Introduction
Granulocytic sarcoma (GS) is a rare, extramedullary
malignant neoplasm consisting of myeloid cells with different
levels of maturation and occurring in anatomic sites other than the
bone marrow or peripheral blood (1).
It represents a distinct entity of AML (2).
The high expression of myeloperoxidase (MPO) makes
these tumors green, hence their alternative name, ‘chloroma’ (from
the Greek word ‘chloros’, meaning green). However, ‘sarcoma’ is the
most commonly used term, as ~30% of these tumors do not contain
MPO, despite the fact that MPO together with cluster of
differentiation (CD)117 represents the marker for myeloid
differentiation (1).
GS may develop de novo (solitary, primary or
non-leukemic GS; 8–20%) (3),
simultaneously to AML (2.5–9.1%) (4),
or as a relapse of leukemia, particularly following allogeneic
hematopoietic stem cell transplant (AHSCT) (4,5). The
reason for this association is still unknown, but may be attributed
to a pattern of graft-versus-leukemia surveillance or to the
biology of high-risk AML treated with transplantation (5).
GS may affect patients of all ages (median age, 56
years; range, 1 month to 89 years), with a male:female ratio 1.2:1
(6). Commonly involved sites include
subperiosteal bone, lymph nodes and skin. The prevalence of head
and neck region involvement is 12–43% of cases (5), and orbit, skull and epidural spaces are
the most frequently involved sites (7). Rare lesions have been described in the
maxilla, soft palate, paranasal sinus, salivary gland, scalp and
temporal bone, whereas only a few cases have been described with
rhinopharyngeal involvement (4).
GS risk factors include specific chromosomal
abnormalities [t(8;21) and inv(16)],
expression of cell-surface markers (CD56, CD2, CD4 and CD7), and
M2, M4 and M5 leukemia subtypes of the French-American-British
classification (4). Additional risk
factors include poor nutritional status, cellular immune
dysfunction, high presenting leukocyte count and decreased blast
Auer rods (4).
Sinonasal congestion and/or hearing loss are the
most common clinical manifestations of rhinopharyngeal GS (4).
The diagnosis of the rhinopharyngeal GS,
particularly when it is poorly differentiated or without
concomitant marrow involvement, is challenging (8), and it is not uncommon for GS to be
misdiagnosed as lymphoma (8,9). To improve the accuracy of diagnosis,
immunohistochemical patterns play a key role. For instance, myeloid
cells are reactive to antibodies against lysozyme, MPO and
chloroacetate esterase. Furthermore, GS myeloblasts typically
express myeloid-associated antigens, such as CD43, but are not
reactive to lymphoid antigens. In addition, flow cytometry and
cytogenetic analysis may aid in determining a definitive diagnosis
(8).
GS is sensitive to focal irradiation and to systemic
chemotherapy, similarly to AML (7,10,11). Systemic treatment should always be
considered due to the high rate of recurrence and progression to
AML (11). Surgery may be a
therapeutic option only for tumors, which cause organ dysfunction
(11). The role of radiotherapy and
AHSCT as a consolidation regimen remains to be clearly established
(10,11).
GS has an unfavorable prognosis. Its course is rapid
with a high mortality rate, particularly when associated with AML,
whereas patients without evidence of leukemia have a better
prognosis (9); cases initially
diagnosed as solitary GS without evidence of leukemia and treated
with systemic chemotherapy have a more favorable prognosis
(9).
The current study reports the case of a 53-year-old
woman who presented with a rhinopharyngeal mass. The mass was
diagnosed as an isolated extramedullary GS as a relapse of AML,
which had been treated 7 years earlier with chemotherapy and AHSCT
and was followed by complete remission. In addition, a review of
the literature is reported, along with the examination of
immunohistochemical features as a tool for the differential
diagnosis of GS compared to other rare tumors of the
rhinopharynx.
Case report
A 53-year-old female non-smoker presented to the
Ear, Nose and Throat Unit of ‘Federico II’ University of Naples
(Naples, Italy) complaining of left otalgy, hearing loss and nasal
obstruction for ~5 months. The patient had a history of AML, which
was in complete remission following high-dose chemotherapy and
AHSCT, administered 7 years earlier.
A nasal endoscopy revealed a left rhinopharyngeal
peritubaric mass obstructing the left Eustachian tube. The
audiological evaluation revealed left conductive hearing loss due
to an ipsilateral middle ear effusion. No lateral cervical palpable
lymph nodes were found. Magnetic resonance imaging and positron
emission tomography-computed tomography (CT) examinations revealed
a mass of the rhinopharynx (6×3.5 cm; standardized uptake value,
5.7) without bone erosion.
