Introduction
Breast cancer is the most common cancer among women
worldwide (1,2). Although genetic, hormonal, lifestyle,
and environmental risk factors have been established, the etiology
and carcinogenesis of breast cancer warrant a more detailed
understanding. The most common treatment for breast cancer includes
breast-conserving surgery followed by a standard radiotherapy
regimen (3). Radiotherapy, the use of
high-energy ionizing radiation to eradicate tumor cells, is the
mainstay of anticancer treatment. Radiotherapy is largely used for
the local control of tumor progression and the preservation of
organs; therefore, it is the major treatment course in the
management of breast cancer. Radiation therapy can reduce the risk
of locoregional recurrences following surgery by ~70%, and has been
shown to improve overall survival for early-stage breast cancer,
after breast-conserving surgery and for locally advanced disease
following mastectomy (4–6). However, radiation hypersensitivity and
the occurrence of radiotherapy-induced toxicity in normal tissue
may affect treatment (7,8). Healthy cells located in the region that
neighbors the tumor inevitably receive a considerable dose of
radiation.
Radiation damage to biological systems is determined
by the type of radiation, total dosage of exposure, dose rate and
region of the body exposed (9). Three
modes of cell death, necrosis, apoptosis, and autophagy, as well as
accelerated senescence, have been demonstrated to occur in
vitro and in vivo in response to radiation in cancerous
and normal cells (10,11). Radiation damage may appear immediately
after radiation therapy or can be delayed in surviving patients.
The number of cancer survivors is steadily rising; thus, assessing
how early- and late-radiation toxicity in normal tissues affects
the quality of life for these patients is becoming increasingly
important (12). The dose of
radiation administered is restricted to avoid excess damage to
healthy tissue, and this has limited its clinical application as
part of modern treatment (13).
Cell death by apoptosis proceeds via 2 major
pathways, the extrinsic (death receptor) and intrinsic
(mitochondrial) pathways. The extrinsic and intrinsic pathways are
initiated by specific ligands or various intracellular stimuli that
activate effector caspases, ultimately fragmenting DNA and inducing
cell death (14). Each pathway is
involved in radiation-induced apoptosis (15). Inhibitor of apoptosis proteins (IAPs)
are a family of endogenous anti-apoptotic proteins characterized by
one or several baculoviral IAP repeat (BIR) domains. Certain IAPs
contain a RING finger domain at the C-terminus, exhibiting E3
ubiquitin ligase activity, which is required for the ubiquitination
and proteasomal degradation of various substrates (16). Cellular inhibitor of apoptosis (cIAP)1
and X-linked inhibitor of apoptosis protein (XIAP)2 tend to be
involved in nuclear factor-κB (NF-κB) activity regulation and the
suppression of caspase-8 activation (17). Aberrant IAP expression occurs in
combination with cancer, particularly in hypoxic microenvironments
(18). Previous studies on IAP
amplification and overexpression demonstrated that IAPs, including
cIAP1 and XIAP, may be novel predictive markers for radiotherapy
resistance in individual cervical squamous cell carcinoma patients.
Therefore, IAPs serve a critical function in interfering with the
efficacy of radiotherapy and chemotherapy (19,20).
In contrast to apoptosis, the role of autophagy is
paradoxical, as it can either induce autophagic cell death or
provide a survival vehicle for the tumor against nutrition- and
energy-deficient environments, as a result of chemotherapy,
endocrine drugs and irradiation (21). During the process of radiotherapy and
chemotherapy, multiple changes occur in the growth arrest and death
pathways (22). Traditionally,
irradiation is considered to induce cell death mainly via
apoptosis. However, more recent studies have suggested that
autophagy is also important in irradiation-induced cell death,
which may aid to restore and improve radiosensitivity (23). For homeostasis, intracellular
organelles and long-lived proteins are degraded by autophagy, a
conserved cellular process (24).
However, autophagy plays paradoxical roles in the initiation,
development and metastasis of cancer. While autophagy exhibits an
antitumor role, it also protects tumor cells against stress
(25). Once autophagy is inhibited,
the therapeutic effect is considered enhanced (26). XIAP and cIAP1 overexpression induces
Beclin-1-dependent autophagy through NF-κB activation, suggesting
that NF-κB signaling is important for radiation-induced cell
apoptosis and autophagy (27).
The present study used a novel recombinant protein,
namely flagellin A (FlaA) N/C, which was developed in an earlier
study and is derived from the flagellin protein of Legionella
pneumophila (28). FlaA N/C has
been shown to increase the expression of several cytoprotective
cytokines, activate the NF-κB signaling pathway and significantly
increase the survival of mice following total body irradiation,
compared with flagellin or amifostine (28). However, in order to assess the
effectiveness of FlaA N/C as a tumor radiation protection agent,
determining whether FlaA N/C has a sensitizing effect on tumor
radiation or has a direct tumoricidal effect is crucial.
The results of the present study found that
pretreatment with FlaA N/C prior to radiation administration
promoted apoptosis, autophagy and tumor radiosensitivity and
regulated the cell cycle in mouse breast cancer 4T1 cells in
vitro and in vivo. Furthermore, the results revealed
that FlaA N/C regulated radiosensitivity by NF-κB signaling via
toll like receptor 5 (TLR5). Treatment with FlaA N/C clearly
prolonged the survival of mice after total body irradiation. Thus,
FlaA N/C may be a useful radiation sensitizer in breast tumor
radiation therapy.
Materials and methods
Cells
Cells of the murine breast cancer 4T1 cell line
(catalog no., CRL-2539; American Type Culture Collection, Manassas,
VA, USA) were cultured in RPMI-1640 medium supplemented with 10%
fetal bovine serum (FBS) and penicillin-streptomycin at 37°C and in
5% CO2. All media and supplements were Invitrogen brand,
purchased from Thermo Fisher Scientific, Inc. (Waltham, MA,
USA).
Mice
Six-week-old female BALB/c mice (n=40) were
purchased from Maccura Biotechnology Co., Ltd. (Chengdu, China).
The animals were housed in groups of 5 or 6 in plastic mouse cages
in a laminar flow housing cabinet, with an automatically controlled
temperature of 22°C and 12 h of light. Specific pathogen free feed
for mice was purchased from Chengdu Dossy Biological Technology
Co., Ltd. (Chengdu, China) and the ddWater for mice was high
pressure treated.
The research was conducted in accordance with the
Declaration of Helsinki and the Guide for Care and Use of
Laboratory Animals as adopted and promulgated by the National
Institutes of Health (Bethesda, MD, USA). All experimental
protocols were approved by the Review Committee for the Use of
Human or Animal Subjects of Chengdu Medical College (Chengdu,
China).
