoma in vitro and the effect of midkine. Cancer Cell Int.
11:182011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao SL, Zhang YJ, Li MH, Zhang XL and
Chen SL: Mesenchymal stem cells with overexpression of midkine
enhance cell survival and attenuate cardiac dysfunction in a rat
model of myocardial infarction. Stem Cell Res Ther. 5:372014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai LC: Midkine translocated to nucleoli
and involved in carcinogenesis. World J Gastroenterol. 15:412–416.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takei Y, Kadomatsu K, Goto T and Muramatsu
T: Combinational antitumor effect of siRNA against midkine and
paclitaxel on growth of human prostate cancer xenografts. Cancer.
107:864–873. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sakamoto K and Kadomatsu K: Midkine in the
pathology of cancer, neural disease, and inflammation. Pathol Int.
62:445–455. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suganthi M, Sangeetha G, Gayathri G and
Ravi Sankar B: Biphasic dose-dependent effect of lithium chloride
on survival of human hormone-dependent breast cancer cells (MCF-7).
Biol Trace Elem Res. 150:477–486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng Z, Ji Z, Mei F, Lu M, Ou Y and Cheng
X: Lithium inhibits tumorigenic potential of PDA cells through
targeting hedgehog-GLI signaling pathway. PLoS One. 8:e614572013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sabancι PA, Ergüven M, Yazιhan N, Aktaş E,
Aras Y, Civelek E, Aydoseli A, Imer M, Gürtekin M and Bilir A:
Sorafenib and lithium chloride combination treatment shows
promising synergistic effects in human glioblastoma multiforme
cells in vitro but midkine is not implicated. Neurol Res.
36:189–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Welshons WV, Engler KS, Taylor JA, Grady
LH and Curran EM: Lithium-stimulated proliferation and alteration
of phosphoinositide metabolites in MCF-7 human breast cancer cells.
J Cell Physiol. 165:134–144. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mann L, Heldman E, Shaltiel G, Belmaker RH
and Agam G: Lithium preferentially inhibits adenylyl cyclase V and
VII isoforms. Int J Neuropsychopharmacol. 11:533–539. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Santos LJ, Garcia JB, Pacheco JS, Vieira
EB and Santos AM: Quality of life, pain, anxiety and depression in
patients surgically treated with cancer of rectum. Arq Bras Cir
Dig. 27:96–100. 2014.(In English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Castillo V, Valenzuela R, Huidobro C,
Contreras HR and Castellon EA: Functional characteristics of cancer
stem cells and their role in drug resistance of prostate cancer.
Int J Oncol. 45:985–994. 2014.PubMed/NCBI
|
|
22
|
Meacham CE and Morrison SJ: Tumour
heterogeneity and cancer cell plasticity. Nature. 501:328–337.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oktem G, Bilir A, Uslu R, Inan SV, Demiray
SB, Atmaca H, Ayla S, Sercan O and Uysal A: Expression profiling of
stem cell signaling alters with spheroid formation in
CD133high/CD44high prostate cancer stem cells. Oncol Lett.
7:2103–2109. 2014.PubMed/NCBI
|
|
24
|
Bilir A, Erguven M, Ermis E, Sencan M and
Yazihan N: Combination of imatinib mesylate with lithium chloride
and medroxyprogesterone acetate is highly active in Ishikawa
endometrial carcinoma in vitro. J Gynecol Oncol. 22:225–232. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hossein G, Zavareh VA and Fard PS:
Combined treatment of androgen-independent prostate cancer cell
line DU145 with chemotherapeutic agents and lithium chloride:
Effect on growth arrest and/or apoptosis. Avicenna J Med
Biotechnol. 4:75–87. 2012.PubMed/NCBI
|
|
26
|
AzimianZavareh V, Hossein G and Janzamin
E: Effect of lithium chloride and antineoplastic drugs on survival
and cell cycle of androgen-dependent prostate cancer LNCap cells.
Indian J Pharmacol. 44:714–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang DG: Understanding cancer stem cell
heterogeneity and plasticity. Cell Res. 22:457–472. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gupta PB, Fillmore CM, Jiang G, Shapira
SD, Tao K, Kuperwasser C and Lander ES: Stochastic state
transitions give rise to phenotypic equilibrium in populations of
cancer cells. Cell. 146:633–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cojoc M, Mäbert K, Muders MH and Dubrovska
A: A role for cancer stem cells in therapy resistance: Cellular and
molecular mechanisms. Semin Cancer Biol. 31:16–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takebe N and Ivy SP: Controversies in
cancer stem cells: Targeting embryonic signaling pathways. Clin
Cancer Res. 16:3106–3112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang XK, He JH, Xu JH, Ye S, Wang F, Zhang
H, Huang ZC, To KK and Fu LW: Afatinib enhances the efficacy of
conventional chemotherapeutic agents by eradicating cancer
stem-like cells. Cancer Res. 74:4431–4445. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carrasco E, Alvarez PJ, Prados J, Melguizo
C, Rama AR, Aránega A and Rodríguez-Serrano F: Cancer stem cells
and their implication in breast cancer. Eur J Clin Invest.
44:678–687. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kahn M: Can we safely target the WNT
pathway? Nat Rev Drug Discov. 13:513–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Silva AK, Yi H, Hayes SH, Seigel GM and
Hackam AS: Lithium chloride regulates the proliferation of
stem-like cells in retinoblastoma cell lines: A potential role for
the canonical Wnt signaling pathway. Mol Vis. 16:36–45.
2010.PubMed/NCBI
|
|
37
|
Cai C and Zhu X: The Wnt/β-catenin pathway
regulates self-renewal of cancer stem-like cells in human gastric
cancer. Mol Med Rep. 5:1191–1196. 2012.PubMed/NCBI
|
|
38
|
Teng Y, Wang X, Wang Y and Ma D:
Wnt/beta-catenin signaling regulates cancer stem cells in lung
cancer A549 cells. Biochem Biophys Res Commun. 392:373–379. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun JL, Chen DL, Hu ZQ, Xu YZ, Fang HS,
Wang XY, Kan L and Wang SY: Arsenite promotes intestinal tumor cell
proliferation and invasion by stimulating epithelial-to-mesenchymal
transition. Cancer Biol Ther. 15:1312–1319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sottoriva A, Spiteri I, Piccirillo SG,
Touloumis A, Collins VP, Marioni JC, Curtis C, Watts C and Tavaré
S: Intratumor heterogeneity in human glioblastoma reflects cancer
evolutionary dynamics. Proc Natl Acad Sci USA. 110:4009–4014. 2013.
View Article : Google Scholar : PubMed/NCBI
|