Introduction
Renal cell carcinoma (RCC), accounting for ~3% of
all malignancies, consists of a wide range of malignancies of
various histological subtypes that arise from the renal parenchyma
(1). The incidence of RCC is
increasing by a rate of ~2.5% each year (2). Despite recent improvements in surgical
and anticancer drugs, the prognosis of RCC remains poor. This is
largely attributed to a lack of complete understanding of the exact
underlying mechanisms that lead to this malignancy (3). Therefore, there is an urgent need to
improve our understanding of the molecular mechanisms underlying
the processes responsible for the development of RCC.
Reactive oxygen species (ROS), including superoxide
and hydrogen peroxide, are able to regulate cysteine-based
phosphatases, for example protein tyrosine phosphatases and lipid
phosphatases, and directly influence cell signaling pathways
(4). ROS are one of the major causes
of tumors and have a significant role in the process of tumor
progression, metastasis and apoptosis. Chronic and persistent
exposure to high ROS levels induces DNA, protein and lipid damage,
and may lead to cellular senescence and apoptosis (5). Therefore, targeting ROS is an important
therapeutic strategy for the treatment of cancer.
Artemisia asiatica has been utilized in
traditional Chinese medicine for centuries (6). Eupatilin (5,7-dihydroxy-3′,4′,
6-trimethoxyflavone) is one of the pharmacologically active
components found in A. asiatica (7). Previous studies have reported that it
has a variety of biological properties, including immune
regulation, anti-inflammatory, anti-ulcer and anti-oxidative
activity (8–11). Furthermore, there is increasing
evidence that eupatilin possesses anti-tumor effects. It has been
demonstrated that eupatilin inhibits the growth of human
endometrial cancer cells through G2/M phase cell cycle arrest via
the upregulation of p21 (12). In
addition, eupatilin also inhibited human gastric cancer cell growth
in a dose- and time-dependent manner, and induced apoptosis with a
concomitant increase in caspase-3 activity (7).
However, the role of eupatilin in RCC remains to be
elucidated. Therefore, the present study investigated the
biological effects and mechanisms underlying eupatilin action in
RCC cell apoptosis. In the present study, it was demonstrated that
eupatilin significantly induces renal cancer cell apoptosis. The
mechanisms of eupatilin's action included ROS accumulation, mitogen
activated protein kinase (MAPK) activation and phosphatidylinositol
3-kinase (PI3K)/AKT activation.
Materials and methods
Cell culture and reagents
The 786-O human RCC cell line was purchased from
American Type Culture Collection (Manassas, VA, USA). The cells
were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and
1% (v/v) penicillin-streptomycin (Sigma-Aldrich, St. Louis, MO,
USA) at 37°C in a humidified atmosphere containing 5%
CO2. Eupatilin was obtained from YuanYe Biotechnology
Co., Ltd., Shanghai, China.
Cell viability assay
Cell viability was assessed by cell counting kit
(CCK)-8 assay (Beyotime Institute of Biotechnology, Haimen, China).
Briefly, cells were seeded into 96-well plates at 5×103
cells per well and cultured for 24 h at 37°C to adhere. Following
treatment with various concentrations of eupatilin (10, 20 and 40
µM) for 72 h, 10 µl of CCK-8 reagent was added to the cells,
followed by incubation for 2 h at 37°C. Subsequently, the optical
density (OD) value was read at 450 nm using a Bio-Rad ELISA
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The viability rate of cells = (the OD values of treated groups /
the OD values of the control group) × 100%.
Nucleosome enzyme-linked immunosorbent
assay (ELISA) assay for detection of apoptosis
For apoptosis assays, 786-O cells at a density of
1×104 cells/well were seeded into 96-well plates at 37°C
overnight and treated with 40 µM eupatilin with or withour
N-acetyl-L-cysteine (NAC; 500 µM; Sigma-Aldrich) for 24 h. Cells
were subsequently harvested and treated with a Nucleosome ELISA kit
(catalog no., QIA25-1EA; Merck Millipore, Darmstadt, Germany),
according to the manufacturer's protocol.
Caspase-3 activity assay
Caspase-3 activity was determined using Caspase
3/caspase 7 Luminescent Assay kit (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. In
brief, cells were lysed using RIPA assay lysis buffer (Takara
Biotechnology Co., Ltd., Dalian, China), and proteins (20 µg) were
incubated with caspase-3 substrate DEVD-AFC (50 µg) at 37°C for 1–2
h. Samples were transferred into black bottom 96-well microplates
and read using a fluorescence plate reader (EMD Millipore,
Billerica, MA, USA). The non-cleaved (blue) and cleaved (green)
substrate emissions were 400 and 505 nm, respectively. Control
reactions were performed without protein in wells and by omitting
the substrate.
Measurement of cellular ROS
Intracellular ROS was measured by flow cytometry
using a Active Oxygen Species Assay kit (catalog no., K0111;
Beyotime Institute of Biotechnology). In brief, 786-O cells were
incubated with various concentrations of eupatilin (10, 20 and 40
µM) with or without NAC (500 µM) for 0.5, 1, 2 or 4 h.