Tumor model and radiation
Mice received a subcutaneous orthotopic injection of
5×105 4T1 tumor cells into the third thoracic mammary
fat pad. After 7 days, purified FlaA N/C was subcutaneously
injected into the BALB/c mice (10 mice per group), and recombinant
protein tags (Beijing Protein Innovation Co., Ltd, Beijing, China)
were used as a control. At 2 h subsequent to administration, the
mice received 10-Gray (Gy) irradiation. Another 5 days later, 2
mice per group were anaesthetized, mices were sacrificed by
cervical dislocation, and tumor tissue was preserved in liquid
nitrogen for further analysis. The tumor volumes and body weights
of the mice were measured every 2 days, and the mortality of mice
was checked every day.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
proliferation assay
4T1 cells were irradiated with 10 Gy γ-rays
following treatment with FlaA N/C for 2 h. Cells were cultured for
2 days (37°C; 5% CO2). MTT reagent was added for 4 h and
was then reduced using mitochondrial reductase (Beyotime Institute
of Biotechnology, Haimen, China). The resulting purple crystals
were dissolved in solubilization buffer and spectrophotometrically
analyzed at 570 nm using a reference of 656 nm in a microplate
reader (SpectraMax M5e; Molecular Devices, LLC, Sunnyvale, CA,
USA). The inhibition index of proliferation was calculated as: 1 -
absorbance of sample/absorbance of sample blank.
Clonogenic assay
Cells were seeded into 6-well plates and allowed to
attach overnight. The cells were then treated with FlaA N/C for 2
h, irradiated with 10 Gy γ-rays, and cultured for 2 weeks. Plates
were then washed with saline, fixed with 100% methanol, stained
with crystal violet, washed with water, and air-dried. Colonies
containing >50 cells were counted.
Western blot analysis
All protein samples for western blot analysis were
resolved by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis on 12% gels and then transferred to nitrocellulose
membranes, which were then blocked for 1 h at room temperature in
Tris-buffered saline containing 0.1% Tween 20 and 5% fat-free milk.
All primary antibody [either mouse monoclonal; dilution, 1:2,000;
catalog no.: β-actin, 60008-1-Ig; or rabbit polyclonal; dilution,
1:2,000; catalog nos.: γ H2A histone family member X (γH2AX),
10856-1-AP; DNA-dependent protein kinases (DNA-PKs), 22129-1-AP; B
cell lymphoma-2 (Bcl-2), 12789-1-AP; Bcl-2-associated X (Bax),
50599-2-Ig; BH3 interacting domain death agonist (Bid), 10988-1-AP;
light chain 3 (LC3), 12135-1-AP; Beclin-1, 11306-1-AP; NF-κB,
14220-1-AP; RELA proto-oncogene, NF-kB subunit (P65), 10745-1-AP;
cyclin dependent kinase inhibitor 1A (P21), 10355-1-AP; and TLR5,
19810-1-AP; ProteinTech Group, Inc., Chicago, IL, USA] incubations
were performed for 18 h at 4°C. Secondary antibody [either, for
β-actin: horseradish peroxidase (HRP)-conjugated goat anti-mouse
immunoglobulin (Ig)G, heavy and light chain (H+L); dilution,
1:5,000; catalog no., SA00001-1; or, for all other primary
antibodies: HRP-conjugated goat anti-rabbit, IgG (H+L); dilution,
1:5,000; catalog no, SA00001-2; ProteinTech Group, Inc.]
incubations were carried out at room temperature for 1 h. Secondary
antibodies conjugated with HRP were used for detection by enhanced
chemiluminescence (SuperSignal; Pierce, Rockford, IL, USA; or ECL
Plus; GE Healthcare Life Sciences, Chalfont, UK) according to the
manufacturers' instructions.
Immunofluorescence
Tissues were dewaxed and antigens retrieved using
high pressure (0.12 MPa) for 3 min and then blocked with
phosphate-buffered saline (PBS) containing 10% normal goat serum
(Beyotime Institute of Biotechnology). Sections were stained with
primary antibodies (rabbit polyclonal; dilution, 1:200; catalog
nos.: γH2AX, 10856-1-AP; DNA-PKs, 22129-1-AP; LC3, 12135-1-AP; and
TLR5, 19810-1-AP; ProteinTech Group, Inc.) for 30 min at 37°C, and
then stained with Cy3-conjugated Affinipure goat anti-rabbit IgG
(H+L) secondary antibody (dilution, 1:500; catalog no., SA00009-2;
ProteinTech Group, Inc.) for 30 min at 37°C. The samples were then
re-stained with 4′,6-diamidino-2-phenylindole for 10 min at 37°C.
All immunofluorescence images were obtained using an Olympus BX51
microscope equipped with either a ×20 or ×40 objective lens and a
DP50 camera (Olympus Corporation, Tokyo, Japan). Images were
processed using Digital Production Control software (BX2 software;
Olympus Corporation).
Cell apoptosis assay
Apoptotic cells were quantified using the Annexin
V/propidium iodide (PI) double staining method and analysis of
phosphatidylserine on the outer surface of apoptotic cell membranes
(29). The treated 4T1 cells received
10-Gy irradiation, were harvested after 72 h, and washed twice with
PBS. Subsequently, 105 cells were resuspended in 100 µl
1X binding buffer and stained with 5 µl fluorescein isothiocyanate
(FITC)-conjugated Annexin V and 10 µl PI simultaneously. The cells
were then incubated for 15 min in the dark and flow cytometry
analysis (BD FACS Calibur flow cytometer with BD FACSComp™
software; BD Biosciences, Franklin Lakes, NJ, USA) was
performed.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
The TUNEL assay was performed using an Apoptosis
Detection kit (Roche Diagnostics, Basel, Switzerland), according to
the manufacturer's instructions. Apoptotic cells contained
brown-yellow granules in the cytoplasm. The ratio of brown-yellow
granules-prositive cells to normal cells in the microscopic field
was used to determine the degree of apoptosis.
Cell cycle assay
The 4T1 cells were cultured for 24 h prior to FlaA
N/C treatment for 2 h. The cells were exposed to 10-Gy irradiation
and were cultured for another 72 h. After the cells were harvested,
they were washed twice with PBS, treated with 50 µg/ml PI
containing 20 µg/ml RNase, incubated at room temperature for 2 h,
and analyzed by flow cytometry, using the aforementioned
method.
Statistical analysis
Each experiment was performed at least 3 times
independently. The effects of various treatments were compared by
one-way analysis of variance and a two-tailed t-test using
GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA,
USA). Differences with a value of P<0.05 were considered to be
statistically significant. The experimental results shown are
presented as the means of multiple individual points from multiple
separate experiments ± standard error of the mean.
