Introduction
Hypopharyngeal squamous cell carcinoma (HSCC) is a
tumor with the poorest prognosis among all head and neck cancers
(1). Advances in surgical techniques
and perioperative management have improved survival rates to some
extent. However, the overall 5-year survival rate remains only
30–35% (2,3), despite the use of multimodal therapy,
such as radiotherapy and chemotherapy (4). Furthermore, HSCC patients have a higher
risk of developing second primary tumors (5). Therefore, it is important to study
factors involved in the biological behavior of HSCC. Improved
understanding may provide useful information for predicting patient
prognosis and identifying specific molecular targets for novel
treatments.
Caveolae are vesicular membrane structures measuring
50–100 nm, which are commonly located in cell plasma membranes
(6,7).
Caveolin-1 (CAV1) is the most important component of caveolae. It
is a multifunctional scaffolding protein with numerous binding
partners coded for by a gene located on chromosome 7q31.1. It is
also associated with cell surface caveolae and involved in
regulating lipid raft domains (8).
CAV1 is associated with numerous physiological and
pathophysiological processes (9). It
is involved in signaling transduction (10), cholesterol homeostasis (11) and vesicular transport (12), as well as tumor oncogenesis and
suppression. Therefore, CAV1 may be important in the development of
cancer.
Previous studies have identified decreased
expression of CAV1 in breast, ovarian and lung cancers, as well as
sarcoma (13–17). Therefore, it has been suggested that.
CAV1 may inhibit cell growth and reduce tumorigenesis in
oncogenically transformed cells and in cancer cells (18–20).
However, overexpression of CAV1 has been observed in pancreatic and
prostate cancer cells (21,22), suggesting that the exact function of
CAV1 may depend on the type of tissue it is expressed in. The aim
of the present study was to investigate the specific function of
CAV1 in HSCC.
Materials and methods
Patients and tumor tissues
All complete surgical specimens of HSCC were
resected and examined from 66 patients (61 male, 5 female), with a
median age of 59.5 years (range, 49–74 years), with no evidence of
metastasis to other organs and without prior anticancer treatment.
All patients underwent hypo-pharyngeal resection at the Qilu
Hospital of Shandong University between January 2013 and August
2015. Forty-four morphologically normal tissues adjacent to the
carcinomas were also collected. The resected tissues were
immediately fixed with 10% buffered formalin overnight, snap-frozen
in liquid nitrogen and stored at −80°C. The specimens were examined
histologically after staining with eosin and hematoxylin. The
pathological stage was classified according to the
tumor-node-metastasis (TNM) classification system of the
International Union Against Cancer (23).
Immunohistochemistry
All specimens were dehydrated and embedded in
paraffin blocks after resection. Sections were cut to 4-µm
thickness and placed on poly-L-lysine-coated glass slides. After
deparaffinization in graded alcohols and xylene, epitope retrieval
was carried out. Target retrieval for activated CAV1 was performed
in 0.01 M sodium citrate buffer (pH 6.0) for 20 min in a microwave
at 92–95°C. Endogenous peroxidase was blocked by immersing the
sections in 3% hydrogen peroxide for 15 min at room temperature.
Sections were washed twice in phosphate-buffered saline (PBS) and
incubated with 10% goat serum (Zhongshan Golden Bridge
Biotechnology Co., Beijing, China). Primary anti-CAV1 rabbit
polyclonal antibody, (Santa Cruz Biotechnology Inc., Dallas, TX,
USA), was immersed in a 1:100 dilution in PBS, and the sections
were incubated at 4°C for 12 h. PBS was used instead of the primary
antibody as a negative control. The polymer is a reagent labeled by
peroxidase and conjugated to goat anti-rabbit antibody (Beyotime
Institute of Biotechnology, Shanghai, China; cat no. A0208; diluted
1:50). After three additional washes, the sections were incubated
with polyvalent biotinylated goat anti-rabbit antibody (Beyotime;
cat no. A0277; 1:100) for 1 h at 37°C. Sections were washed three
times in PBS and incubated with streptavidin-conjugated peroxidase
(Zhongshan; cat no. SP-9000) for 30 min. Sections were then
visualized by incubation with 3,3-diaminobenzidine (Zhongshan; cat
no. ZLI-9019) as a chromogen for 5 min followed by washing in
distilled water. After that, sections were counterstained with
Mayer's hematoxylin for 5 min. Immunoreactivity was detected with
use of a standard ABC method. The immunohistochemistry kit used was
from Zhongshan Golden Bridge Biotechnology Co. (Beijing,
China).