Subsequently, the cells were washed twice with phosphate-buffered
saline (PBS), and incubated with 10 µM dichlorofluorescein
diacetate for 30 min at 37°C. The cells were subsequently
trypsinized and analyzed by the FACSCaliber flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA). Intracellular ROS levels
were expressed as the mean dichlorofluorescein fluorescence
intensity of the cells.
Measurement of glutathione
(GSH)/oxidized glutathione (GSSG) ratio
Total GSH was measured using
5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB) according to a
previously described method (13). In
brief, 786-O cells were cultured with or without various
concentrations of eupatilin (10, 20 and 40 µM) for 24 h at room
temperature. Subsequently, the cells were treated with 5%
5-sulfosalicylic acid for 30 sec. Following centrifugation (6,000 ×
g for 10 min at room temperature), the resultant extract was
assayed, and the GSSG concentration was obtained by quantifying the
reduction of DTNB due to its conversion to 5-thio-2-nitrobenzoic
acid at 412 nm.
Western blot analysis
Proteins were collected from 786-O cells treated
with various concentrations of eupatilin (10, 20 and 40 µM). Cells
were washed in PBS and lysed using RIPA assay lysis buffer. The
concentration of protein was measured by bicinchoninic acid assay
kit (Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Protein samples (40 µg) were subjected to
12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred to polyvinylidene difluoride (PVDF) membranes (EMD
Millipore). PVDF membranes were blocked with 5% bovine serum
albumin (Sigma-Aldrich) in PBS with tween, followed by incubation
with primary antibody at 4°C overnight. Detection of p38 (1:1,500;
catalog no., sc-81621), phosphorylated (p)-p38 (1:1,500; catalog
no., sc-7973), c Jun N terminal kinases (JNK; 1:1,000; catalog no.,
sc-7345), p-JNK (1:1,000; catalog no., sc-6254), extracellular
signal-regulated kinase 1/2 (ERK; 1:1,500; catalog no., sc-514302),
p-ERK (1:1,500; catalog no., sc-7383), PI3K (1:2,000; catalog no.,
sc-365290), AKT (1:2,000; catalog no., sc-5298) and β-actin
(1:1,500; catalog no., sc-21733) was performed using mouse
monoclonal antibodies from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA). p-PI3K (1:2,000; catalog no., sc-293115) and p-AKT
(1:2,000; catalog no., sc-135650) were detected using rabbit
monoclonal and rabbit polyclonal antibodies, respectively, (Santa
Cruz Biotechnology, Inc.). Subsequently, the blots were washed with
TBST and incubated with bovine anti-rabbit and rabbit anti-mouse
horseradish peroxidase-conjugated secondary antibodies (1:3,000;
catalog nos., sc-2379 and sc-358914, respectively, Santa Cruz
Biotechnology, Inc.) at room temperature for 2 h. An enhanced
chemiluminescence system was applied to visualize the blots
according to the manufacturer's protocol (Roche Applied Science,
Mannheim, Germany). The relative protein expression levels were
quantified using Image-Pro Plus version 6.0 software (Media
Cybernetics, Silver Spring, MD, USA) and normalized to β-actin.
Statistical analysis
Results are expressed as the mean ± standard
deviation. Comparisons between two groups were performed using the
Student's t-test and between multiple groups using analysis
of variance. SPSS version 13.0 software (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Eupatilin inhibits proliferation in
786-O cells
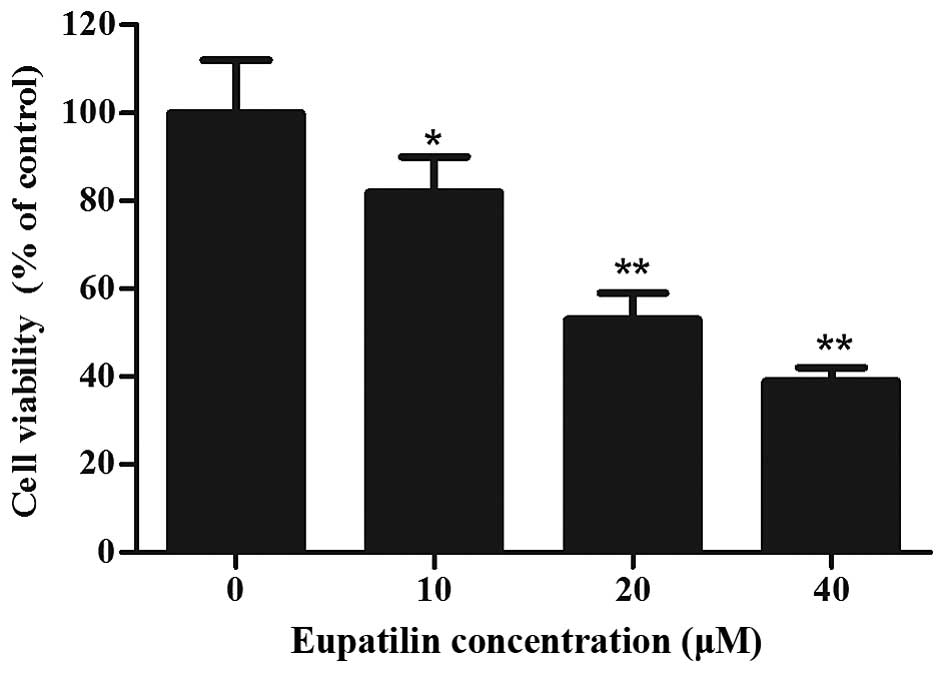
To investigate the cytotoxicity of eupatilin, 786-O
cells were treated with various concentrations of eupatilin for 72
h, followed by CCK-8 assay. As shown in Fig. 1, eupatilin significantly inhibited
cell viability in a concentration-dependent manner (P<0.05), and
the cell viability of 20 and 40 µM eupatilin-treated 786-O cells
was decreased by 47.2 and 61.3%, respectively. Due to the prominent
proliferation inhibition of 786-O cells, 40 µM of eupatilin was
used for the majority of the subsequent assays.