Laboratory studies, including a normal complete
blood count, unremarkable serum chemistry and normal liver enzyme
levels, did not reveal any alteration. No evidence of increased
blast cell count was observed in the bone marrow aspiration
sample.
The specimen was formalin fixed and paraffin
embedded. Immunohistochemistry was performed using the avidin
biotin complex as a visualization system and 3,3′-diaminobenzidine
as chromogen for the reaction, with pre-diluted antibodies
(dilution, 1:100) against B-cell lymphoma-2 (Bcl-2; 790–4604, clone
SP66; rabbit; Ventana Medical Systems, Inc., Tucson, AZ, USA), CD3
(790–4341; clone 2GV6; rabbit; Ventana Medical Systems, Inc.), CD5
(790–4451; clone SP19; rabbit; Ventana Medical Systems, Inc.), CD10
(790–4506; clone SP67; rabbit; Ventana Medical Systems, Inc.), CD20
(760–2531; clone L26; mouse; Ventana Medical Systems, Inc.), CD34
(790–2927; clone QBEnd/10; mouse; Ventana Medical Systems, Inc.),
CD43 (760–2511; clone L60; mouse; Ventana Medical Systems, Inc.),
CD56 (790–4465; clone 123C3; mouse; Ventana Medical Systems, Inc.),
CD99 (790–4452; clone O13; mouse; Ventana Medical Systems, Inc.),
CD117 (790–2951; clone 9.7; rabbit; Ventana Medical Systems, Inc.),
Ki67 (M724029; clone MIB1; mouse; Dako, Glostrup, Denmark) and MPO
(760–2659; rabbit polyclonal; Ventana Medical Systems, Inc.). The
patient underwent biopsy of the rhinopharyngeal lesion, which
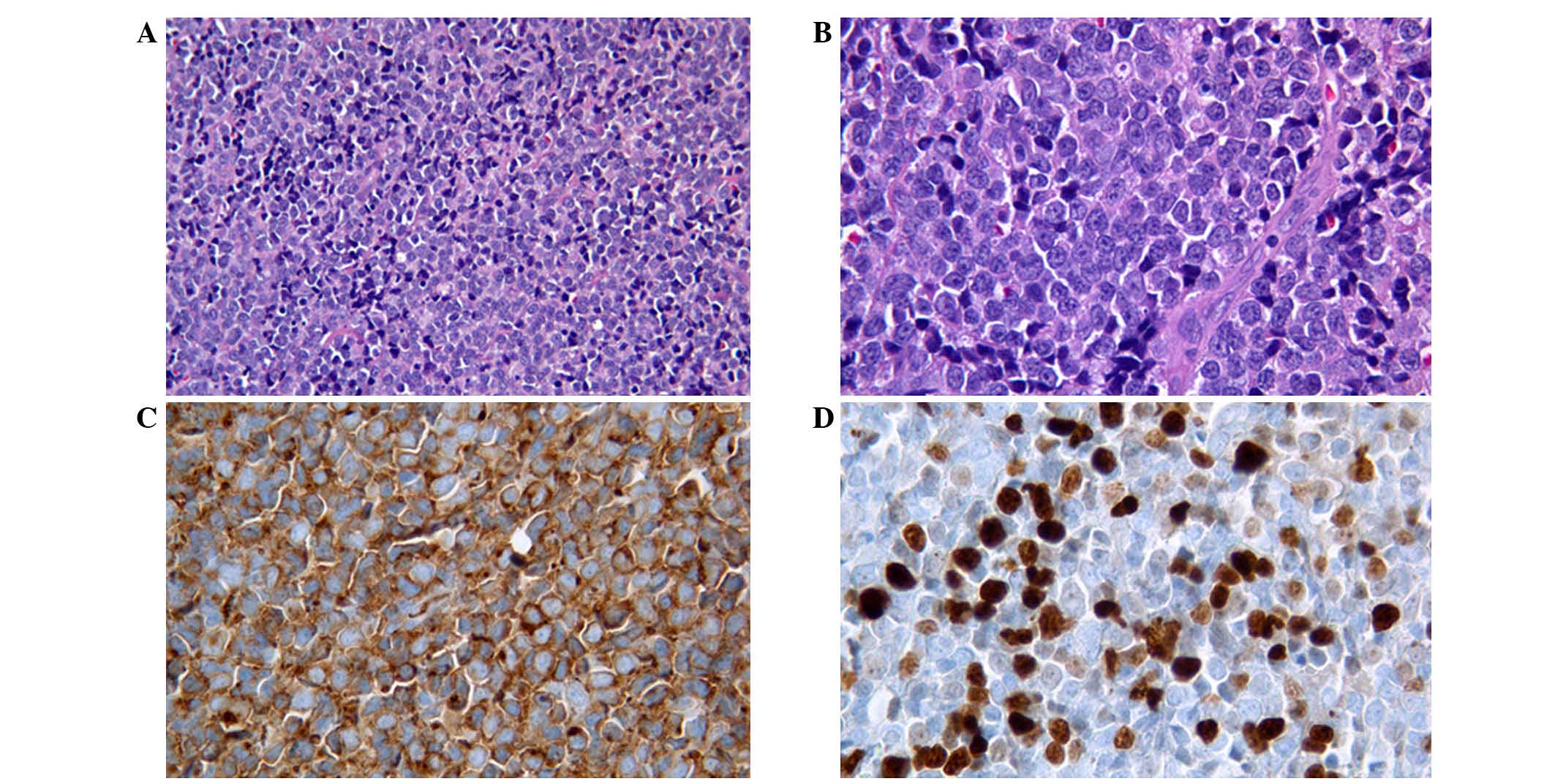
revealed neoplastic proliferation of medium- and small-sized cells.
