Introduction
Gastric cancer (GC) is the fifth most prevalent
cancer and the third leading cause of cancer-associated mortality
(1). The prognosis of GC is generally
poor, since GC metastasize frequently from gastric glands to other
parts of the body. The 5-year survival rate of GC is <10%
(2). Therefore, study concerning the
treatment of GC is of great importance.
Treatments for GC include surgery, radiation therapy
and chemotherapy (3). Drugs used in
GC treatment primarily consist of fluorouracil or its analog
capecitabine, carmustine, mitomycin C, semustine, doxorubicin,
cisplatin and taxotere (3,4). However, the results of these treatments
are unsatisfactory, and GC remains incurable; therefore, requiring
an alternative treatment method (2).
Celecoxib is a classic nonsteroidal
anti-inflammatory drug (NSAID) targeting cyclooxygenase-2 (COX-2).
Previous epidemiological studies have demonstrated that prolonged
treatment with NSAIDs may reduce the risk of GC (5,6).
Reportedly, celecoxib possesses anticancer effects, since it
downregulates AKT serine/threonine kinase 1, glycogen synthase
kinase 3β and forkhead box O1, and upregulates caspase-9 in the
mitochondrial apoptotic pathway (7).
In addition, celecoxib regulates cell cycle arrest, mitochondrial
cytochrome C release and caspase activation in cancer cells
(8). Furthermore, celecoxib
suppresses the invasion of GC by affecting the expression of
E-cadherin, vascular endothelial growth factor and COX-2, and
interfering with nuclear factor-κB signaling, Snail signaling and
microvessel density (9,10). However, the anti-GC mechanism of
celecoxib remains unclear and requires further study.
The present study aimed to improve the understanding
of the anti-GC mechanism of celecoxib using bioinformatics methods.
Two gene expression datasets (GSE56807, GC vs. normal gastric
tissues; GSE54657, celecoxib-treated vs. non-treated GC cells) were
downloaded from the Gene Expression Omnibus (GEO) database.
GSE56807 was uploaded by Wang et al (11), who investigated hypoxia inducible
factor-1α-regulated transcription factors and regulatory signaling
pathways in GC. The present study identified two sets of
differentially expressed genes (DEGs) and their overlapped DEGs
from the two datasets. Furthermore, pathway enrichment analysis was
performed, and a protein-protein interaction (PPI) network was
constructed to predict the targets of celecoxib.
Materials and methods
Microarray data
Two gene expression datasets, GSE56807 (11) and GSE54657 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE54657),
were downloaded from the GEO database (www.ncbi.nlm.nih.gov/geo/) (12). Dataset GSE56807 consisted of 10
samples, which included 5 pairs of GC and normal gastric tissues.
Its corresponding platform is GPL5175 [HuEx-1_0-st] Affymetrix
Human Exon 1.0 ST Array [transcript (gene) version]. Dataset
GSE54657 consisted of 6 samples, which included 3 celecoxib-treated
human GC epithelial AGS cell line samples and 3 non-treated AGS
cell line samples. The celecoxib-treated AGS samples were harvested
following incubation with 20 µM celecoxib for 24 h in triplicate.
The corresponding platform for GSE54657 is GPL6244 [HuGene-1_0-st]
Affymetrix Human Gene 1.0 ST Array [transcript (gene) version].
Data preprocessing and DEGs
screening
The downloaded raw gene expression data were
preprocessed based on R language (13). Data in different chips were normalized
using the Robust Multichip Averaging algorithm (14). Subsequently, limma version 3.22.1
software (www.bioconductor.org/packages/release/bioc/html/limma.html)
(15) in R language was applied to
identify DEGs. For DEGs between GC tissues and normal gastric
tissues (GSE56807), the P-value was adjusted using the
Benjamini-Hochberg method (16).
Adjusted P<0.05, also known as false discovery rate (FDR), was
set as the criterion for DEG screening. For DEGs between
celecoxib-treated AGS samples and non-treated AGS samples
(GSE54657), P<0.01 was set as the cut-off criterion. DEGs shared
by the two DEG groups were defined as overlapped DEGs, which
exhibited the same or opposite regulation directions in the two
datasets.
Hierarchical clustering analysis
In order to determine the sample-specificity of the
overlapped DEGs, bidirectional hierarchical clustering analysis
(BHCA) (17) was performed using
pheatmap version 1.0.8 package (https://cran.r-project.org/web/packages/pheatmap/) in
R language. DEGs with similar expression patterns were
clustered.
Pathway enrichment analysis
Pathway enrichment analysis of the overlapped DEGs
was performed using Database for Annotation, Visualization and
Integrated Discovery software version 6.7 (david.abcc.ncifcrf.gov/) (18) based on the Kyoto Encyclopedia of Genes
and Genomes (KEGG) pathway database (19). P<0.1 and gene count ≥2 were set as
the cut-off criteria.