The number of stained cells per 500 cells was
ascertained using an Olympus microscope (Olympus Corporation,
Tokyo, Japan) in five visual fields at a magnification of ×200.
Fields with no stained cells were given a score of 0; fields with
>5% stained cells, 1; fields with 5–25% stained cells, 2; fields
with 26–50% stained cells, 3; fields with >50% stained cells, 4.
The intensity of staining was then graded by comparison with a
positive control. The positive control was derived from a male
patient with hypopharyngeal carcinoma which was diagnosed with a
moderately differentiated HSCC. No staining was given a score of 0;
weak staining, 1; moderate staining, 2; strong staining, 3. The
expression result was considered negative when the sum of two
factors was ≤3. The expression result was considered positive when
the sum of two factors was ≥4 (24).
The assessment of staining intensity was based on the same positive
slides and performed independently by two observers who were
blinded to the patients' clinical information. The final result was
obtained by consensus between the two observers.
Statistical analysis
The Fisher exact test or chi square test was used to
analyze the correlation between the patients' parameters and CAV1
histopathological expression using the statistical software SPSS
ver. 13.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Patient clinicopathological
parameters
Specimens from 66 patients were included in the
current study (61 males and 5 females). The median patient age was
59.5 years (range, 49–74 years). 32% of patients (21 patients) had
early stage disease, and 74% patients (44 patients) had metastases
in their lymph nodes. Details of tumor pathological parameters are
shown in Table I.
 | Table I.Association between caveolin-1
expression and clinicopathological variables in the 66
patients. |
Table I.
Association between caveolin-1
expression and clinicopathological variables in the 66
patients.
|
|
| Caveolin-1 |
|
|---|
|
|
|
|
|
|---|
| Variable | No. patients | Negative | Positive | P-value |
|---|
| Age |
|
|
| P>0.05 |
| ≤50 | 32 | 9 | 23 |
|
|
>50 | 34 | 10 | 24 |
|
| Gender |
|
|
| P>0.05 |
| Male | 61 | 17 | 44 |
|
|
Female | 5 | 2 | 3 |
|
| Site of
expression |
|
|
| P>0.05 |
| Pyriform
sinus | 53 | 32 | 21 |
|
| Posterior
wall | 8 | 6 | 2 |
|
|
Postcricoid area | 5 | 4 | 1 |
|
| Histological
grade |
|
|
| P<0.05 |
| High | 11 | 9 | 2 |
|
|
Medium | 31 | 21 | 10 |
|
| Low | 24 | 9 | 15 |
|
| Clinical stage |
|
|
| P<0.05 |
| I | 9 | 2 | 7 |
|
| II | 12 | 4 | 8 |
|
| III | 27 | 8 | 19 |
|
| IV | 18 | 5 | 13 |
|
| Lymph node
status |
|
|
| P<0.05 |
|
Positive | 49 | 12 | 37 |
|
|
Negative | 17 | 7 | 10 |
|
Expression of caveolin-1
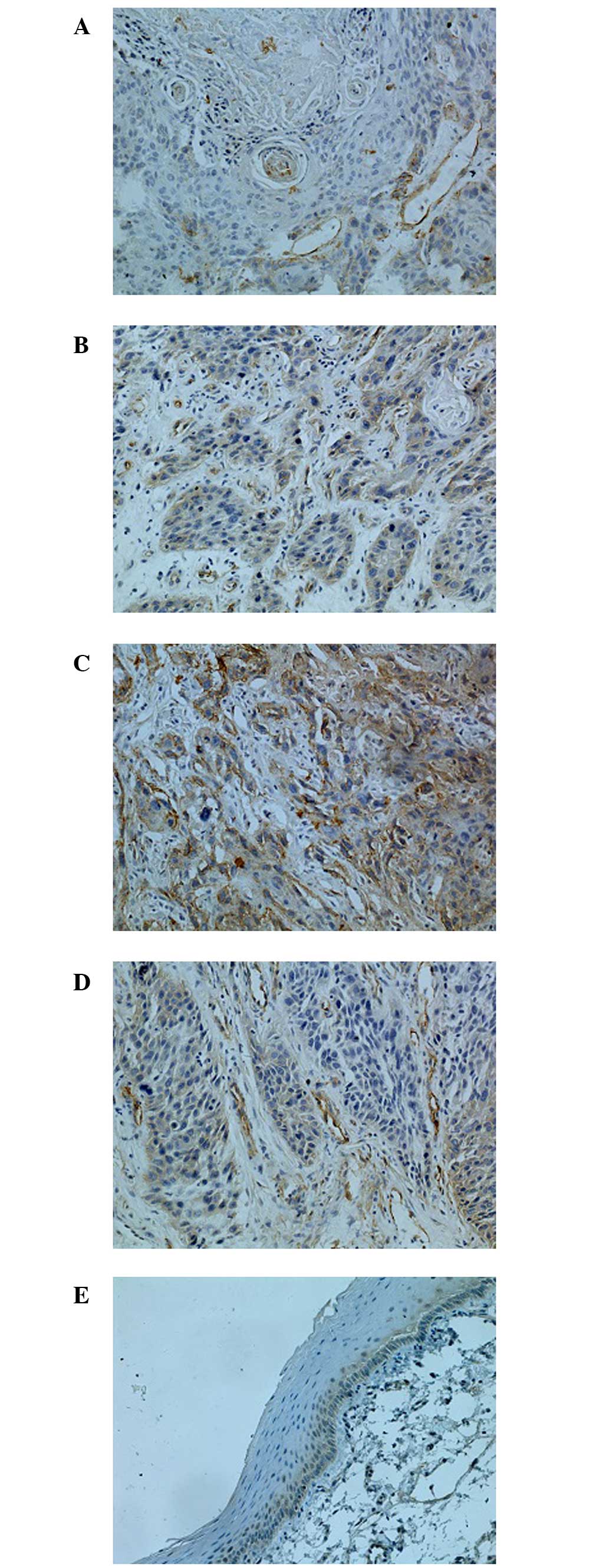
Immunoreactivity, indicating expression of CAV1, was
detected in the cytoplasm and at the cell membrane, and was