Eupatilin induces apoptosis in 786-O
cells
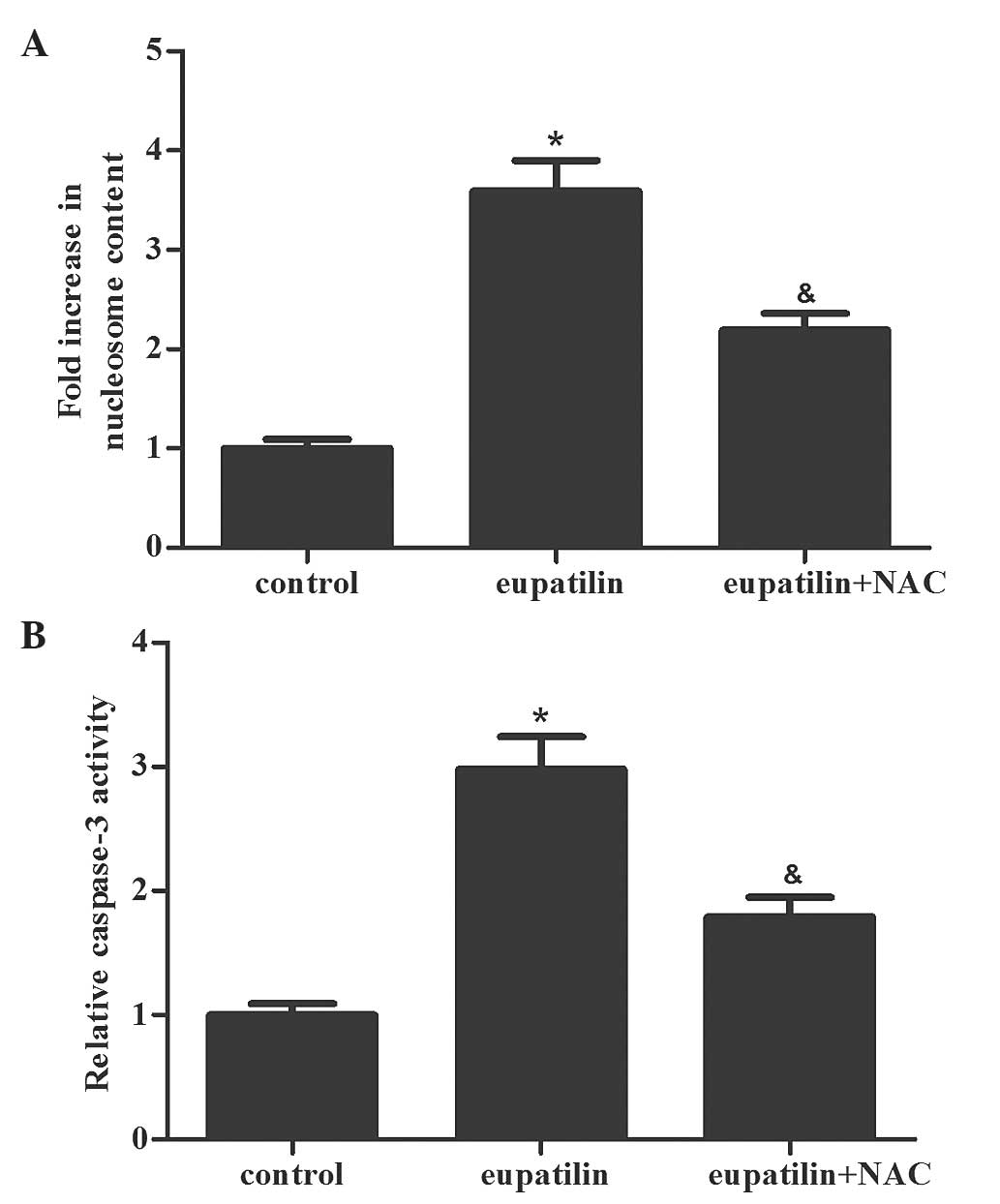
Subsequently. Nucleosome ELISA assays were performed
to measure the effect of eupatilin treatment on cell apoptosis. As
shown in Fig. 2A, the eupatilin
treatment group had an increased number of apoptotic cells compared
with the control group (P<0.05). In addition, the caspase-3
activity was determined in the various groups. The activity of
caspase-3 was markedly increased in the eupatilin treatment group
compared with the control group (Fig.
2B; P<0.05). The results of the present study indicated that
eupatilin induced apoptosis in 786-O cells.