Results
FlaA N/C increased radiosensitivity in
4T1 cells
A previous study demonstrated that, through the
activation of NF-κB signaling, FlaA N/C regulates the expression of
various cytokines, suppresses the inflammatory response and
regulates multiple cellular functions important for
radiation-induced intestinal injury (28). In addition, the effect of FlaA N/C on
the radiosensitivity of tumor cells is currently unclear. In the
present study, pre-treatment with FlaA N/C prior to radiation
inhibited the proliferation of 4T1 cells (Fig. 1A). A clonogenic assay also showed that
FlaA N/C inhibited the survival of 4T1 cells (Fig. 1B-C). The present study detected the
expression of associated biomarkers of radiosensitivity and found
that FlaA N/C clearly increased γH2AX and DNA-PKs in 4T1 cells
(Fig. 1D).
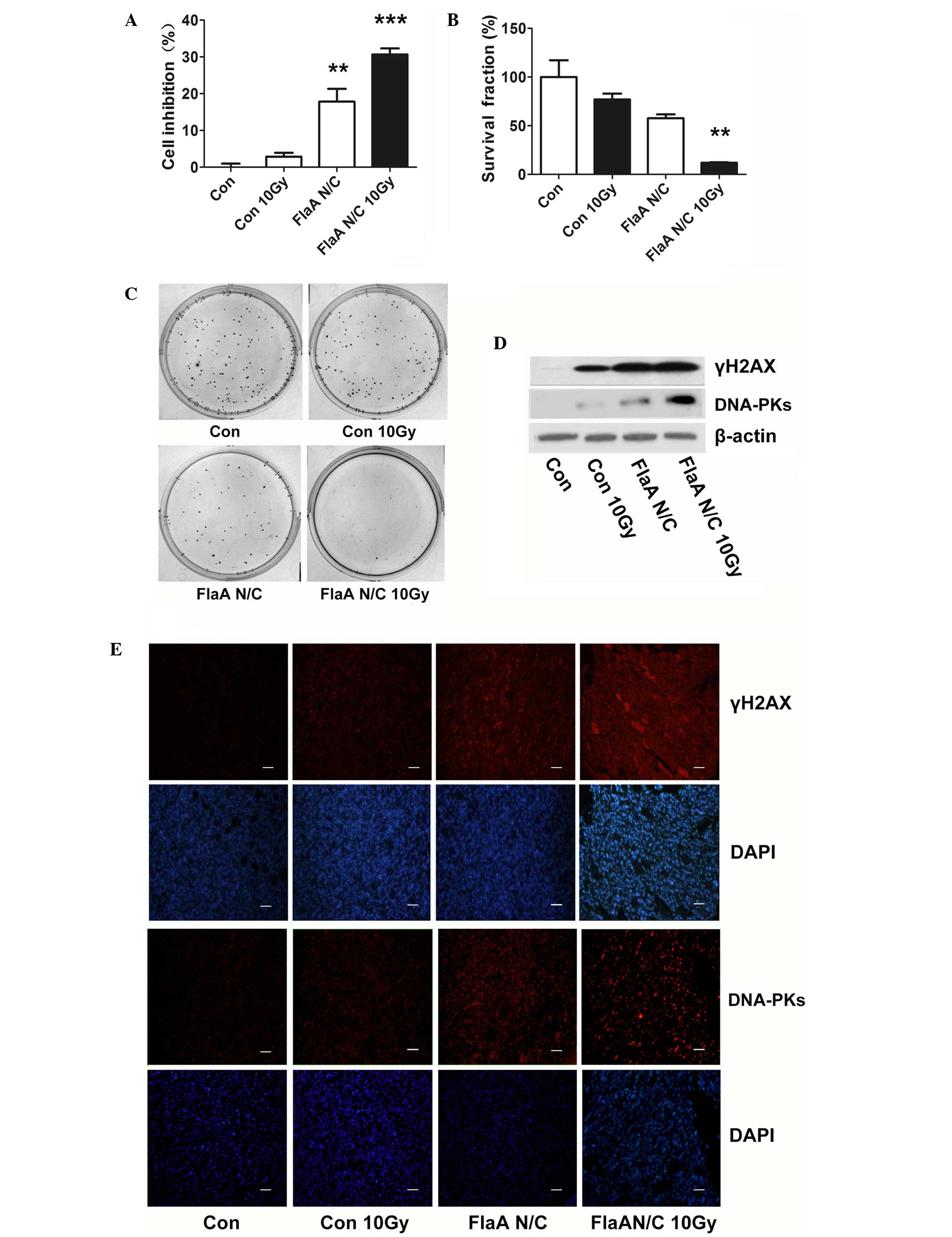 | Figure 1.FlaA N/C increased radiosensitivity
in 4T1 cells. (A)
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
showed that pre-treatment with FlaA N/C prior to radiation
inhibited the proliferation of 4T1 cells; **P<0.01,
***P<0.001. (B) Bar graph of the clonogenic assay showed that
pre-treatment with FlaA N/C prior to radiation inhibited cell
survival of 4T1 cells; **P<0.01. (C) Clonogenic assay showed
that pre-treatment with FlaA N/C prior to radiation inhibited cell
survival of 4T1 cells. (D) Western blot analysis showed that FlaA
N/C increased γH2AX and DNA-PK expression. (E) Immunofluorescence
staining showed that FlaA N/C increased γH2AX and DNA-PK expression
in vivo. Scale bars represent 50 µm. Con, control; Gy, Gray;
FlaA, flagellin; γH2AX, γ H2A histone family member X; DAPI,
4′,6-diamidino-2-phenylindole; DNA-PKs, DNA-dependent protein
kinases. |
FlaA N/C treatment prior to total body irradiation
was studied in vivo. As shown in Fig. 1E, treatment with FlaA N/C increased
the expression of γH2AX and DNA-PKs, suggesting that FlaA N/C has a
marked capacity to increase tumor radiosensitivity.