Construction of a PPI network
Based on the PPI data downloaded from IntAct
(www.ebi.ac.uk/intact/) (20), Database of Interacting Proteins
(dip.doe-mbi.ucla.edu/dip/Main.cgi) (21), Biomolecular Interaction Network
Database (http://bind.ca) (22) and Human Protein Reference Database
(http://www.hprd.org/) (23), and the studies by Rual et al
(24), Stelzl et al (25) and Ramani et al (26), a PPI set was established, which
investigated the PPIs among the overlapped DEGs. Subsequently, the
PPI network of DEGs was visualized using Cytoscape version 3.2.0
software (www.cytoscape.org/) (27).
Results
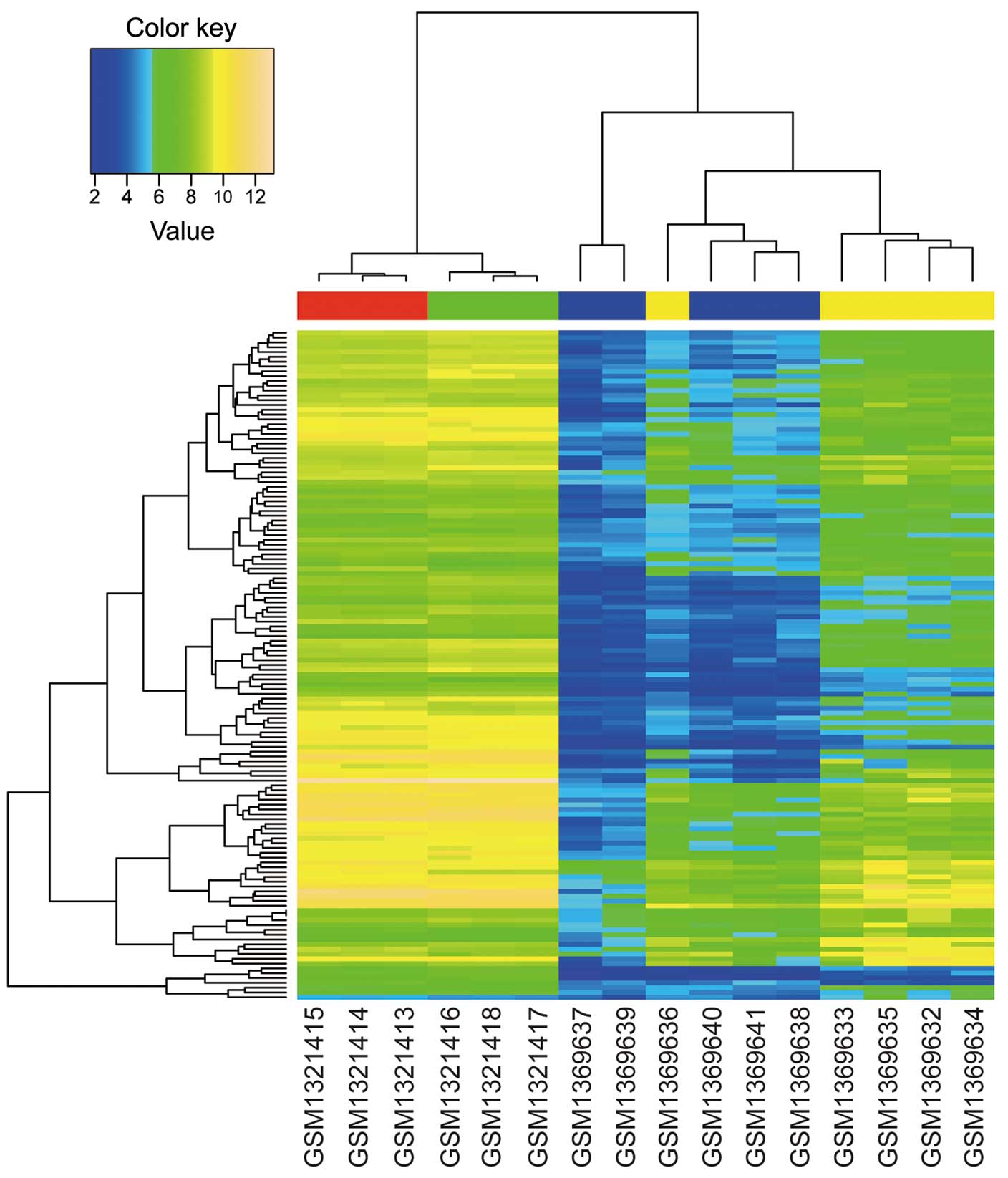
DEG screening and BHCA
Based on the aforementioned analysis, 5190 DEGs (FDR
<0.05) between GC tissues and normal gastric tissues, and 540
DEGs (P<0.01) between celecoxib-treated AGS samples and
non-treated AGS samples were identified. In total, 137 overlapped
DEGs were obtained by investigating the intersection of the two DEG
groups. Furthermore, BHCA was performed, and the genes with similar
expression patterns are shown in Fig.
1. The two datasets used in the present study were based on
different platforms; therefore, gene expression levels exhibited a
large difference between the two datasets. However, qualitative
conclusions were obtained from Fig.
1: The overlapped DEGs could differentiate between GC and
normal gastric tissues, and between celecoxib-treated and
non-treated AGS samples; and celecoxib did affect the gene
expression patterns of AGS cells during treatment.
