Introduction
Gastrointestinal stromal tumors (GISTs) are rare
tumors located in the alimentary tract, which originate from
mesenchymal tissues, and have gene mutations, most often mast/stem
cell growth factor receptor (CD117) and platelet-derived growth
factor receptor-α (1). In Europe, the
estimated annual incidence of clinically detected GISTs is 10 cases
per million individuals, and the age-adjusted incidence is 7 cases
per million individuals in Europe and the USA. However, at present
the global incidence of GISTs remains unknown (2). GISTs may arise anywhere along the
gastrointestinal tract, but are identified primarily in the stomach
(50–60%) and small intestine (30–35%), and less frequently in the
colon and rectum (5%) and oesophagus (<1%) (3). Previously, a few cases of GISTs were
diagnosed outside the gastrointestinal tract, usually in the
omentum, mesentery or retroperioneum (4). This type of GIST is referred to as an
extra-gastrointestinal stromal tumor (EGIST), which contributes to
<5% of all GISTs (3). In addition,
to the tumor size and mitotic index, the malignant behavior of a
GIST is associated with the primary location (3). EGISTs possess a higher malignant
potential and risk of recurrence following surgery compared with
GISTs in the alimentary tract (3).
Other unusual primary anatomic locations of GISTs have also been
reported, including the liver (5),
pancreas (6), mediastinum (7) and gall bladder (8). The standard treatment for resectable
GISTs is en bloc resection. The majority of patients may
also benefit from perioperative administration of tyrosine kinase
inhibitors (2). The estimated 5- and
15-year recurrence-free survival rates for GISTs treated with
surgery alone are 70.5 and 59.9%, respectively (2). As with GIST, complete surgical resection
is the primary treatment for EGIST. However, compared with GIST,
EGIST is considered to exhibit a worse prognosis (9). To the best of our knowledge, only 10
patients with primary liver GIST had been reported in the world
(5,10–18). The
present study reports a novel case of primary liver GIST and
reviews the literature concerning all primary liver GISTs
previously reported. Written informed consent was obtained from the
patient.
Case report
A 63-year-old man was admitted to The First
Affiliated Hospital of Zhejiang University (Hangzhou, China) on
September 5, 2011, with a liver mass, which was detected by
ultrasound. No clinical manifestations were present, including
abdominal pain and distention, nausea and vomiting. The patient had
suffered from hypertension for 10 years, which was well regulated
with oral plendil (5 mg) taken daily. A cholecystectomy was
performed 12 years ago, due to gallbladder polyps, and an
appendectomy was performed 25 years ago, due to acute appendicitis.
A review of the medical history of the patient excluded systemic
disease and any history of other malignancies. Laboratory values
were within normal limits. Serum concentration of tumor markers
revealed the following: Alpha-fetoprotein, 2.3 ng/ml (normal range,
<20.0 ng/ml); carcinoembryonic antigen, 2.2 ng/ml (normal range,
<5.0 ng/ml); CA125, 8.0 U/ml (normal range, <35.0 U/ml); and
CA199, 23.0 U/ml (normal range, <37.0 U/ml).