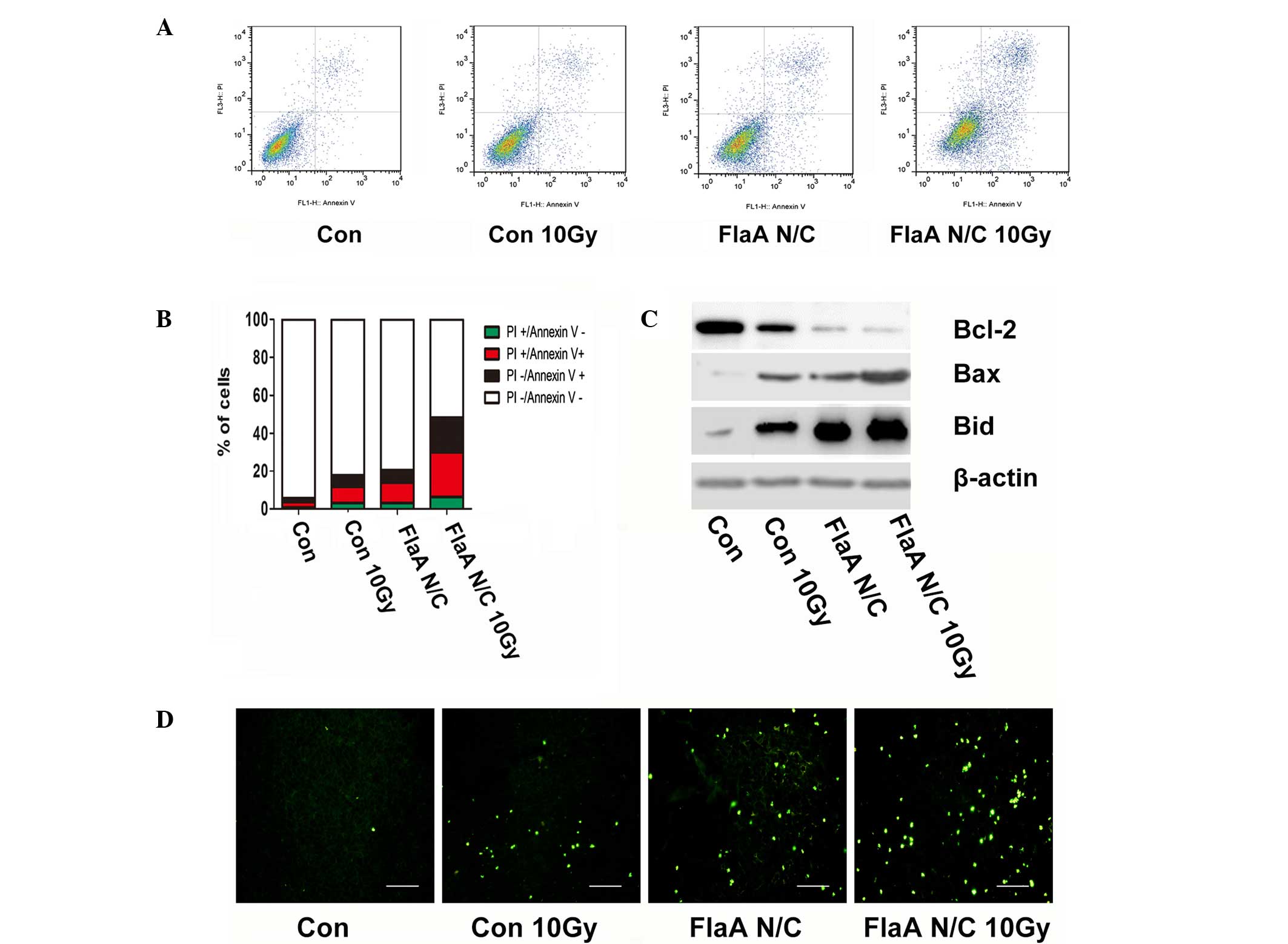
FlaA N/C promoted apoptosis in 4T1
cells
A large number of studies have shown that apoptosis
is important in tumor radiotherapy (30,31).
Therefore, the present study further tested whether FlaA N/C
affects cell apoptosis after radiation. Treatment with FlaA N/C
prior to radiation was found to increase apoptosis in 4T1 cells
(Fig. 2A-B). When the expressions of
Bcl-2, Bax and Bid were analyzed in 4T1 cells, FlaA N/C was found
to increase Bax and Bid expression, while inhibited Bcl-2
expression (Fig. 2C).
In vivo experiments using the TUNEL assay in
a 4T1-bearing animal model also proved that treatment with FlaA N/C
increased tumor apoptosis. Compared with the control group, the
tumor-bearing mice receiving FlaA N/C treatment prior to
irradiation demonstrated significantly increased apoptosis in tumor
cells (Fig. 2D).
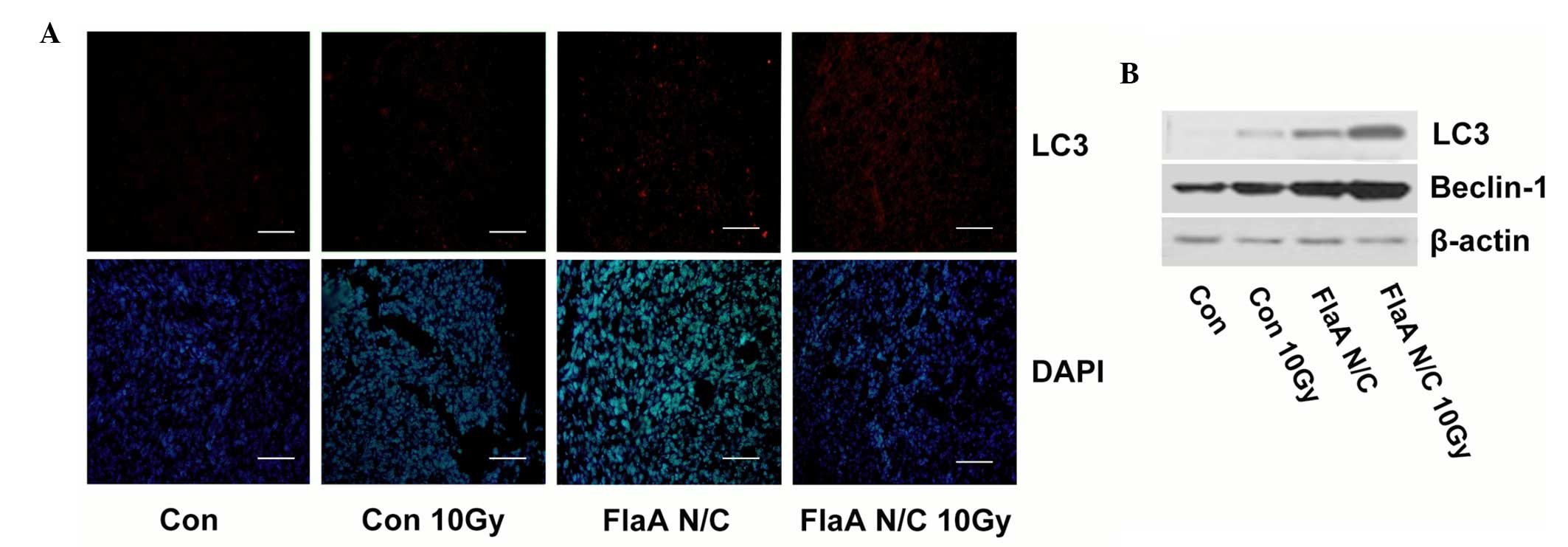
FlaA N/C promoted autophagy in 4T1
cells
Cell death as a result of autophagy is important in
radiation therapy for tumors. Therefore, the function of autophagy
after treatment with FlaA N/C was analyzed. Following irradiation,
FlaA N/C was found to increase the expression of LC3 and Beclin-1
in 4T1 cells (Fig. 3A). Furthermore,
the present study investigated tumor autophagy in tumor-bearing
mice. As shown in Fig. 3B, FlaA N/C
wa found to increase LC3 expression in tumors, which indicated that
FlaA N/C could promote cell death in the process of irradiation
through cell autophagy.