Pathway enrichment analysis
To investigate the mechanism underlying the effects
of celecoxib on GC cells, KEGG pathway enrichment analysis of the
137 overlapped DEGs was performed. As shown in Table I, the overlapped DEGs were primarily
enriched in four pathways (P<0.1; gene count ≥2), including
lysosome, other glycan degradation, focal adhesion and leukocyte
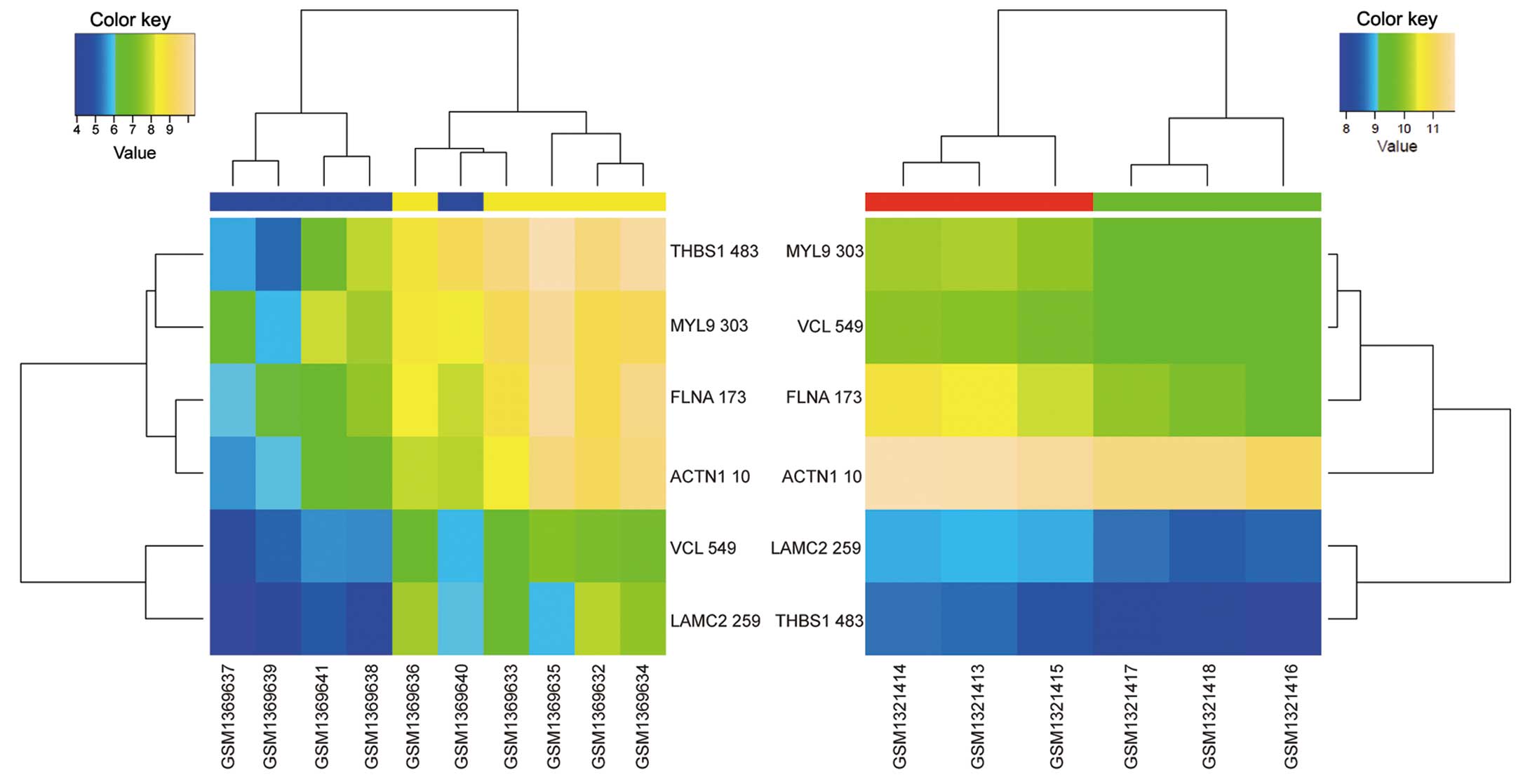
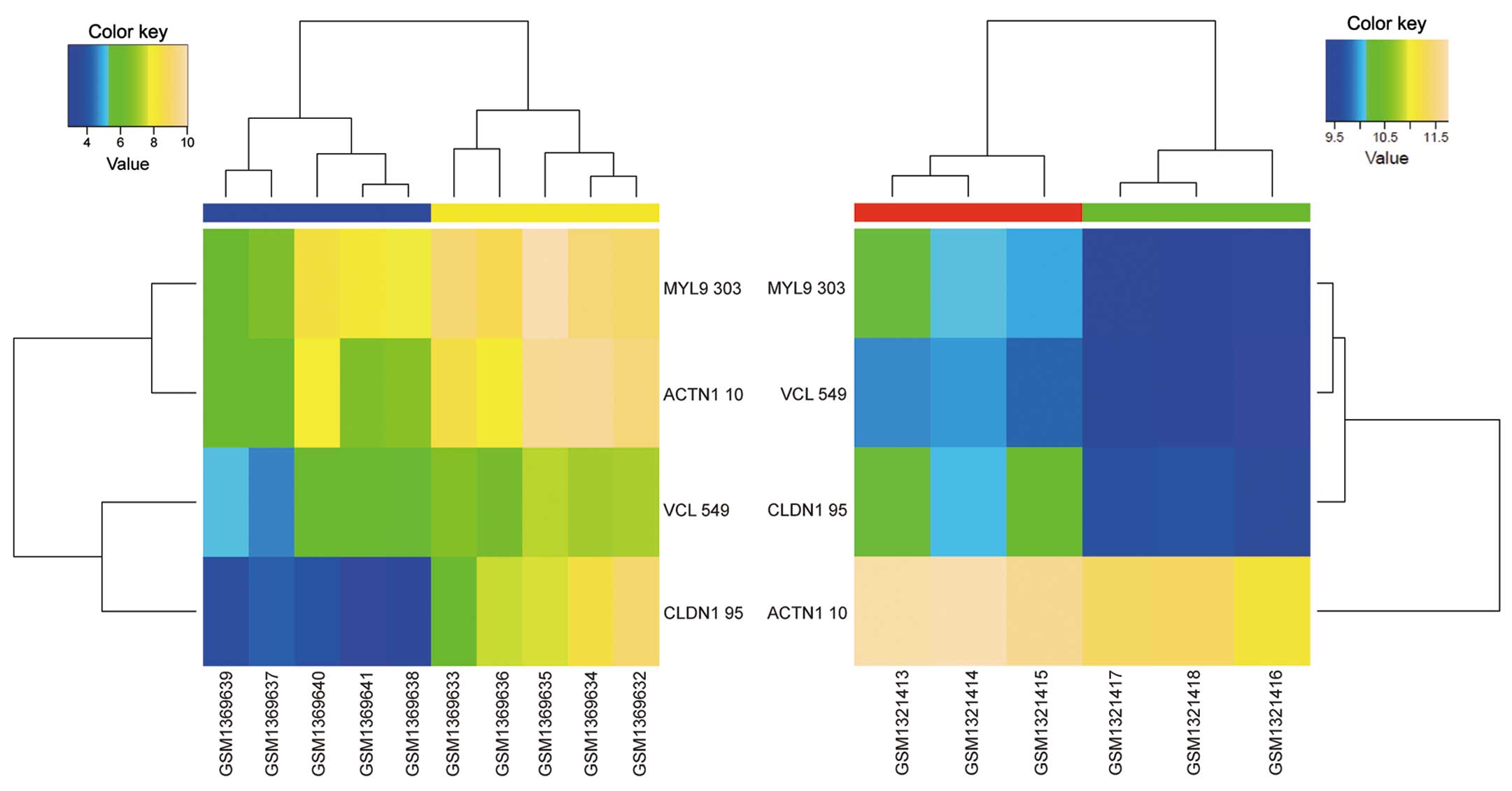
transendothelial migration pathways. Among these pathways, focal
adhesion (Fig. 2) and leukocyte
transendothelial migration (Fig. 3)
were enriched by DEGs that exhibited opposite regulation directions
in the two datasets. In fact, 6 DEGs enriched in focal adhesion
[thrombospondin 1 (THBS1), myosin light chain 9
(MYL9), filamin A (FLNA), actinin alpha 1
(ACTN1), vinculin (VCL), laminin subunit gamma 2
(LAMC2)] and 4 DEGs enriched in leukocyte transendothelial
migration [MYL9, VCL, ACTN1 and claudin 1
(CLDN1)] were significantly upregulated in GC tissues
compared with normal gastric tissues, and significantly
downregulated in celecoxib-treated AGS cells compared with
non-treated AGS cells.
 | Table I.Significantly enriched pathways in
overlapped DEGs between two datasets used by the present study. |