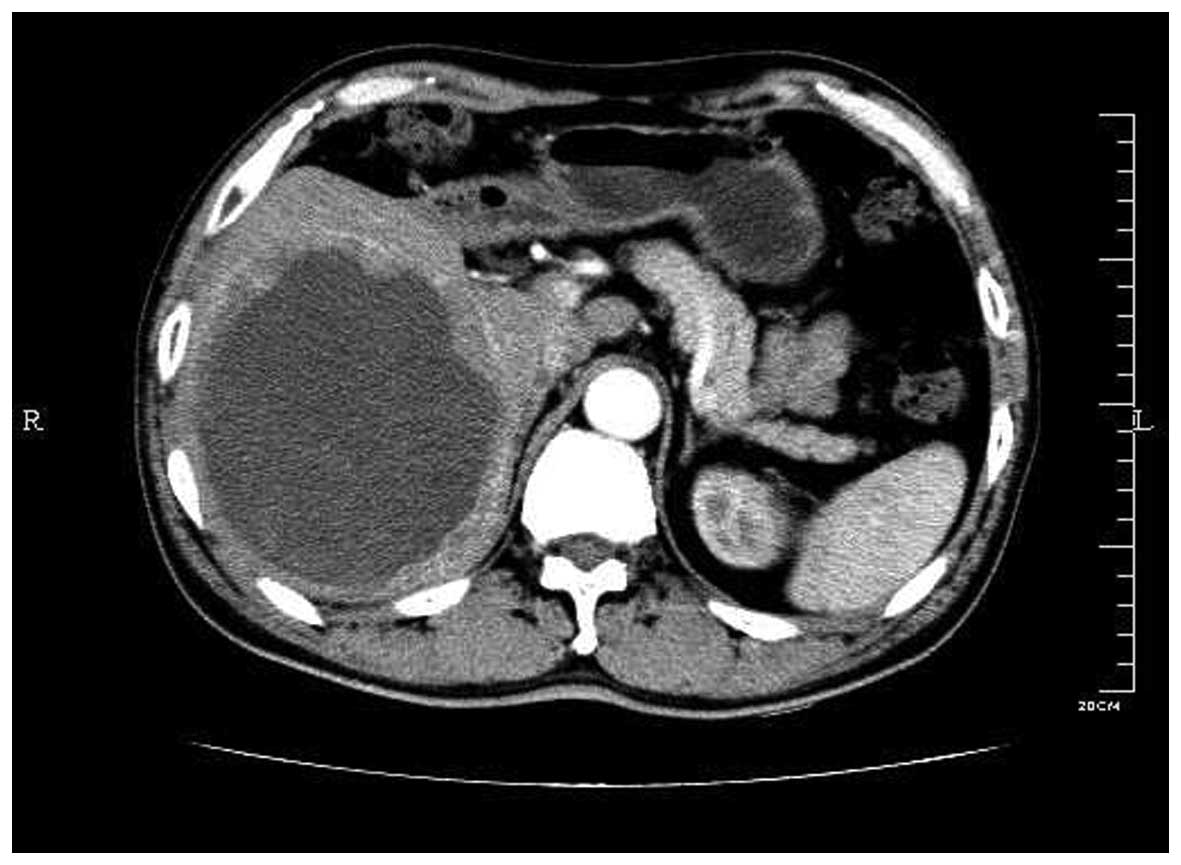
Abdominal contrast-enhanced computed tomography (CT)
scan (Aquilion 16; Toshiba, Tokyo, Japan) revealed the presence of
a cystic-solid mixed mass with a suspected internal hemorrhage in
the right liver lobe (Fig. 1). The
mass was measured at 11×13×15 cm at the widest point. The
peripheral margin was irregular and thickened with heterogeneous
enhancement on the CT scan. No tumors were detected in other
organs, with the exception of multiple cysts in the liver and left
kidney. A right hepatectomy was performed to remove the tumor
completely on September 20, 2011. On gross examination, the tumor
contained numerous blood clots. Microscopically (DM2500; Leica
Microsystems, Wetzlar, Germany), the tumor cells were atypical and
spindle-shaped. There was a high cellular density and infiltrative
growth was observed. The mitotic count was >5/50 high power
fields (HPF). Resected tissues were formalin-fixed,
paraffin-embedded and cut into 4-mm sections. The sections were
incubated with primary monoclonal mouse anti-human CD34 (cat. no.
M0117; 1:200; ChangDao, Shanghai, China) and monoclonal mouse
anti-human CD117 (cat. no. ZM0437; 1:200; Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) at 4°C overnight.