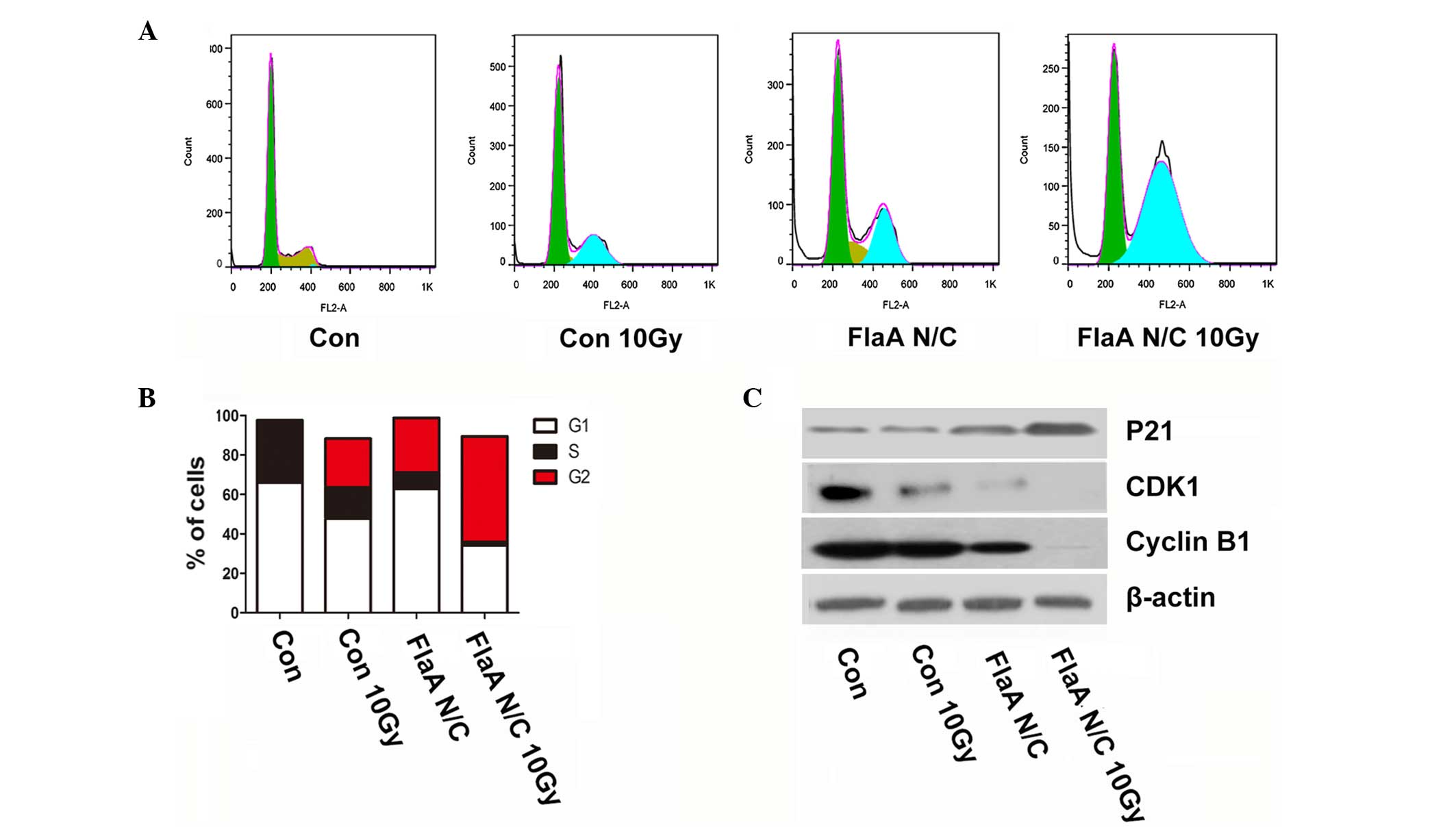
FlaA N/C regulated the cell cycle in
4T1 cells
Changes in the cell cycle, particularly during the
G2/M phase arrest, are critical in tumor radiation sensitization.
The present study found that treatment with FlaA N/C promoted G2/M
phase arrest (Fig. 4A-B). With
regards to cell cycle biomarkers, FlaA N/C clearly increased the
expression of P21 and inhibited the expression of cyclin dependent
kinase 1 (CDK1) and Cyclin B1 (Fig.
4C), which further showed that treatment with FlaA N/C prior to
radiation promotes cell radiosensitivity.
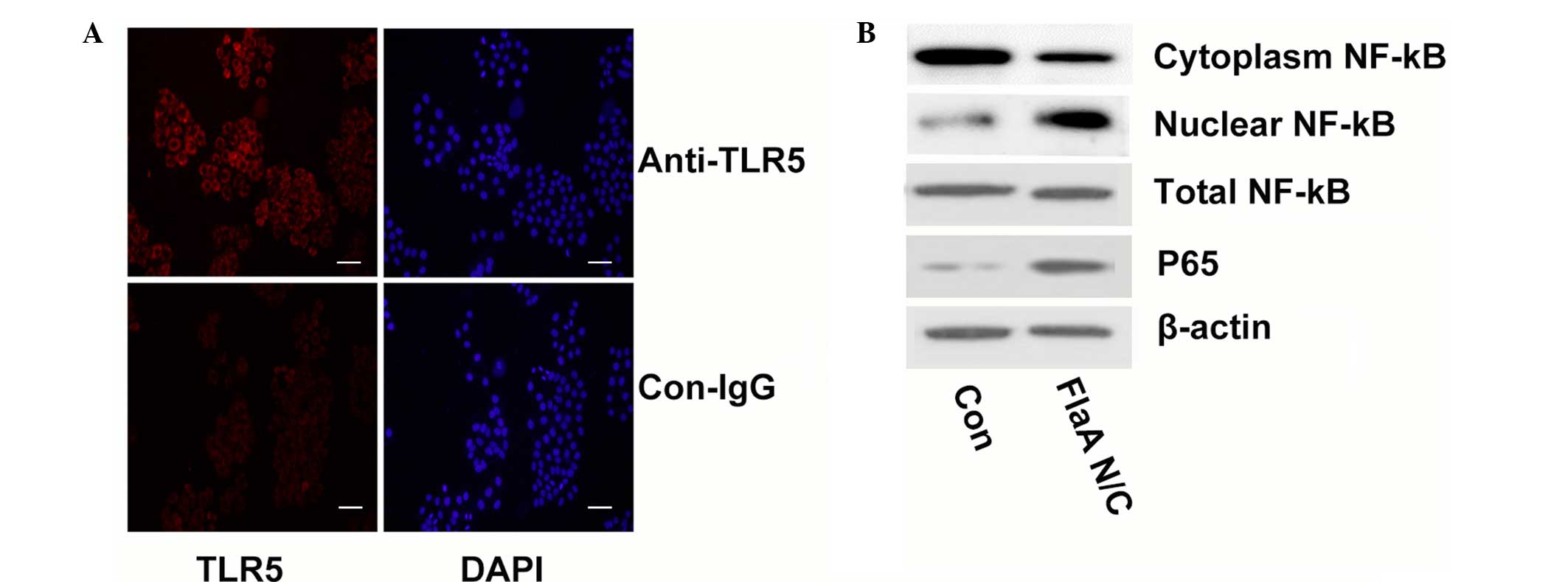
FlaA N/C increased radiosensitivity
though NF-κB signaling
Flagellin activates NF-κB signaling through
interaction with TLR5 (32);
therefore, TLR5 expression was first tested in 4T1 cells. As shown
in Fig. 5A, 4T1 cells were positive
for TLR5 expression. When the present study assessed the activation
of NF-κB signaling following treatment with FlaA N/C, P65
expression was found to be upregulated. In addition, the
subcellular localization of NF-κB was assessed by western blot
analyses of the cytosolic and nuclear extracts. The analyses found
that FlaA N/C clearly increased NF-κB levels in the nuclear
fraction while decreasing NK-κB levels in the cytosolic fraction,
and the total NF-κB expression remained unchanged (Fig. 5B).