identified by the presence of stained granular immunoreaction
products (Fig. 1). Overexpression of
CAV1 was observed in vascular endothelial cells (Fig. 1D). Forty-seven specimens (71.2%) were
immunoreactive for CAV1 indicating high expression, and CAV1 levels
at peripheral parts of the cancer cell nests were higher than at
the central parts; only four specimens (9.5%) were immunoreactive
in paracancerous tissues. Compared with adjacent tissues, CAV1
protein expression was significantly higher in HSCC
(P<0.05).
Expression of CAV1 was markedly associated with
tumor differentiation grade, TNM stage and lymph nodes metastasis.
The CAV1 staining results (Fig. 1)
demonstrated a trend of gradually increasing CAV1 intensity as the
pathological grading increased. Additionally, CAV1 expression in
well-differentiated HSCC was significantly lower than in the group
with poorly differentiated HSCC. Expression of CAV1 in patients
with lymph node metastasis (78.7%) was significantly higher than in
patients without metastasis (58.8%, P<0.05). There was no
correlation between CAV1 expression with age, gender or site of
CAV1 expression (P>0.05).
Discussion
Caveolae are a subdomain of lipid rafts that are
defined as ‘small (50–200 nm) heterogeneous membrane domains
enriched in sphingolipids and sterol that are involved in the
compartmentalization of various cellular processes’ (25). CAV1 is a 21–24 kDa protein that is an
important structural component of caveolae membranes in vivo
and participates in transduction processes and vesicular
trafficking. It also has a regulatory role in a number of signaling
pathways, such as epidermal growth factor receptor, protein kinase
C, G-proteins, endothelial nitric oxide synthase (eNOS), and Src
family tyrosine kinase, which may induce development of human
cancer.
The differential expression of CAV1 is not a novel
finding in relation to the neoplastic histotype. As stated
previously, downregulation of CAV1 has been identified and reported
in a variety of tumors (13–17), however, its overexpression has been
identified in other types of tumor (21,22),
indicating that the behavior of CAV1 in carcinogenesis may depend
on the type of tissue it is expressed in.
The present study suggests that overexpression of
CAV1 in HSCC is comparable to that observed in prostate carcinoma.
CAV1 overexpression was also identified to correlate with lymph
node involvement, TNM stage, Gleason score, and a positive surgical
margin. Previous studies have demonstrated that immunoreactivity
for CAV1 is an important and independent predictor for disease
prognosis in lymph node negative patients with prostate carcinomas
(21). Thus, HSCC seems to share
common features with prostate carcinoma in CAV1
immunoreactivity.
It is well known that the incidence of HSCC in men
is higher than that of women (26).