Following three washes with phosphate-buffered saline (PBS), the
sections were incubated with ready-to-use horseradish
peroxidase-conjugated goat anti-mouse IgG secondary antibody (cat.
no. A0216; 1:50; Beyotime Institute of Biotechnology, Shanghai,
China) for 20 min at 37°C. After three washes with PBS (Beyotime
Institute of Biotechnology), the sections were then incubated with
diaminobenzidine (Beyotime Institute of Biotechnology) substrate
for 5 min at room temperature and staining was visualized using a
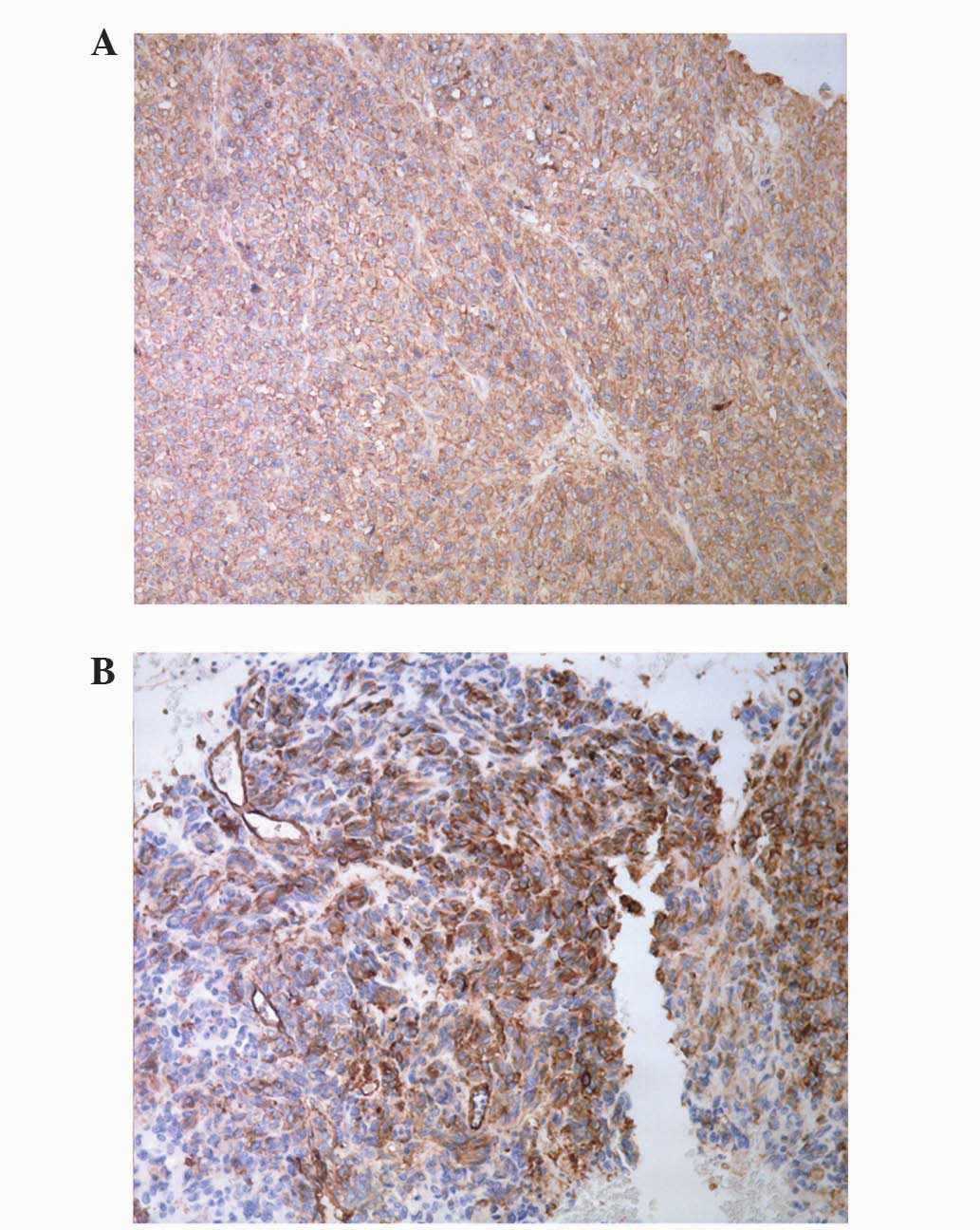
microscope (DM2500; Leica Microsystems). Immunohistochemistry
revealed positive staining for cluster of differentiation (CD) 34
and CD117 (Fig. 2). The
immunohistochemistry results lead to a diagnosis of GIST. A liver
metastatic lesion was considered and a positron emission
tomography-CT was administered to detect the latent primary lesion.