Table I.
Significantly enriched pathways in
overlapped DEGs between two datasets used by the present study.
| Pathway ID | Pathway | Gene count | DEGs | RD | P-value |
|---|
| hsa04142 | Lysosome | 6 | NEU1, GLB1,
FUCA1, CLN5, ATP6AP1, CTSD | Same | 0.002834 |
| hsa00511 | Other glycan
degradation | 3 | NEU1, GLB1,
FUCA1 | Same | 0.007776 |
| hsa04510 | Focal adhesion | 6 | THBS1, MYL9,
FLNA, ACTN1, VCL, LAMC2 | Opposite | 0.026095 |
| hsa04670 | Leukocyte
transendothelial migration | 4 | MYL9, VCL,
ACTN1, CLDN1 | Opposite | 0.076932 |
Construction of PPI network
To further investigate the underlying mechanism of
celecoxib in GC treatment, a PPI network of the 137 overlapped DEGs
was constructed, consisting of 8 DEGs and 5 PPIs (Fig. 4). Cysteine and glycine rich protein 1
(CSRP1), VCL and ACTN1 exhibited opposite
regulation directions in the two datasets. In fact, CSRP1,
VCL and ACTN1 were significantly upregulated in GC
tissues compared with normal gastric tissues, while significantly
downregulated in celecoxib-treated AGS cells compared with
non-treated AGS cells. Comprehensively, a total of 8 key DEGs
(CSRP1, THBS1, MYL9, FLNA,
ACTN1, VCL, LAMC2 and CLDN1) were
upregulated in GC tissues and downregulated in celecoxib-treated
cells.