Eupatilin induces oxidative stress in
786-O cells
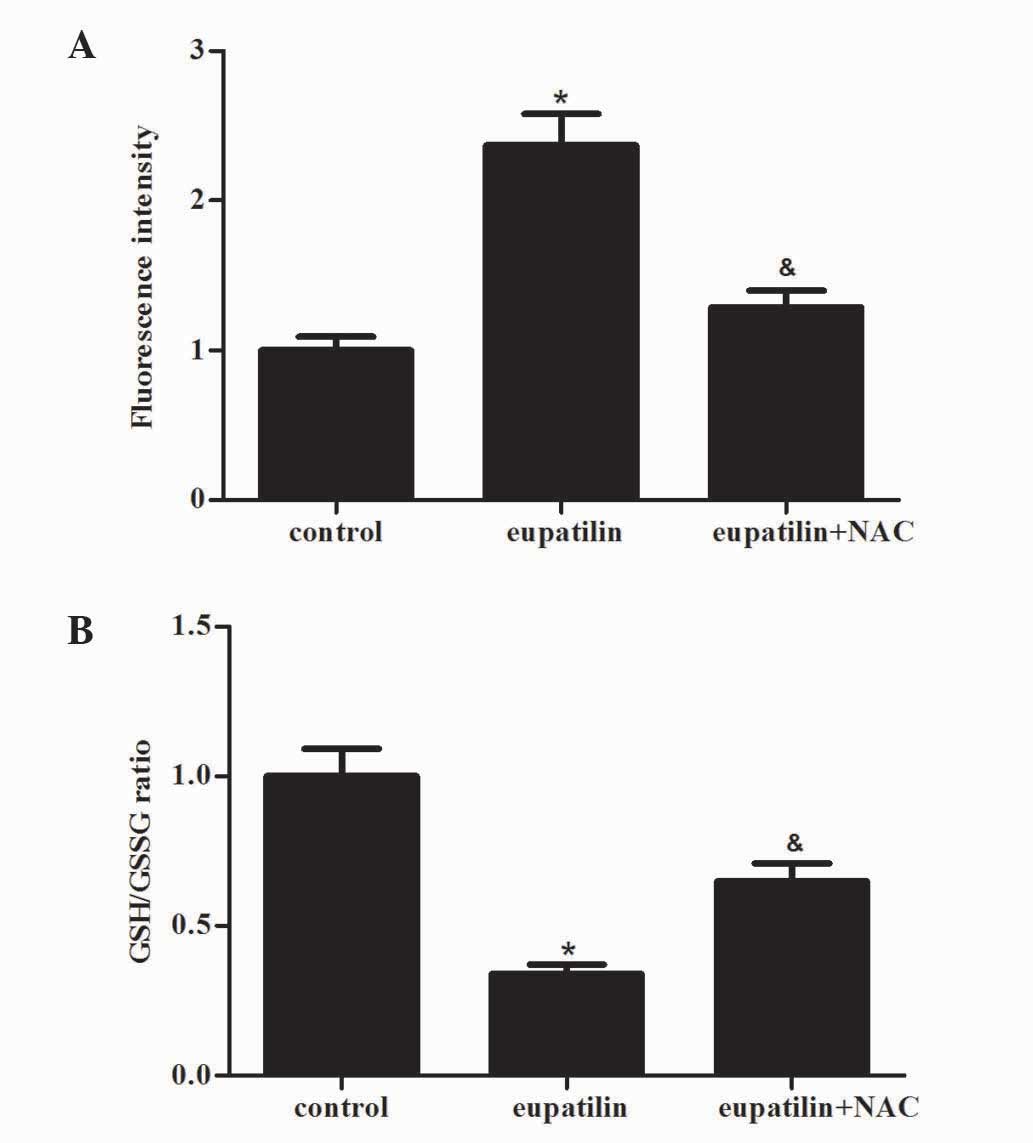
ROS have a significant role in the processes of
tumor progression, metastasis and apoptosis (14). Therefore, the present study
investigated the effect of eupatilin treatment on ROS production in
786-O cells. When 786-O cells were exposed to eupatilin for 24 h,
eupatilin significantly increased the levels of ROS in 786-O cells
compared with the control group (Fig.
3A; P<0.05). In addition, the GSH/GSSG ratio was markedly
decreased by eupatilin (Fig. 3B). The
antioxidant NAC (500 µM), a precursor of GSH, effectively prevented
eupatilin-induced ROS production and GSH/GSSG ratio reduction
(Fig. 3A and B).