However, no tumor signs were observed and a consultation with the
Department of Pathology and Radiology resulted in a diagnosis of
primary liver GIST.
The post-operative recovery of the patient was
uneventful. Adjuvant therapy of imatinib (400 mg/day, oral
administration) was initiated one month subsequent to surgery,
which lasted for two years. For follow-up, the patients underwent
abdominal CT three times a year. After 5 years of follow-up, there
was no evidence of tumor recurrence, and the patient is currently
alive and well.
Discussion
A literature review performed by the present study
revealed a total of 11 cases diagnosed with primary liver GIST
worldwide, including the current case (Table I) (5,10–18). In these studies, primary liver GISTs
exhibited a male predominance to females (72.7 vs. 27.3%), and the
median age of occurrence was 63 years (range, 17–79 years).
Similarly to GISTs located in other organs, primary liver GISTs
have no specific clinical manifestation, such as abdominal
distention and pain, and shortness of breath (possibly occurring
due to upward displacement of the diaphragm) may be the initial
clinical manifestation owing to the large size of the primary liver
lesions. Liver GISTs may not be identified if they are smaller in
size, and require identification by magnetic resonance, computed
tomography or ultrasound imaging. Microscopically, 10 out of the 11
cases reported data concerning cell morphology, which revealed that
7 tumors were of a spindle type, 1 was of a epithelioid type and 2
had spindle and epithelioid cells. The majority of the tumors in
the 11 cases were of a large volume (>10 cm) and 1 patient with
a small tumor, and the median diameter of 18.0 cm (range, 5.1–44.0
cm). In addition, cells in the mitotic phase was commonly detected.
Excepting 1 case without any mitosis and one case with no data, the
median mitotic index was 20/50 HPF (range, 1–75/50 HPF).
Immunohistochemical results revealed positive staining for CD117
(10/11) and CD34 (9/11) in the majority of the primary liver GIST
cases, except 1 case and 2 cases that presented with negative
staining for CD117 and CD34, respectively.
 | Table I.Clinicopathological characteristics of
patients with primary gastrointestinal stromal tumors of the liver,
as reported in the literature. |
Table I.
Clinicopathological characteristics of
patients with primary gastrointestinal stromal tumors of the liver,
as reported in the literature.
|
|
|
|
| Pathology |
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Author, year | Gender | Age, years | Presentation | Cell type | IH | Size, cm | Mitotic, HPF | Treatment | IM | Outcome | RFS, months | (Ref.) |
|---|
| Hu et al,
2003 | F | 79 | Shortness of
breath | S |
CD117/34+ | 15 | 20/50 | EBR | NA | Portal lymph node
metastasis | 16 | (10) |
| De Chiara et
al, 2006 | M | 37 | NA | S |
CD117/34+ | 18 | 20/50 | EBR | Yes | Lung metastases | 14 | (11) |
| Ren et al,
2006 | M | 45 | Abdominal pain | S |
CD117/34+ | 20 | 35/50 | Drainage | NA | Live with tumor | 3 | (12) |
| Hu et al,
2007 | M | 67 | Abdominal distension
and shortness of breath | NA |
CD117/34+ | 44 | 3/50 | EBR | NA | DF | 4 | (13) |
| Luo et al,
2009 | M | 17 | No symptoms | S |
CD117/34+ | 5 | 0/50 | RFA | NA | DF | 3 | (14) |
| Ochiait et al,
2009 | M | 30 | Abdominal
distension | M |
CD117/34+ | 27 | 75/50 | EBR | Yes | Recurrence residual
liver | 24 | (15) |
| Yamamoto et
al, 2010 | M | 70 | Loss of appetite | E |
CD117−/34+ | 20 | 1/50 | EBR | NA | NA | NA | (5) |
| Yang et al,
2010 | F | 40 | Abdominal pain | M |
CD117+/34− | 18 | 5/50 | EBR | Yes | DF | 12 | (16) |
| Xue et al,
2013 | F | 66 | Abdominal distension
and pain | S |
CD117+/34− | 9 | 35/50 | EBR | NA | Succumbed to brain
metastasis | 12 | (17) |
| Lu et al,
2013 | M | 71 | Sortness of
breath | S |
CD117/34+ | 10 | NA | EBR | NA | NA | NA |
(18) |
| Current case | M | 63 | No symptoms | S |
CD117/34+ | 15 | 5/50 | EBR | Yes | DF | 60 | – |
The majority of the patients were defined as
possessing high-risk GIST, according to the National Institute of
Health criteria (19), except for 1
case that was classed as low-risk. Curative surgery remains the
mainstay of treatment for resectable high-risk lesions, and 9 out
of 11 patients underwent resection of the tumor. In total, 4
patients received adjuvant therapy with imatinib post-operatively.