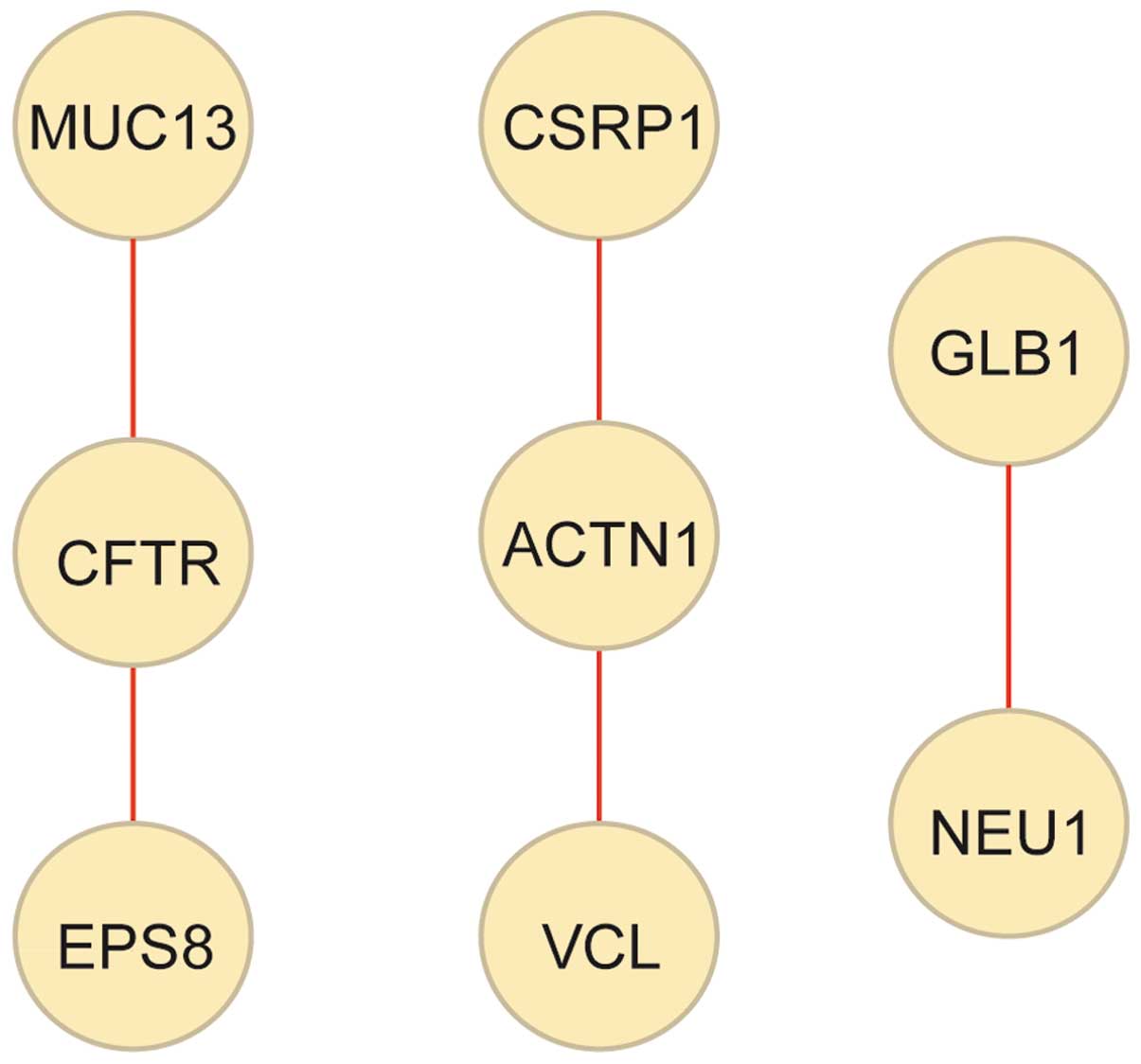 | Figure 4.Protein-protein interaction network
of the overlapped DEGs. Yellow nodes, DEGs (proteins); red lines,
interactions between DEGs (proteins). DEGs, differently expressed
genes; MUC13, mucin 13, cell surface associated; CSRP1, cysteine
and glycine rich protein 1; GLB1, galactosidase beta 1; CFTR,
cystic fibrosis transmembrane conductance regulator; ACTN1, actinin
alpha 1; NEU1, neuraminidase 1; EPS8, epidermal growth factor
receptor pathway substrate 8; VCL, vinculin. |
Discussion
GC is a prevalent and malignant cancer, which
remains incurable. As a NSAID, celecoxib is a potentially effective
chemotherapy for GC. However, the anti-GC mechanism of celecoxib
remains unclear. To gain insight into the anti-GC mechanism of
celecoxib, the present study systematically analyzed the gene
expression data of gastric tissue and human gastric cancer
epithelial AGS cells using bioinformatics methods. Consequently, a
total of 137 genes were identified to be differentially expressed
between GC and normal gastric tissues, and between
celecoxib-treated and non-treated AGS cells. Following pathway
enrichment analysis and PPI network construction, 8 key DEGs
(CSRP1, THBS1, MYL9, FLNA,
ACTN1, VCL, LAMC2 and CLDN1) were
identified, which were upregulated in GC tissues and downregulated
in celecoxib-treated cells, and enriched in focal adhesion and
leukocyte transendothelial migration pathways.