Sex hormones play an important role in this disease. Nuclear
estrogen receptor α levels and epidermal growth factor receptor
expression are significantly increased in head and neck squamous
cell carcinomas (27). Prostate
carcinoma growth is androgen dependent. CAV1 mediates testosterone
stimulated clonal or survival growth and promotes metastatic
activity in prostate carcinoma (28).
Additionally, overexpression of CAV1 potentiates ligand-dependent
androgen receptor activation (29).
Similar mechanisms may be involved in HSCC.
The results of the current study indicate that
overexpression of CAV1 may be a marker for poor HSCC cancer
prognosis and lymph node metastasis. CAV1 overexpression alone
cannot be relied upon to predict lymph node metastases. However,
practitioners may use the tumor markers of biopsy specimens to
improve current therapeutic strategies. Moreover, overexpression of
CAV1 has been identified in vascular endothelial cells. The defects
in activation of eNOS with vascular endothelial growth factor
(VEGF) stimulation played a role in the inability of
Cav−/− mice to stimulate angiogenesis
(30). The result was demonstrated in
endothelial cells (ECs) from these mice. The ability of
Cav−/− ECs to form tubes on Matrigel with
VEGF stimulation was significantly lower than that of
Cav+/+ ECs. VEGF-induced nitric oxide
production was greatly inhibited in Cav−/−
ECs (30). VEGF-induced
phosphorylation of eNOS on Ser1177 and dephosphorylation on Thr495
were abrogated in Cav−/− ECs but not in
Cav+/+ ECs, both of which were considered
hallmarks of eNOS activation (30).
Thus, the overexpression of CAV1 may play an important role in
tumorigenesis of HSCC by positively regulating angiogenesis.
Nonetheless, whether this overexpression is a crucial factor
requires further study.
In conclusion, the present study demonstrates that
CAV1 expression is significantly higher in HSCC than that in
paracancerous tissues and is associated with tumor invasion and
metastasis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 30772411/H1625).
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoffman HT, Karnell LH, Shah JP, Ariyan S,
Brown GS, Fee WE, Glass AG, Goepfert H, Ossoff RH and Fremgen AM:
Hypopharyngeal cancer patient care evaluation. Laryngoscope.
107:1005–1017. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bova R, Goh R, Poulson M and Coman WB:
Total pharyngolaryngectomy for squamous cell carcinoma of
hypopharynx: A review. Laryngoscope. 115:864–869. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Godballe C, Jørgensen K, Hansen O and
Bastholt L: Hypopharyngeal cancer: Results of treatment based on
radiation therapy and salvage surgery. Laryngoscope. 112:834–838.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dikshit RP, Boffetta P, Bouchardy C,
Merletti F, Crosignani P, Cuchi T, Ardanaz E and Brennan P: Risk
factors for the development of second primary tumors among men
after laryngeal and hypopharyngeal carcinoma. Cancer.
103:2326–2333. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anderson RG: The caveolae membrane system.
Annu Rev Biochem. 67:199–225. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamada E: The fine structure of the gall
bladder epithelium of the mouse. J Biophys Biochem Cytol.
1:445–458. 1955. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rothberg KG, Heuser JE, Donzell WC, Ying
YS, Glenney JR and Anderson RG: Caveolin, a protein component of
caveolae membrane coats. Cell. 68:673–682. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rothberg KG, Heuser JE, Donzell WC, Ying
YS, Glenney JR and Anderson RG: Caveolin, a protein component of
caveolae membrane coats. Cell. 68:673–682. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smart EJ, Graf GA, McNiven MA, Sessa WC,
Engelman JA, Scherer PE, Okamoto T and Lisanti MP: Caveolins,
liquid-ordered domains and signal transduction. Mol Cell Biol.
19:7289–7304. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pike LJ, Han X, Chung KN and Gross RW:
Lipid rafts are enriched in arachidonic acid and
plasmenylethanolamine and their composition is independent of
caveolin-1 expression: A quantitative electrospray ionization/mass
spectrometric analysis. Biochemistry. 41:2075–2088. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Orlandi PA and Fishman PH:
Filipin-dependent inhibition of cholera toxin: Evidence for toxin
internalization and activation through caveolae-like domains. J
Cell Biol. 141:905–915. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park SS, Kim JE, Kim YA, Kim YC and Kim
SW: Caveolin-1 is down-regulated and inversely correlated with HER2
and EGFR expression status in invasive ductal carcinoma of the
breast. Histopathology. 47:625–630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen ST, Lin SY, Yeh KT, Kuo SJ, Chan WL,
Chu YP and Chang JG: Mutational, epigenetic and expressional
analyses of caveolin-1 gene in breast cancers. Int J Mol Med.