Eupatilin activates ERK, JNK and p38
in 786-O cells
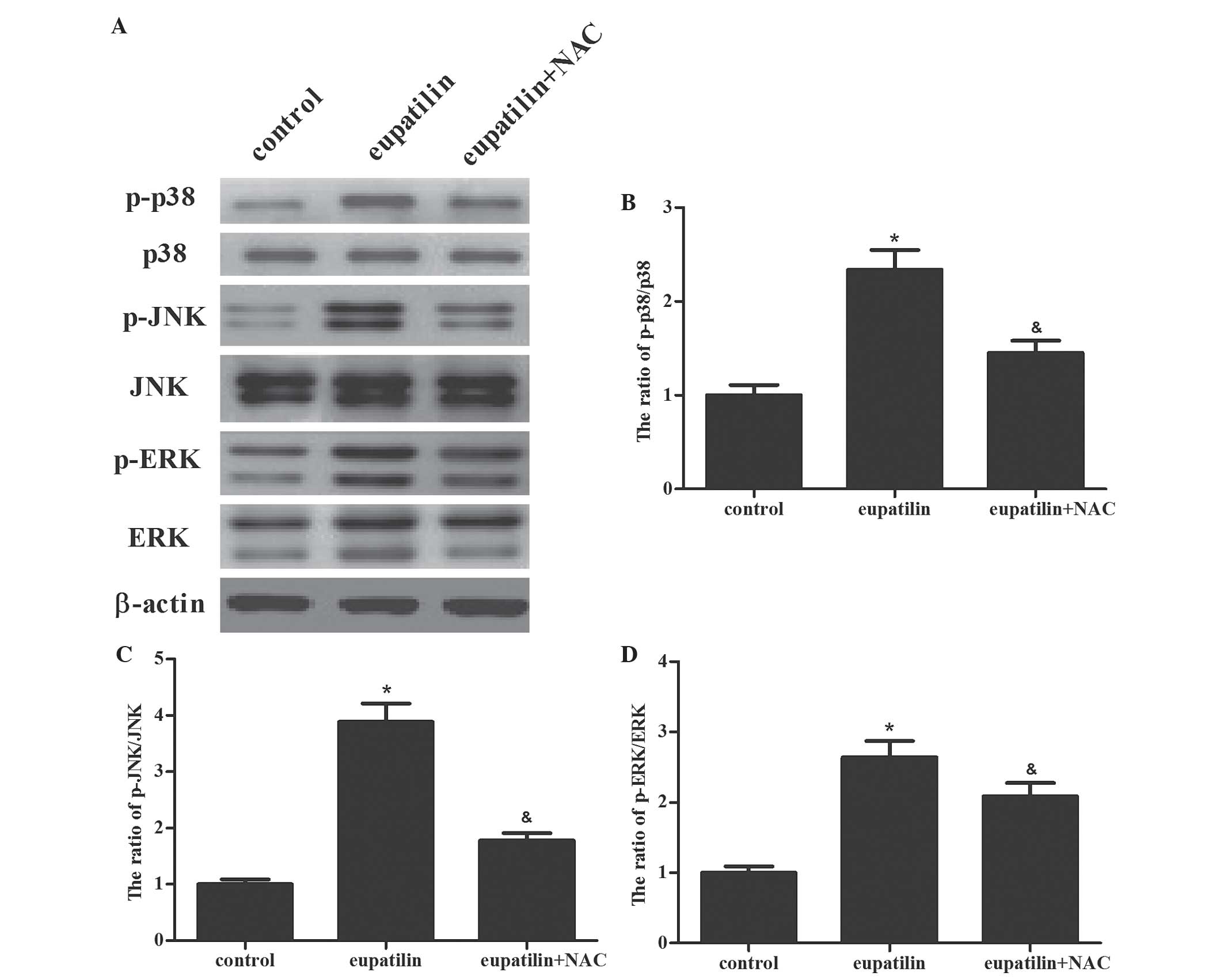
ROS have been demonstrated to be an inducer or
mediator of the activation of mitogen-activated protein kinase
(MAPK) family members, including JNK, p38 and ERK1/2 (15). In addition, the MAPK signaling pathway
has a significant role in the regulation of a number of cellular
processes, including cell growth and proliferation,
differentiation, and apoptosis (16).
Therefore, the present study investigated whether eupatilin was
able to induce the activation of MAPK cascades in 786-O cells. As
shown in Fig. 4, it was observed that
eupatilin induced phosphorylation of p38α (Thr180/Tyr182), ERK1/2
and JNK1/2 (Thr183/Tyr185) in 786-O cells. Subsequently, the role
of ROS in eupatilin-mediated ERK, JNK, and p38 MAPK activation was
investigated in 786-O cells. The results of the present study
demonstrated that pretreatment with NAC clearly prevented the
activation of the protein kinases induced by eupatilin
treatment.