Among the 8 DEGs, THBS1, MYL9,
FLNA, LAMC2 and CLDN1 have been reported to
participate in the development of GC, as follows: THBS1
encodes thrombospondin-1, and its expression is a prognostic factor
in advanced GC (28); MYL9
encodes myosin light chain 9, which is an upregulated
apoptosis-associated protein and biomarker in GC (29); FLNA encodes filamin A, which is
aberrantly regulated in GC tissue and regulates migration and
invasion of GC cells in vitro (30); LAMC2 encodes the γ2 chain of
laminin-5, which is a major component of the basement membrane, and
the expression of LAMC2 is frequently upregulated by
promoter demethylation in GC (31);
CLDN1 encodes claudin 1, a tight junction protein that is
critical in the maintenance of epithelial integrity. The
overexpression of CLDN1 has been identified in GC patients
with lymph node metastasis, and is associated with decreased
overall survival (32). In the
present study, THBS1, MYL9, FLNA, LAMC2
and CLDN1 were aberrantly upregulated in GC tissue. This is
consistent with previous studies, particularly studies concerning
MYL9, LAMC2 and CLDN1 (29,31,32). In
addition, these genes were significantly downregulated in
celecoxib-treated cells, indicating that celecoxib may exhibit an
anti-GC effect by targeting these genes.
Following PPI network construction by the present
study, it was revealed that ACTN1 interacted with
CSRP1 and VCL. VCL encodes vinculin, an
important focal adhesion protein that is responsible for
cell-matrix junction and signal transduction on the membrane.
Vinculin forms a vinculin-talin-actin scaffolding complex and
promotes the malignancy and invasiveness of various cancers,
including breast (33), pancreatic
(34) and prostate (35) cancer. Additionally, the
dephosphorylation of vinculin in gastric epithelial cells results
in altered cell-matrix adhesion, contractility, motility and wound
repair (36). In the present study,
the gene expression value of VCL was significantly
upregulated in GC tissues [log2 fold change (FC)=1.59;
FDR=0.0043], indicating that VCL may participate in GC
progression by promoting tumor malignancy and invasiveness.
ACTN1 encodes actinin α1, which triggers the
unmasking of vinculin, allowing for F-actin binding and vinculin
activation. In this way, the transmission of extracellular or
intracellular forces and integrin-mediated mechano-chemical
signaling are enabled (37). In the
present study, the gene expression value of ACTN1 was
significantly upregulated in GC tissues (log2FC=2.43;
FDR=0.0044), indicating that ACTN1 may participate in GC
progression by activating VCL and promoting tumor malignancy and
invasiveness.
CSRP1 is a member of the CSRP family,
which encodes a group of proteins with LIM (Lin11, Isl-1 and Mec-3)
domains. CSRPs are generally transcription regulators associated
with gene regulation, cell growth and somatic differentiation
(38). In the present study,
CSRP1 was upregulated in GC tissues (log2FC=2.88;
FDR=0.0061), and interacted with ACTN1, indicating that the
upregulation of CSRP1 may promote GC progression by
regulating the expression of ACTN1. It should be noted that
CSRP1 (log2FC=−0.53; P=0.0002), VCL
(log2FC=−0.48; P=0.0011) and ACTN1
(log2FC=−0.39; P=0.0058) were significantly
downregulated in celecoxib-treated AGS cells, indicating that
celecoxib may exhibit an anti-GC effect by targeting CSRP1,
VCL and ACTN1.
Furthermore, THBS1, MYL9, FLNA,
ACTN1, VCL and LAMC2 were identified to be
significantly enriched in focal adhesion pathways by the present
study, while MYL9, VCL, ACTN1, and
CLDN1 were significantly enriched in leukocyte
transendothelial migration pathways. Focal adhesion is essential in
various important biological processes, including cell survival and
apoptosis (39), and leukocyte
transendothelial migration is generally activated in cancer
progression, which hampers the anti-tumour responses of the host
(40). In the present study, the DEGs
enriched in focal adhesion and leukocyte transendothelial migration
were significantly upregulated in GC tissues and downregulated in
celecoxib-treated AGS cells, suggesting that these pathways were
activated in GC progression, and celecoxib may exhibit an anti-GC
effect by suppressing these pathways.