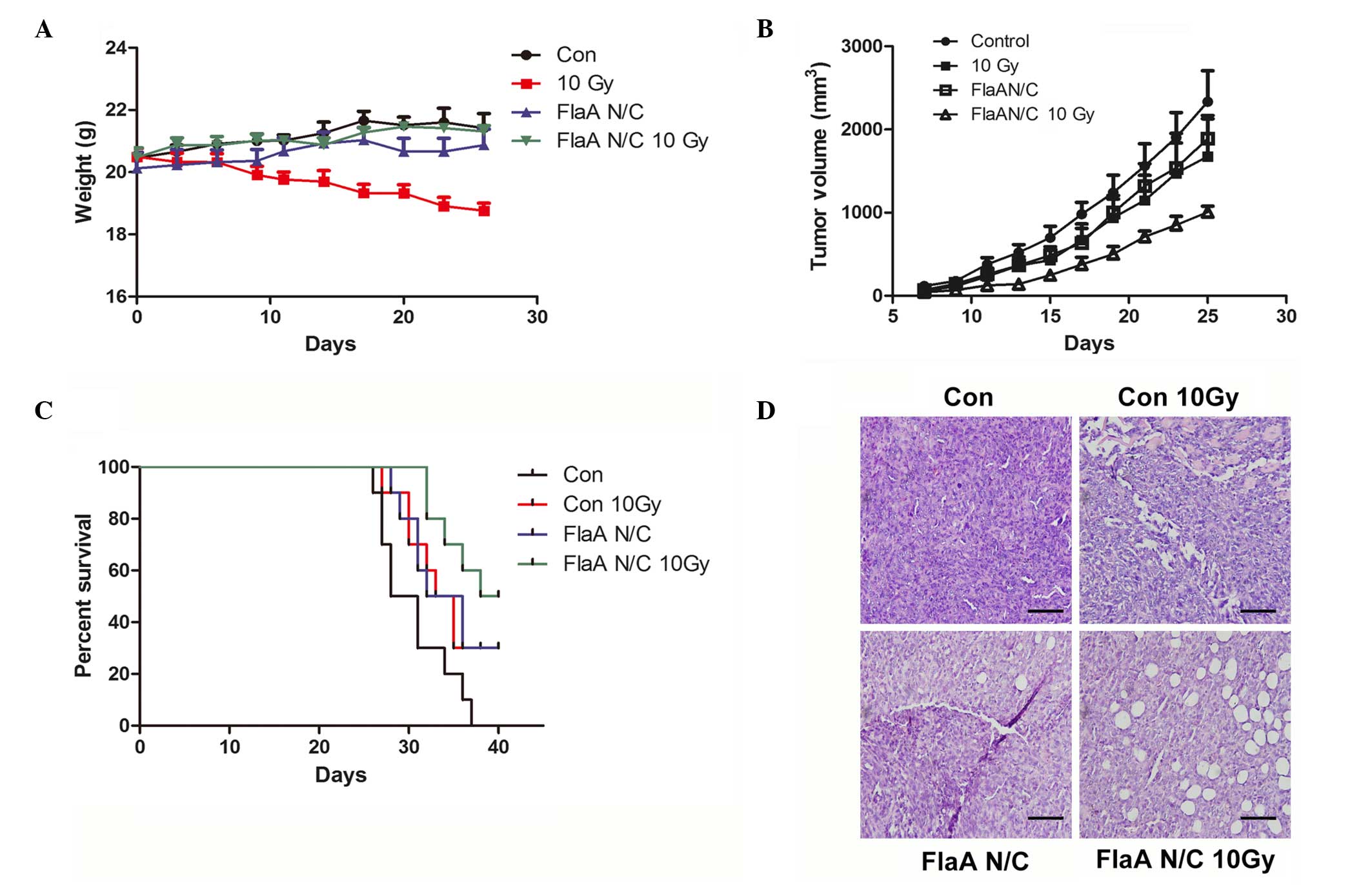
FlaA N/C increases animal
survival
In the in vivo studies of FlaA N/C function,
irradiation reduced the weight of mice, while the weight of mice in
the FlaA N/C group remained unchanged (Fig. 6A). Treatment with FlaA N/C inhibited
the 4T1 tumor growth prior to irradiation and further increased the
overall survival of mice that received 10 Gy irradiation (Fig. 6B-C). As shown in Fig. 6D, hematoxylin-eosin staining further
proved that FlaA N/C inhibited tumor growth, and is another
indicator that FlaA N/C has a notable effect on tumor radiation
sensitization.
Discussion
Radiotherapy is a cornerstone of breast cancer
treatment (33). Radiotherapy greatly
reduces the risk of ductal carcinoma recurrence in situ and
in lymph node-positive breast cancer (34). Even though radiotherapy shows promise
in the treatment of all breast cancer subtypes, radiotherapy is
associated with an increased risk of cardiovascular disease
(35). In addition, breast tumors can
acquire radioresistance (36).
Finding agents that sensitize malignant cells to radiation may
increase tumor response while minimizing toxicity to surrounding
organs by lowering effective therapeutic doses.
Flagellin has been reported to increase the
radiosensitization of cancer cells in several tumor types. Total
flagellin activates the inflammatory reaction of normal tissues
(37). In an earlier study, a
recombinant protein, FlaA N/C, was constructed to reduce the side
effects of flagellin. The study showed that FlaA N/C can reduce
intestinal inflammation in total body irradiation (28). Thus far, whether FlaA N/C has an
inhibitory effect on tumors that receive radiation therapy has
remained unknown. The present study tested the irradiation effect
of FlaA N/C in 4T1 cells in vitro and in vivo.