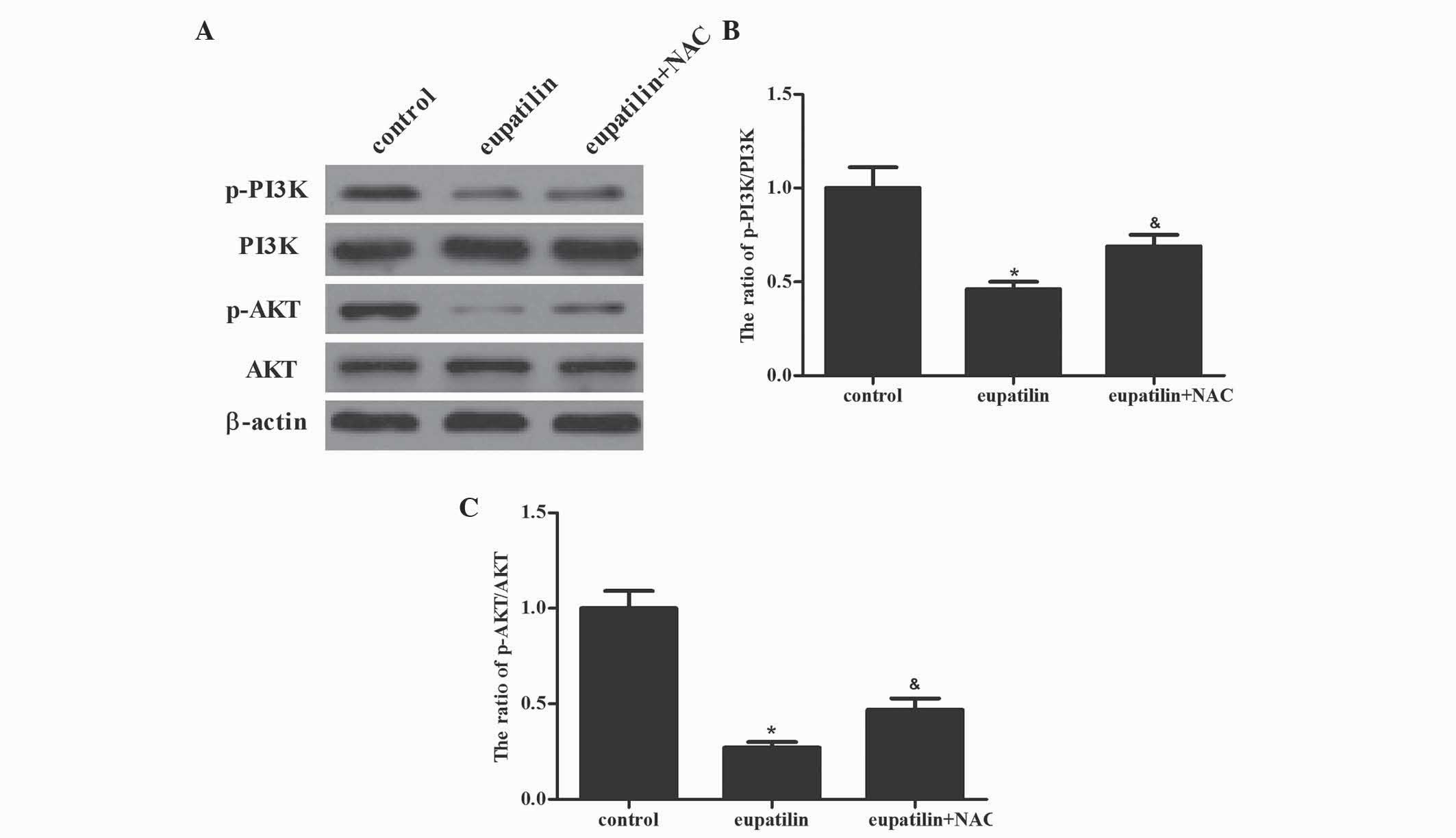
Eupatilin suppresses the PI3K/AKT
signaling cascade in 786-O cells
Besides MAPK signaling pathways, the PI3K/AKT signal
transduction pathway additionally has a critical role in cell
survival and the enhanced protection of cancer cells from apoptosis
during tumorigenesis (17). The
present study investigated whether eupatilin downregulated PI3K/AKT
activation in 786-O cells. Western blotting demonstrated that
eupatilin significantly suppressed the activation of PI3K and AKT.
Furthermore, pretreatment with NAC reversed the eupatilin-inhibited
activation of PI3K and AKT in 786-O cells (Fig. 5; P<0.05). The results of the
present study indicated that the inhibition of the PI3K/AKT
signaling cascade by eupatilin led to the suppression of cell
proliferation in 786-O cells.
Discussion
Previous studies have demonstrated that eupatilin
inhibits cell proliferation, migration and invasion, and promotes
apoptosis in various cancer cells (7,12). In the
present study, it was demonstrated that eupatilin treatment
significantly induced 786-O cell apoptosis. The anticancer effect
of eupatilin depends on enhanced ROS in 786-O cells. ROS-dependent
MAPK activation and inhibition of PI3K/AKT contribute to
eupatilin's effects on RCC cell apoptosis.
ROS are primarily produced in mitochondria as a
product of normal cellular metabolism of oxygen, and function as
significant molecules for mediating normal physiological signaling
(18). In addition, elevated ROS
production followed by oxidative stress is involved in the
initiation and promotion of multistep carcinogenesis (19). Apoptosis has been recognized as one of
the most important biological features of RCC progression, and the
execution of apoptosis requires the accurate coordination of the
caspase protein family (20).
Caspase-3, also known as the death enzyme, plays a critical role in
the controlled execution of programmed cell death (21). The results of the present study reveal
that eupatilin significantly inhibited RCC cell proliferation, and
induced RCC apoptosis, as well as the activity of caspase-3. In
addition, eupatilin treatment dramatically induced the production
of ROS and decreased the ratio of GSH/GSSG in 786-O cells. The
present study subsequently investigated whether ROS was involved in
eupatilin-induced apoptosis. As expected, pretreatment with NAC
clearly prevented eupatilin-induced apoptosis, as evidenced by
decreased caspase-3 cleavage. The results of the present study
suggested that ROS has a significant role in eupatilin-induced
apoptosis in RCC cells.