Of the 11 patients, prognostic data on 7 patients were reported: 3
patients had no recurrent signs during follow-up; 1 patient
underwent recurrence twice at 2 and 5 years following the first
surgery, followed by a second and third surgery with adjuvant
imatinib treatment, and the patient was alive with no recurrence at
the end of follow-up; 1 patient presented with lung metastasis 14
months post-surgery and received imatinib, which lead to the
metastasis becoming reduced and the patient had disease-free
survival for the following 39 months; 1 patient presented with
portal lymph node metastases 16 months post-surgery, and received a
second curative surgery with no recurrence in subsequent
follow-ups; 1 patient succumbed to brain metastasis 1 year
post-operatively. For the above 7 patients, the median
recurrence-free survival was 16 months (95% confidence interval,
11.8–20.2 months; range, 4.0–60.0 months). Radiofrequency ablation
(RFA) was administered to the patient with the small low-risk
lesion. This patient recovered quickly and had no recurrence during
3 months of follow-up. There was 1 patient with an unresectable
high-risk lesion, which appeared to be a thick-walled cyst. The
patient received percutaneous transhepatic drainage and nutritional
support, and was alive with the tumor during 3 months of
follow-up.
Primary liver GISTs are extremely uncommon compared
to their alimentary counterparts, and EGISTs are gradually being
diagnosed more regularly, with lesions in the omentum, mesentery
and retroperioneum already described (4). The current study reported a case of a
male patient with a large primary liver GIST confirmed by
immunohistochemistry and CT imaging. The high mitotic index and
large tumor size contributed to a malignant characterization, thus
classifying the tumor as high-risk. The patient received en
bloc resection and post-operative adjuvant treatment of
imatinib for 2 years. The patient had a good prognosis without
recurrence in 5-years of follow-up. Primary liver GISTs are
extremely rare; therefore, the possibility of metastasis in the
current case was excluded. The present authors hypothesize that the
current case is a true primary liver GIST for the following
reasons: The lesion was completely limited to the liver; a CT scan
and intraoperative exploration excluded the possibility of another
primary lesion; and the patient had no history of malignancies,
thus excluding the possibility of recurrence.
There are certain hypotheses concerning the origin
of EGISTs. Since Min and Leabu (20)
confirmed the presence of interstitial cells of Cajal (ICC) in the
extra-digestive tract, including in the urinary bladder, gall
bladder, uterus, omentum, prostate and myocardium, it is reasonable
to presume that EGISTs originate from common precursor cells of
ICC. Other authors suggest that EGISTs may originate from
pluripotent mesenchymal stem cells located outside alimentary tract
(9). Consequently, these observations
may demonstrate the origin and existence of primary liver
GISTs.
During an extensive literature retrieval, the
present study identified 11 cases of primary liver GIST (5,10–18), including the current case. Similarly
to alimentary GISTs, primary liver GISTs possess a male
predilection and have no distinguishing symptoms, such as the
presence of a swollen abdomen. The liver lesion may become large in
size, since there is no evident discomfort or clinical
manifestation in the early stages. Therefore, primary liver GISTs
are often identified when they are large, while small lesions may
be observed during periodical examination. In addition, primary
liver GISTs possess clear proliferative activity, leading to
high-risk classification in numerous primary liver GISTs.