Treatment with FlaA N/C was found to inhibit cell proliferation in
4T1 cells. The staining of radiosensitivity biomarkers also proved
that FlaA N/C increased the radiosensitivity of 4T1 cells in
vivo.
Apoptosis is crucial for cell death following
radiotherapy, and autophagy is termed the ‘second apoptosis’
(38). The IAP family proteins, XIAP
and cIAP1, induce autophagy by upregulating the transcription of
Beclin-1, an essential autophagy gene. The E3 ubiquitin ligase
activity of the two proteins activates NF-κB signaling, which
results in the direct binding of P65 to the promoter of Beclin-1
and to its transcriptional activation (26). In cancer therapy, the role of
autophagy is paradoxical; this cellular process may serve as a
pro-survival or pro-death mechanism to counteract or mediate the
cytotoxic effect of anticancer agents (39). The present study found that treatment
with FlaA N/C prior to cell radiation increased cell apoptosis in
4T1 cells. In vivo experiments also proved that treatment
with FlaA N/C 2 h after total body irradiation clearly increased
tumor apoptosis. FlaA N/C increased cell autophagy in 4T1 cells and
in vivo experiments.
Shortening of the G2 checkpoint leads to decreased
repair of radiation-induced damage, which can occur prior to cell
division (39). For the
radiosensitization of cancer cells, pharmacological agents that can
inhibit specific checkpoint components, particularly the G2/M
transition, have received attention recently. The checkpoint kinase
(Chk) inhibitor, AZD7762, has demonstrated synergy with ionizing
radiation to inhibit cancer growth by abrogating the G2/M
checkpoint through selective inhibition of Chk1 (40). The present study found that FlaA N/C
regulates the cell cycle and increases the G2/M phase arrest in 4T1
cells.
NF-κB can activate a great number of genes involved
in stress responses, inflammation, apoptosis and autophagy. NF-κB
subunit 1 (P50) homodimers, or P50/P65 or P50/c-Rel heterodimers,
bind to the NF-κB DNA-binding sites in the promoter regions of
numerous stress-response genes, suggesting a complex regulation
network at gene and physiological levels, controlled by NF-κB in
stress response (41). Accumulated
evidence indicates that the transcription factor NF-κB is critical
for cellular protection against a variety of genotoxic agents,
including irradiation, and that the inhibition of NF-κB may result
in radiosensitization in radioresistant cancer cells (42). In a previous study, human breast
cancer cells treated with fractional γ-irradiation displayed
enhanced NF-κB activation (43).
In conclusion, the present study inidcates that,
through activating NF-κB signaling, FlaA N/C regulates the function
of apoptosis and autophagy and regulates cell cycle of radiation
sensitization. The radiosensitization effects of FlaA N/C appear
promising and indicate its clinical potential for use as a novel
radiation sensitizer in tumor radiation therapy.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant no. 81001345), the Foundation of
Sichuan Province Science and Technology Agency (grant no.
2014JY0039), the Foundation of Sichuan Province Education Office
(grant no. 15ZA0248) and the Founding of Sichuan Province Academic
and Technology Leaders in 2014.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang R and Li JC: TRAIL suppresses human
breast cancer cell migration via MADD/CXCR7. Asian Pac J Cancer
Prev. 16:2751–2756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Esposito E, Anninga B, Harris S, Capasso
I, D'Aiuto M, Rinaldo M and Douek M: Intraoperative radiotherapy in
early breast cancer. Br J Surg. 102:599–610. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clarke M, Collins R, Darby S, Elphinstone
P, Evans V, Godwin J, Gray R, Hicks C, James S, MacKinnon E, et al:
Effects of radiotherapy and of differences in the extent of surgery
for early breast cancer on local recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 366:2087–2106. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paszat LF, Vallis KA, Benk VM, Groome PA,
Mackillop WJ and Wielgosz A: A population-based case-cohort study
of the risk of myocardial infarction following radiation therapy
for breast cancer. Radiother Oncol. 82:294–300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kongsiang A, Tangvoraphonkchai V,
Jirapornkul C, Promthet S, Kamsa-Ard S and Suwanrungruang K:
Survival time and molecular subtypes of breast cancer after
radiotherapy in Thailand. Asian Pac J Cancer Prev. 15:10505–10508.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanaka H, Hayashi S and Hoshi H: Cardiac
counterclockwise rotation is a risk factor for high-dose
irradiation to the left anterior descending coronary artery in
patients with left-sided breast cancer who receiving adjuvant
radiotherapy after breast-conserving surgery. Nagoya J Med Sci.
76:265–272. 2014.PubMed/NCBI
|
|
8
|
Hanna GG and Kirby AM: Intraoperative
radiotherapy in early stage breast cancer: Potential indications
and evidence to date. Br J Radiol. 88:201406862015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marinko T, Dolenc J and Bilban-Jakopin C:
Cardiotoxicity of concomitant radiotherapy and trastuzumab for
early breast cancer. Radiol Oncol. 48:105–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Ming W, Wang D, Li X, Wang W, Lou H
and Yuan H: Malformin A1 promotes cell death through induction of
apoptosis, necrosis and autophagy in prostate cancer cells. Cancer
Chemother Pharmacol. 77:1–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nikoletopoulou V, Markaki M, Palikaras K
and Tavernarakis N: Crosstalk between apoptosis, necrosis and
autophagy. Biochim Biophys Acta. 1833:3448–3459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stone HB, Coleman CN, Anscher MS and
McBride WH: Effects of radiation on normal tissue: Consequences and
mechanisms. Lancet Oncol. 4:529–536. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu TQ, Wang GB, Li ZJ, Tong XD and Liu
HX: Silencing of rac3 inhibits proliferation and induces apoptosis
of human lung cancer cells. Asian Pac J Cancer Prev. 16:3061–3065.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim MY, Park SJ, Shim JW, Yang K, Kang HS
and Heo K: Naphthazarin enhances ionizing radiation-induced cell
cycle arrest and apoptosis in human breast cancer cells. Int J
Oncol. 46:1659–1666. 2015.PubMed/NCBI
|
|
15
|
Seo TW, Lee JS and Yoo SJ: Cellular
inhibitor of apoptosis protein 1 ubiquitinates endonuclease G but
does not affect endonuclease G-mediated cell death. Biochem Biophys
Res Commun. 451:644–649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao WJ, Deng BY, Wang XM, Miao Y and Wang
JN: XIAP associated factor 1 (XAF1) represses expression of
X-linked inhibitor of apoptosis protein (XIAP) and regulates
invasion, cell cycle, apoptosis and cisplatin sensitivity of
ovarian carcinoma cells. Asian Pac J Cancer Prev. 16:2453–2458.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng HT, Fu YC, Yu W, Lin JM, Zhou L, Liu
L and Wang W: SIRT1 prevents atherosclerosis via liver-X-receptor
and NF-κB signaling in a U937 cell model. Mol Med Rep. 8:23–28.