MAPKs, including ERK1/2, JNK and p38, are a family
of serine/threonine kinases that regulate a variety of cellular
events, including proliferation and apoptosis (16,22,23). A
previous study demonstrated that various stress stimuli, including
the oxidative stress caused by ROS, may induce potential activation
of MAPK signaling pathways (24). In
addition, the activation of JNK contributes to stress-induced
apoptosis (25). p38 MAPK has been
demonstrated to either promote apoptosis or enhance cell survival
depending on the cell type and stimulus (26,27). The
PI3K/AKT signaling pathway is one of the major signaling pathways
associated with RCC progression (28–30). It is
involved in propagation of signals from various cell membrane
receptor tyrosine kinases into the nucleus, and regulates various
cellular processes, including cell proliferation, differentiation
and apoptosis (31). In numerous
cancer tissues and cells, the PI3K/AKT signaling pathway is
overactive, reducing apoptosis and allowing proliferation (32). ROS have been demonstrated to be
associated with apoptosis induced by anticancer drugs and may be
upstream of MAPK and PI3K/AKT signaling pathways (33). Hao et al (34) reported that licochalcone A, a
flavonoid extracted from licorice root, inhibited cell
proliferation and induced gastric cell apoptosis via modulation of
ROS-mediated MAPKs and PI3K/AKT signaling pathways. The results of
the present study demonstrate that eupatilin induces
phosphorylation of p38α (Thr180/Tyr182), ERK1/2 and JNK1/2
(Thr183/Tyr185), and decreases the phosphorylation of PI3K and AKT
in 786-O cells in a concentration-dependent manner. It was also
observed that the ROS inhibitor NAC was able to rescue the MAPK
activation, PI3K/AKT inhibition and apoptosis induced by inhibition
of eupatilin. The results of the present study suggest that
eupatilin induces ROS-mediated MAPK activation, inhibits the
PI3K/AKT signaling pathway and leads to RCC cell apoptosis.
In conclusion, the results of the present study
provide evidence that inhibition of eupatilin induces apoptosis in
human RCC through ROS-mediated activation of the MAPK signaling
pathway and inhibition of the PI3K/AKT signaling pathway.
Therefore, eupatilin may serve as a potential therapeutic agent for
the treatment of human RCC. Xenograft nude mice may be employed in
the future to examine the role of eupatilin in tumorigenesis of
RCC.
References
|
1
|
Larkin J, Goh XY, Vetter M, Pickering L
and Swanton C: Epigenetic regulation in RCC: Opportunities for
therapeutic intervention? Nat Rev Urol. 9:147–155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Penticuff JC and Kyprianou N: Therapeutic
challenges in renal cell carcimoma. Am J Clin Exp Urol. 3:77–90.
2015.PubMed/NCBI
|
|
4
|
Salmeen A and Barford D: Functions and
mechanisms of redox regulation of cysteine-based phosphatases.
Antioxid Redox Signal. 7:560–577. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Waris G and Ahsan H: Reactive oxygen
species: Role in the development of cancer and various chronic
conditions. J Carcinog. 5:142006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jang HJ, Jeong EK, Kim SS, Lee JH, Oh MY,
Kang KS, Kwan HC, Song KI, Eom DW and Han DJ: Protective effect of
Artemisia asiatica extract against renal ischemia-reperfusion
injury in mice. Exp Clin Transplant Suppl. 1:377–82. 2015.
|
|
7
|
Park BB, Yoon JS, Kim ES, Choi J, Won YW,
Choi JH and Lee YY: Inhibitory effects of eupatilin on tumor
invasion of human gastric cancer MKN-1 cells. Tumor Biol.
34:875–885. 2013. View Article : Google Scholar
|
|
8
|
Kim YD, Choi SC, Oh TY, Chun JS and Jun
CD: Eupatilin inhibits T-cell activation by modulation of
intracellular calcium flux and NF-kappaB and NF-AT activity. J Cell
Biochem. 108:225–236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi EJ, Lee S, Chae JR, Lee HS, Jun CD
and Kim SH: Eupatilin inhibits lipopolysaccharide-induced
expression of inflammatory mediators in macrophages. Life Sci.
88:1121–1126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoon KD, Chin YW, Yang MH and Kim J:
Separation of anti-ulcer flavonoids from Artemisia extracts by
high-speed countercurrent chromatography. Food Chem. 129:679–683.
2011. View Article : Google Scholar
|
|
11
|
Choi EJ, Oh HM, Na BR, Ramesh TP, Lee HJ,
Choi CS, Choi SC, Oh TY, Choi SJ, Chae JR, et al: Eupatilin
protects gastric epithelial cells from oxidative damage and
down-regulates genes responsible for the cellular oxidative stress.
Pharm Res. 25:1355–1364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho JH, Lee JG, Yang YI, Kim JH, Ahn JH,
Baek NI, Lee KT and Choi JH: Eupatilin, a dietary flavonoid,
induces G2/M cell cycle arrest in human endometrial cancer cells.
Food Chem Toxicol. 49:1737–1744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin Y, Yang J, Lin L, Lin Y and Zheng C:
The attenuation of Scutellariae radix extract on oxidative stress
for colon injury in lipopolysaccharide-induced RAW264.7 cell and
2,4,6-trinitrobenzene sulfonic acid-induced ulcerative colitis
rats. Pharmacogn Mag. 12:153–159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiong Y, Ye T, Wang M, Xia Y, Wang N, Song
X, Wang F, Liu L, Zhu Y, Yang F, et al: A novel cinnamide YLT26
induces breast cancer cells apoptosis via ROS-mitochondrial
apoptotic pathway in vitro and inhibits lung metastasis in vivo.