Immunohistochemically, the majority of primary liver lesions reveal
positive staining for CD117. Overall, regarding the
clinicopathological information available, primary liver GISTs are
similar to conventional GISTs that originate in the alimentary
tract.
Curative surgery remains the primary treatment
option for resectable primary liver lesions. However, the prognosis
of patients is not favorable, and 4 out of 7 patients identified in
the literature search with post-operative data had recurrence. This
may be due to the strong proliferative activity of primary lesions
and potential satellite lesions, as described by Hu et al
(10). Furthermore, EGISTs have been
reported to be frequently accompanied by adverse prognostic
factors, including a high proliferative index, large size, lymph
node involvement and distant metastasis (9). Consequently, patients with EGISTs are
considered to have a poorer prognosis compared to alimentary GISTs
(9). Imatinib treatment may be
considered preoperatively in order to inhibit tumor activity, and
works by minimizing the tumor load and eliminating potential
satellite lesions (21).
Post-operatively, imatinib may be administered for adjuvant
treatment for patients with a high recurrence risk, which is
similar to the guidelines for their alimentary counterparts
(22). Although imatinib exhibits a
satisfactory curative effect in certain cases (11,16), the
clinical value of imatinib on primary liver GISTs requires
verification. Previously, a mutational analysis of EGISTs reported
by Yamamoto et al (4) revealed
an infrequent mutation of CD117 on exon 11, which suggests a good
response to imatinib (9). Only 1 case
among the primary liver GISTs reported has been identified with a
platelet-derived growth factor receptor, alpha polypeptide exon 12
mutation and 1 case with a CD117 exon 11 mutation (5,15).
Deficient data on the analysis of gene mutations in GISTs impedes
the understanding of the behavior and intervention therapeutics for
this disease. RFA, hepatic arterial embolization or
chemoembolization are usually administered for the management of
liver metastases of GISTs (23,24).
Following the identification of primary liver GISTs, these
techniques may also be considered for unresectable lesions.
Notably, RFA may be a useful and minimally invasive interventional
therapy for curative treatment of small lesions (14).
In conclusion, the present study described a case of
primary liver GIST, and discussed the associated
immunohistochemical and molecular features, in addition to
treatment options. En bloc resection remains the optimal
treatment for resectable masses. The value of imatinib and other
intervention therapeutics requires further verification via future
investigation.
Acknowledgements
This study was supported by the Natural Science
Foundation of Zhejiang Province (grant no. LY13H030004).
References
|
1
|
Kitamura Y: Gastrointestinal stromal
tumors: Past, present and future. J Gastroenterol. 43:499–508.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Joensuu H, Hohenberger P and Corless CL:
Gastrointestinal stromal tumour. Lancet. 382:973–983. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Joensuu H, Vehtari A, Riihimäki J, Nishida
T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C,
et al: Risk of recurrence of gastrointestinal stromal tumour after
surgery: An analysis of pooled population-based cohorts. Lancet
Oncol. 13:265–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamamoto H, Oda Y, Kawaguchi K, Nakamura
N, Takahira T, Tamiya S, Saito T, Oshiro Y, Ohta M, Yao T and
Tsuneyoshi M: c-kit and PDGFRA mutations in extragastrointestinal
stromal tumor (gastrointestinal stromal tumor of the soft tissue).