14:577–582. 2004.PubMed/NCBI
|
|
15
|
Wiechen K, Sers C, Agoulnik A, Arlt K,
Dietel M, Schlag PM and Schneider U: Down-regulation of caveolin-1,
a candidate tumor suppressor gene, in sarcomas. Am J Pathol.
158:833–839. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bélanger MM, Roussel E and Couet J:
Caveolin-1 is down-regulated in human lung carcinoma and acts as a
candidate tumor suppressor gene. Chest. 125(5 Suppl): 106S2004.
View Article : Google Scholar
|
|
17
|
Wiechen K, Diatchenko L, Agoulnik A,
Scharff KM, Schober H, Arlt K, Zhumabayeva B, Siebert PD, Dietel M,
Schäfer R and Sers C: Caveolin-1 is down-regulated in human ovarian
carcinoma and acts as a candidate tumor suppressor gene. Am J
Pathol. 159:1635–1643. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Engelman JA, Wykoff CC, Yasuhara S, Song
KS, Okamoto T and Lisanti MP: Recombinant expression of caveolin-1
in oncogenically transformed cells abrogates anchorage-independent
growth. J Biol Chem. 272:16374–16381. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee SW, Reimer CL, Oh P, Campbell DB and
Schnitzer JE: Tumor cell growth inhibition by caveolin
re-expression in human breast cancer cells. Oncogene. 16:1391–1397.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bender FC, Reymond MA, Bron C and Quest
AF: Caveolin-1 levels are down-regulated in human colon tumors, and
ectopic expression of caveolin-1 in colon carcinoma cell lines
reduces cell tumorigenicity. Cancer Res. 60:5870–5878.
2000.PubMed/NCBI
|
|
21
|
Yang G, Truong LD, Wheeler TM and Thompson
TC: Caveolin-1 expression in clinically confined human prostate
cancer: A novel prognostic marker. Cancer Res. 59:5719–5723.
1999.PubMed/NCBI
|
|
22
|
Suzuoki M, Miyamoto M, Kato K, Hiraoka K,
Oshikiri T, Nakakubo Y, Fukunaga A, Shichinohe T, Shinohara T, Itoh
T, et al: Impact of caveolin-1 expression on prognosis of
pancreatic ductal adenocarcinoma. Br J Cancer. 87:1140–1144. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sobin LH and Wittekind CH: TNM
lassification of head and neck carcinomaInternational Union Against
Cancer, TNM: Classification of Malignant Tumours. 1. 5th. Wiley;
New York, NY: pp. 17–50. 1997
|
|
24
|
Zhao T, Zhu MG and Huang ZY: Comparative
study of expression of oncogene protein products in lung cancer.
Chinese Journal of Cancer. 1:13–15. 1995.
|
|
25
|
Pike LJ: Rafts defined: A report on the
keystone symposium on lipid rafts and cell function. J Lipid Res.
47:1597–1598. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang WL, Lee CT, Lee YC, Hwang TZ, Wang
CC, Hwang JC, Tai CM, Chang CY, Tsai SS, Wang CP, et al: Risk
factors for developing synchronous esophageal neoplasia in patients
with head and neck cancer. Head Neck. 33:77–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Egloff AM, Rothstein ME, Seethala R,
Siegfried JM, Grandis JR and Stabile LP: Cross-talk between
estrogen receptor and epidermal growth factor receptor in head and
neck squamous cell carcinoma. Clin Cancer Res. 15:6529–6540. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li L, Yang G, Ebara S, Satoh T, Nasu Y,
Timme TL, Ren C, Wang J, Tahir SA and Thompson TC: Caveolin-1
mediates testosterone-stimulated survival/clonal growth and
promotes metastatic activities in prostate cancer cells. Cancer
Res. 61:4386–4392. 2001.PubMed/NCBI
|
|
29
|
Lu ML, Schneider MC, Zheng Y, Zhang X and
Richie JP: Caveolin-1 interacts with androgen receptor. A positive
modulator of androgen receptor mediated transactivation. J Biol
Chem. 276:13442–13451. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sonveaux P, Martinive P, DeWever J, Batova
Z, Daneau G, Pelat M, Ghisdal P, Grégoire V, Dessy C, Balligand JL
and Feron O: Caveolin-1 expression is critical for vascular
endothelial growth factor-induced ischemic hindlimb
collateralization and nitric oxide-mediated angiogenesis. Circ Res.
95:154–161. 2004. View Article : Google Scholar : PubMed/NCBI
|