Overall, the present study proposes that celecoxib
may exhibit an anti-GC effect by suppressing the expression of
CSRP1, THBS1, MYL9, FLNA, ACTN1,
VCL, LAMC2 and CLDN1, and inhibiting leukocyte
transendothelial migration and focal adhesion. Although limitations
exist in the present study, including the small sample size and
lack of validation, the predictions proposed, based on
bioinformatics analysis, provide novel directions for the
understanding of the anti-GC mechanism of celecoxib. Future studies
by the present authors may focus on enlarging the sample size and
validating the conclusions in vitro and in vivo.
References
|
1
|
Fock KM: Review article: The epidemiology
and prevention of gastric cancer. Aliment Pharmacol Ther.
40:250–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Orditura M, Galizia G, Sforza V,
Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J,
Savastano B, Mabilia A, et al: Treatment of gastric cancer. World J
Gastroenterol. 20:1635–1649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wagner AD, Unverzagt S, Grothe W, Kleber
G, Grothey A, Haerting J and Fleig WE: Chemotherapy for advanced
gastric cancer. Cochrane Database Syst Rev. CD0040642010.PubMed/NCBI
|
|
4
|
Scartozzi M, Galizia E, Verdecchia L,
Berardi R, Antognoli S, Chiorrini S and Cascinu S: Chemotherapy for
advanced gastric cancer: Across the years for a standard of care.
Expert Opin Pharmacother. 8:797–808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heravi R Entezari, Hadizadeh F, Sankian M,
Afshari J Tavakol, Taghdisi SM, Jafarian H and Behravan J: Novel
selective Cox-2 inhibitors induce apoptosis in Caco-2 colorectal
carcinoma cell line. Eur J Pharm Sci. 44:479–486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fischer SM, Hawk ET and Lubet RA: Coxibs
and other nonsteroidal anti-inflammatory drugs in animal models of
cancer chemoprevention. Cancer Prev Res (Phila). 4:1728–1735. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim N, Kim CH, Ahn DW, Lee KS, Cho SJ,
Park JH, Lee MK, Kim JS, Jung HC and Song IS: Anti-gastric cancer
effects of celecoxib, a selective COX-2 inhibitor, through
inhibition of Akt signaling. J Gastroenterol Hepatol. 24:480–487.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang YJ, Niu XP, Yang L, Han Z and Ma YJ:
Effects of celecoxib on cycle kinetics of gastric cancer cells and
protein expression of cytochrome C and caspase-9. Asian Pac J
Cancer Prev. 14:2343–2347. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou Y, Ran J, Tang C, Wu J, Honghua L,
Xingwen L, Ning C and Qiao L: Effect of celecoxib on E-cadherin,
VEGF, Microvessel density and apoptosis in gastric cancer. Cancer
Biol Ther. 6:269–275. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Z, Liu M, Liu X, Huang S, Li L, Song
B, Li H, Ren Q, Hu Z, Zhou Y and Qiao L: COX-2 regulates E-cadherin
expression through the NF-κB/Snail signaling pathway in gastric
cancer. Int J Mol Med. 32:93–100. 2013.PubMed/NCBI
|
|
11
|
Wang J, Ni Z, Duan Z, Wang G and Li F:
Altered expression of hypoxia-inducible factor-1α (HIF-1α) and its
regulatory genes in gastric cancer tissues. PLoS One. 9:e998352014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barrett T, Wilhite SE, Ledoux P,
Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH,
Sherman PM and Holko M: NCBI GEO: Archive for functional genomics
data sets-update. Nucleic Acids Res. 41:(Database Issue).
D991–D995. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
R Development Core Team: R: A language and
environment for statistical computing. The R Foundation for
Statistical Computing. Vienna. 2012.
|
|
14
|
Irizarry RA, Hobbs B, Collin F,
Beazer-Barclay YD, Antonellis KJ, Scherf U and Speed TP:
Exploration, normalization, and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smyth GK: limma: Linear models for
microarray dataBioinformatics and Computational Biology Solutions
Using R and Bioconductor. Gentleman R, Carey VJ, Huber W, Irizarry
RA and Dudoit S: Springer; New York: pp. 397–420. 2005, View Article : Google Scholar
|
|
16
|
Benjamini Y and Hochberg Y: Controlling
the false discovery rate: A practical and powerful approach to
multiple testing. J Royal Statistical Soc, Series B
(Methodological). 289–300. 1995.
|
|
17
|
Szekely GJ and Rizzo ML: Hierarchical
clustering via joint between-within distances: Extending Ward's
minimum variance method. J Classification. 22:151–183. 2005.
View Article : Google Scholar
|
|
18
|
Huangda W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kerrien S, Aranda B, Breuza L, Bridge A,
Broackes-Carter F, Chen C, Duesbury M, Dumousseau M, Feuermann M,
Hinz U, et al: The IntAct molecular interaction database in 2012.