Am J Surg Pathol. 28:479–488. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamamoto H, Miyamoto Y, Nishihara Y,
Kojima A, Imamura M, Kishikawa K, Takase Y, Ario K, Oda Y and
Tsuneyoshi M: Primary gastrointestinal stromal tumor of the liver
with PDGFRA gene mutation. Hum Pathol. 41:605–609. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vij M, Agrawal V and Pandey R: Malignant
extra-gastrointestinal stromal tumor of the pancreas. A case report
and review of literature. JOP. 12:200–204. 2011.PubMed/NCBI
|
|
7
|
Lee JR, Anstadt MP, Khwaja S and Green LK:
Gastrointestinal stromal tumor of the posterior mediastinum. Eur J
Cardiothorac Surg. 22:1014–1016. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park JK, Choi SH, Lee S, Min KO, Yun SS
and Jeon HM: Malignant gastrointestinal stromal tumor of the
gallbladder. J Korean Med Sci. 19:763–767. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yi JH, Sim J, Park BB, Lee YY, Jung WS,
Jang HJ, Ha TK and Paik SS: The primary extra-gastrointestinal
stromal tumor of pleura: A case report and a literature review. Jpn
J Clin Oncol. 43:1269–1272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu X, Forster J and Damjanov I: Primary
malignant gastrointestinal stromal tumor of the liver. Arch Pathol
Lab Med. 127:1606–1608. 2003.PubMed/NCBI
|
|
11
|
De Chiara A, De Rosa V, Lastoria S, Franco
R, Botti G, Iaffaioli VR and Apice G: Primary gastrointestinal
stromal tumor of the liver with lung metastases successfully
treated with STI-571 (imatinib mesylate). Front Biosci. 11:498–501.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ren SY, Huang ZQ and Dong BZ: Primary
gastrointestinal stromal tumor of the liver: One case report.
Zhonghua Yi Xue Za Zhi. 76:33112006.(In Chinese).
|
|
13
|
Hu ZQ, Wei YS, Zhu HH, Yang Z, Deng ZZ,
You M and Xiang Z: Huge malignant liver mesenchymal tumor: One case
report. Zhongguo Shi Yong Wai Ke Za Zhi. 27:4172007.(In
Chinese).
|
|
14
|
Luo XL, Liu D, Yang JJ, Zheng MW, Zhang J
and Zhou XD: Primary gastrointestinal stromal tumor of the liver: A
case report. World J Gastroenterol. 15:3704–3707. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ochiai T, Sonoyama T, Kikuchi S, et al:
Primary large gastrointestinal stromal tumor of the liver: Report
of a case. Surg Today. 39:633–636. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Y, Xing CP, Liu B, Su QJ and Dong L:
Diagnosis and differential diagnosis of primary liver
gastrointestinal stromal tumors. Modern Oncology. 18:2432–420.
2010.
|
|
17
|
Xue YJ and Hu XJ: Primary gastrointestinal
stromal tumor of the liver: One case report. Zhong Liu Xue Za Zhi.
19:159–160. 2013.(In Chinese).
|
|
18
|
Lu Y and Guo SL: One case: Primary
malignant stromal tumor of the liver. Shi Yong Fang She Xue Za Zhi.
29:1368–1369. 2013.(In Chinese).
|
|
19
|
Joensuu H: Risk stratification of patients
diagnosed with gastrointestinal stromal tumor. Hum Pathol.
39:1411–1419. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Min KW and Leabu M: Interstitial cells of
Cajal (ICC) and gastrointestinal stromal tumor (GIST): Facts,
speculations and myths. J Cell Mol Med. 10:995–1013. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wilkinson MJ, Fitzgerald JE, Strauss DC,
Hayes AJ, Thomas JM, Messiou C, Fisher C, Benson C, Tekkis PP and
Judson I: Surgical treatment of gastrointestinal stromal tumour of
the rectum in the era of imatinib. Br J Surg. 102:965–971. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
von Mehren M, Randall RL, Benjamin RS,
Boles S, Bui MM, Casper ES, Conrad EU III, DeLaney TF, Ganjoo KN,
George S, et al: Gastrointestinal stromal tumors, version 2.2014. J
Natl Compr Canc Netw. 12:853–862. 2014.PubMed/NCBI
|
|
23
|
Kobayashi K, Szklaruk J, Trent JC, Ensor
J, Ahrar K, Wallace MJ, Madoff DC, Murthy R, Hicks ME and Gupta S:
Hepatic arterial embolization and chemoembolization for
imatinib-resistant gastrointestinal stromal tumors. Am J Clin
Oncol. 32:574–581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jones RL, McCall J, Adam A, O'Donnell D,
Ashley S, Al-Muderis O, Thway K, Fisher C and Judson IR:
Radiofrequency ablation is a feasible therapeutic option in the
multi modality management of sarcoma. Eur J Surg Oncol. 36:477–482.
2010. View Article : Google Scholar : PubMed/NCBI
|