Cell Physiol Biochem. 34:1863–1876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi EK, Yeo JS, Park CY, Na HI, Lim JA,
Lee JE, Hong SW, Park SS, Lim DG and Kwak KH: Inhibition of
reactive oxygen species downregulates the MAPK pathway in rat
spinal cord after limb ischemia reperfusion injury. Int J Surg.
22:74–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang D, Ding Y, Luo WM, Bender S, Qian
CN, Kort E, Zhang ZF, VandenBeldt K, Duesbery NS, Resau JH and Teh
BT: Inhibition of MAPK kinase signaling pathways suppressed renal
cell carcinoma growth and angiogenesis in vivo. Cancer Res.
68:81–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo H, German P, Bai S, Barnes S, Guo W,
Qi X, Lou H, Liang J, Jonasch E, Mills GB and Ding Z: The PI3K/AKT
pathway and renal cell carcinoma. J Genet Genomics. 42:343–353.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thannickal VJ and Fanburg BL: Reactive
oxygen species in cell signaling. Am J Physiol Lung Cell Mol
Physiol. 279:L1005–L1028. 2000.PubMed/NCBI
|
|
19
|
Essick EE and Sam F: Oxidative stress and
autophagy in cardiac disease, neurological disorders, aging and
cancer. Oxid Med Cell Longev. 3:168–177. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J and Yuan J: Caspases in apoptosis and
beyond. Oncogene. 27:6194–6206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tawa P, Hell K, Giroux A, Grimm E, Han Y,
Nicholson DW and Xanthoudakis S: Catalytic activity of caspase-3 is
required for its degradation: Stabilization of the active complex
by synthetic inhibitors. Cell Death Differ. 11:439–447. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ballif BA and Blenis J: Molecular
mechanisms mediating mammalian mitogen-activated protein kinase
(MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ.
12:397–408. 2001.PubMed/NCBI
|
|
24
|
Liu J, Chang F, Li F, Fu H, Wang J, Zhang
S, Zhao J and Yin D: Palmitate promotes autophagy and apoptosis
through ROS-dependent JNK and p38 MAPK. Biochem Biophys Res Commun.
463:262–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ki YW, Park JH, Lee JE, Shin IC and Koh
HC: JNK and p38 MAPK regulate oxidative stress and the inflammatory
response in chlorpyrifos-induced apoptosis. Toxicol Lett.
218:235–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Porras A, Zuluaga S, Black E, Valladares
A, Alvarez AM, Ambrosino C, Benito M and Nebreda AR: P38 alpha
mitogen-activated protein kinase sensitizes cells to apoptosis
induced by different stimuli. Mol Biol Cell. 15:922–933. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park EJ, Park SW, Kim HJ, Kwak JH, Lee DU
and Chang KC: Dehydrocostuslactone inhibits LPS-induced
inflammation by p38MAPK-dependent induction of hemeoxygenase-1 in
vitro and improves survival of mice in CLP-induced sepsis in vivo.
Int Immunopharmacol. 22:332–340. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Porta C and Figlin RA:
Phosphatidylinositol-3-kinase/Akt signaling pathway and kidney
cancer, and the therapeutic potential of
phosphatidylinositol-3-kinase/Akt inhibitors. J Urol.
182:2569–2577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Banumathy G and Cairns P: Signaling
pathways in renal cell carcinoma. Cancer Biol Ther. 10:658–664.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sourbier C, Lindner V, Lang H, Agouni A,
Schordan E, Danilin S, Rothhut S, Jacqmin D, Helwig JJ and
Massfelder T: The phosphoinositide 3-kinase/Akt pathway: A new
target in human renal cell carcinoma therapy. Cancer Res.
66:5130–5142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brzezianska E and Pastuszak-Lewandoska D:
A minireview: The role of MAPK/ERK and PI3K/Akt pathways in thyroid
follicular cell-derived neoplasm. Fronti Biosci (Landmark Ed).
16:422–439. 2011. View
Article : Google Scholar
|
|
32
|
Shi Y, Song Q, Hu D, Zhuang X, Yu S and
Teng D: Oleanolic acid induced autophagic cell death in
hepatocellular carcinoma cells via PI3K/Akt/mTOR and ROS-dependent
pathway. Korean J Physiol Pharmacol. 20:237–243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mi Y, Xiao C, Du Q, Wu W, Qi G and Liu X:
Momordin Ic couples apoptosis with autophagy in human
hepatoblastoma cancer cells by reactive oxygen species
(ROS)-mediated PI3K/Akt and MAPK signaling pathways. Free Radic
Biol Med. 90:230–422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hao W, Yuan X, Yu L, Gao C, Sun X, Wang D
and Zheng Q: Licochalcone A-induced human gastric cancer BGC-823
cells apoptosis by regulating ROS-mediated MAPKs and PI3K/AKT
signaling pathways. Sci Rep. 5:103362015. View Article : Google Scholar : PubMed/NCBI
|