Nucleic Acids Res. 40:(Database Issue). D841–D846. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xenarios I, Salwinski L, Duan XJ, Higney
P, Kim SM and Eisenberg D: DIP, the database of interacting
proteins: A research tool for studying cellular networks of protein
interactions. Nucleic Acids Res. 30:303–305. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bader GD, Betel D and Hogue CW: BIND: The
biomolecular interaction network database. Nucleic Acids Res.
31:248–250. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peri S, Navarro JD, Kristiansen TZ,
Amanchy R, Surendranath V, Muthusamy B, Gandhi TK, Chandrika KN,
Deshpande N, Suresh S, et al: Human protein reference database as a
discovery resource for proteomics. Nucleic Acids Res. 32:(Database
Issue). D497–D501. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rual JF, Venkatesan K, Hao T,
Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze
M, Ayivi-Guedehoussou N, et al: Towards a proteome-scale map of the
human protein-protein interaction network. Nature. 437:1173–1178.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stelzl U, Worm U, Lalowski M, Haenig C,
Brembeck FH, Goehler H, Stroedicke M, Zenkner M, Schoenherr A,
Koeppen S, et al: A human protein-protein interaction network: A
resource for annotating the proteome. Cell. 122:957–968. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ramani AK, Bunescu RC, Mooney RJ and
Marcotte EM: Consolidating the set of known human protein-protein
interactions in preparation for large-scale mapping of the human
interactome. Genome Biol. 6:R402005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kohl M, Wiese S and Warscheid B:
Cytoscape: Software for visualization and analysis of biological
networks. Methods Mol Biol. 696:291–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakao T, Kurita N, Komatsu M, Yoshikawa K,
Iwata T, Utsunomiya T and Shimada M: Expression of thrombospondin-1
and Ski are prognostic factors in advanced gastric cancer. Int J
Clin Oncol. 16:145–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bai Z, Ye Y, Liang B, Xu F, Zhang H, Zhang
Y, Peng J, Shen D, Cui Z, Zhang Z and Wang S: Proteomics-based
identification of a group of apoptosis-related proteins and
biomarkers in gastric cancer. Int J Oncol. 38:375–383.
2011.PubMed/NCBI
|
|
30
|
Sun GG, Sheng SH, Jing SW and Hu WN: An
antiproliferative gene FLNA regulates migration and invasion of
gastric carcinoma cell in vitro and its clinical significance.
Tumour Biol. 35:2641–2648. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kwon OH, Park JL, Kim M, Kim JH, Lee HC,
Kim HJ, Noh SM, Song KS, Yoo HS, Paik SG, et al: Aberrant
up-regulation of LAMB3 and LAMC2 by promoter demethylation in
gastric cancer. Biochem Biophys Res Commun. 406:539–545. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang J, Li J, Qu Y, Zhang J, Zhang L,
Chen X, Liu B and Zhu Z: The expression of Claudin 1 correlates
with β-catenin and is a prognostic factor of poor outcome in
gastric cancer. Int J Oncol. 44:1293–1301. 2014.PubMed/NCBI
|
|
33
|
Rubashkin MG, Cassereau L, Bainer R,
DuFort CC, Yui Y, Ou G, Paszek MJ, Davidson MW, Chen YY and Weaver
VM: Force engages vinculin and promotes tumor progression by
enhancing PI3K activation of phosphatidylinositol
(3,4,5)-triphosphate. Cancer Res. 74:4597–4611. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Kuramitsu Y, Ueno T, Suzuki N,
Yoshino S, Iizuka N, Zhang X, Akada J, Oka M and Nakamura K:
Proteomic differential display identifies upregulated vinculin as a
possible biomarker of pancreatic cancer. Oncol Rep. 28:1845–1850.
2012.PubMed/NCBI
|
|
35
|
Ruiz C, Holz DR, Oeggerli M, Schneider S,
Gonzales IM, Kiefer JM, Zellweger T, Bachmann A, Koivisto PA, Helin
HJ, et al: Amplification and overexpression of vinculin are
associated with increased tumour cell proliferation and progression
in advanced prostate cancer. J Pathol. 223:543–552. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moese S, Selbach M, Brinkmann V, Karlas A,
Haimovich B, Backert S and Meyer TF: The Helicobacter pylori CagA
protein disrupts matrix adhesion of gastric epithelial cells by
dephosphorylation of vinculin. Cell Microbiol. 9:1148–1161. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Goldmann WH, Auernheimer V, Thievessen I
and Fabry B: Vinculin, cell mechanics and tumour cell invasion.
Cell Biol Int. 37:397–405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weiskirchen R and Günther K: The
CRP/MLP/TLP family of LIM domain proteins: Acting by connecting.
Bioessays. 25:152–162. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Petit V and Thiery JP: Focal adhesions:
Structure and dynamics. Biol Cell. 92:477–494. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Enarsson K, Lundin BS, Johnsson E,
Brezicka T and Quiding-Järbrink M: CD4+ CD25high regulatory T cells
reduce T cell transendothelial migration in cancer patients. Eur J
Immunol. 37:282–291. 2007. View Article : Google Scholar : PubMed/NCBI
|