2013.PubMed/NCBI
|
|
18
|
Sekine K, Takubo K, Kikuchi R, Nishimoto
M, Kitagawa M, Abe F, Nishikawa K, Tsuruo T and Naito M: Small
molecules destabilize cIAP1 by activating auto-ubiquitylation. J
Biol Chem. 283:8961–8968. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Owens TW, Gilmore AP, Streuli CH and
Foster FM: Inhibitor of apoptosis proteins: Promising targets for
cancer therapy. J Carcinog Mutagen. Suppl 14:pii:S140042013.
|
|
20
|
Bao L, Jaramillo MC, Zhang Z, Zheng Y, Yao
M, Zhang DD and Yi X: Induction of autophagy contributes to
cisplatin resistance in human ovarian cancer cells. Mol Med Rep.
11:91–98. 2015.PubMed/NCBI
|
|
21
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim KW, Hwang M, Moretti L, Jaboin JJ, Cha
YI and Lu B: Autophagy upregulation by inhibitors of caspase-3 and
mTOR enhances radiotherapy in a mouse model of lung cancer.
Autophagy. 4:659–668. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim R, Emi M, Tanabe K, Uchida Y and
Arihiro K: The role of apoptotic or nonapoptotic cell death in
determining cellular response to anticancer treatment. Eur J Surg
Oncol. 32:269–277. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu K, Dunner K Jr and McConkey DJ:
Proteasome inhibitors activate autophagy as a cytoprotective
response in human prostate cancer cells. Oncogene. 29:451–462.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rao R, Balusu R, Fiskus W, Mudunuru U,
Venkannagari S, Chauhan L, Smith JE, Hembruff SL, Ha K, Atadja P
and Bhalla KN: Combination of pan-histone deacetylase inhibitor and
autophagy inhibitor exerts superior efficacy against
triple-negative human breast cancer cells. Mol Cancer Ther.
11:767–774. 2012. View Article : Google Scholar
|
|
26
|
Lin F, Ghislat G, Luo S, Renna M, Siddiqi
F and Rubinsztein DC: XIAP and cIAP1 amplifications induce Beclin
1-dependent autophagy through NFκB activation. Hum Mol Genet.
24:2899–2913. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peulen HI, Hanbeukers B, Boersma L, van
Baardwijk A, van den Ende P, Houben R, Jager J, Murrer L and Borger
J: Forward intensity-modulated radiotherapy planning in breast
cancer to improve dose homogeneity: Feasibility of class solutions.
Int J Radiat Oncol Biol Phys. 82:394–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ying X, Wu D, Fan Y, Li P, Du H, Shi J,
Wang D and Zhou X: Novel recombinant protein FlaA N/C protects
against radiation injury via NF-κB signaling. Radiat Res.
185:77–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu DM, Zhang P, Xu GC, Tong AP, Zhou C,
Lang JY and Wang CT: Pemetrexed induces G1 phase arrest and
apoptosis through inhibiting Akt activation in human non small lung
cancer cell line A549. Asian Pac J Cancer Prev. 16:1507–1513. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu M, Ma S, Hou Y, Liang B, Su X and Liu
X: Synergistic killing of lung cancer cells by cisplatin and
radiation via autophagy and apoptosis. Oncol Lett. 7:1903–1910.
2014.PubMed/NCBI
|
|
31
|
Yang L, Liu Y, Sun C, Yang X, Yang Z, Ran
J, Zhang Q, Zhang H and Wang X and Wang X: Inhibition of DNA-PKcs
enhances radiosensitivity and increases the levels of ATM and ATR
in NSCLC cells exposed to carbon ion irradiation. Oncol Lett.
10:2856–2864. 2015.PubMed/NCBI
|
|
32
|
Forstnerič V, Ivičakkocjan K, Ljubetič A,
Jerala R and Benčina M: Distinctive recognition of flagellin by
human and mouse toll-like receptor 5. Plos One. 11:e01588942016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Steward LT, Gao F, Taylor MA and
Margenthaler JA: Impact of radiation therapy on survival in
patients with triple-negative breast cancer. Oncol Lett. 7:548–552.
2014.PubMed/NCBI
|
|
34
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG); Correa C, McGale P, Taylor C, Wang Y,
Clarke M, Davies C, Peto R, Bijker N, Solin L and Darby S: Overview
of the randomized trials of radiotherapy in ductal carcinoma in
situ of the breast. J Natl Cancer Inst Monogr. 2010:162–177. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hooning MJ, Botma A, Aleman BM, Baaijens
MH, Bartelink H, Klijn JG, Taylor CW and van Leeuwen FE: Long-term
risk of cardiovascular disease in 10-year survivors of breast
cancer. J Natl Cancer Inst. 99:365–375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jameel JK, Rao VS, Cawkwell L and Drew PJ:
Radioresistance in carcinoma of the breast. Breast. 13:452–460.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Uchida M, Oyanagi E, Kawanishi N, Iemitsu
M, Miyachi M, Kremenik MJ, Onodera S and Yano H: Exhaustive
exercise increases the TNF-α production in response to flagellin
via the upregulation of toll-like receptor 5 in the large intestine
in mice. Immunol Lett. 158:151–158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Velentzas AD, Nezis IP, Stravopodis DJ,
Papassideri IS and Margaritis LH: Apoptosis and autophagy function
cooperatively for the efficacious execution of programmed nurse
cell death during Drosophila virilis oogenesis. Autophagy.
3:130–132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Degenhardt Y and Lampkin T: Targeting
Polo-like kinase in cancer therapy. Clin Cancer Res. 16:384–389.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Morgan MA, Parsels LA, Zhao L, Parsels JD,
Davis MA, Hassan MC, Arumugarajah S, Hylander-Gans L, Morosini D,
Simeone DM, et al: Mechanism of radiosensitization by the Chk1/2
inhibitor AZD7762 involves abrogation of the G2 checkpoint and
inhibition of homologous recombinational DNA repair. Cancer Res.
70:4972–4981. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun YS, Zhao Z and Zhu HP: Hispolon
inhibits TPA-induced invasion by reducing MMP-9 expression through
the NF-κB signaling pathway in MDA-MB-231 human breast cancer
cells. Oncol Lett. 10:536–542. 2015.PubMed/NCBI
|
|
42
|
Ahmed KM and Li JJ: NF-kappa B-mediated
adaptive resistance to ionizing radiation. Free Radic Biol Med.
44:1–13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guo G, Yan-Sanders Y, Lyn-Cook BD, Wang T,
Tamae D, Ogi J, Khaletskiy A, Li Z, Weydert C, Longmate JA, et al:
Manganese superoxide dismutase-mediated gene expression in
radiation-induced adaptive responses. Mol Cell Biol. 23:2362–2378.
2003. View Article : Google Scholar : PubMed/NCBI
|