These cells exhibited inconspicuous cytoplasm, nuclei with
irregular membranes, occasionally a small nucleolus, diffuse
karyorrhexis and high mitotic activity (Fig. 1).
The immunohistochemical evaluation revealed strong
reactivity for CD43, CD34 and CD99, whereas CD20, CD3, CD5, CD10,
Bcl-2, MPO, CD117 and CD56 were all negative. The Ki-67
proliferative index was ~40%. Fluorescence in situ
hybridization (FISH) analysis was performed on 4-µm-thick
formalin-fixed, paraffin-embedded (FFPE) tissue sections, using the
Vysis LSI Dual Color probe (Abbott Molecular, Inc., Des Plaines,
IL, USA) specific for runt-related transcription factor 1 (RUNX1)
labeled with Spectrum Green and for RUNX1T1 labeled with Spectrum
Orange. The slides were hybridized overnight according to the
manufacturer's protocol. Image analysis was then conducted. The
FISH analysis of FFPE tumor slides did not identify either the
t(8;21) translocation or MLL gene dissociation.
The patient's medical history and immunophenotype
suggested the presence of a poorly differentiated extramedullary GS
of the rhinopharynx as a relapse of AML, without bone marrow
disease.
The patient was treated with conventional induction
AML therapy: Combined idarubicin (12 mg/m2/day, days
1–2) and cytarabine (200 mg/m2/day, days 1–7), followed
by one course of consolidation therapy with idarubicin (12
mg/m2, day 1) and cytarabine (1 g/m2/12 h,
days 1–5), as well as radiation treatment (1,500 cGy in 5
fractions).
A CT scan performed at 1 month after
chemoradiotherapy revealed complete resolution of the
rhinopharyngeal mass. At the 3-year follow-up, the patient was
asymptomatic and without signs of recurrence. At present, the
patient is under a regular surveillance protocol; she is closely
monitored through a multidisciplinary ‘short time’ follow-up
protocol, consisting of nasal endoscopy and blood studies every 3
months.
Written informed consent was obtained from the
patient for publication of this case report and the accompanying
images.
Discussion
GS, also known as ‘chloroma’ or ‘extramedullary
myeloblastoma’, is a rare solid tumor consisting of primitive
precursors of the granulocytic series of white blood cells, which
include myeloblasts, promyelocytes, and myelocytes (1). Rhinopharyngeal localization of GS is
extremely rare; only a few cases have been previously reported in
the literature (4), and the present
study reports the 15th case (Table
I).
 | Table I.Case reports of GS involving the
nasopharynx. |
Table I.
Case reports of GS involving the
nasopharynx.
| Author | Age,
years/gender | Site/symptoms | Associated
diagnosis | Cytogenetic
findings |
Treatment/outcome | Ref. |
|---|
| Vishnu et
al | 63/F | Conductive hearing
loss; nasopharyngeal mass | Developed AML 1 year
later | Normal | Radiation therapy
only for GS; chemotherapy for AML; CR at 18 months | (4) |
| Raphael et
al | 73/M | Left maxillary sinus,
posterior ethmoidal cells | Synchronous MDS | t(3;21) | Chemotherapy; AML
after 3 months; mortality during chemotherapy. | (10) |
| Bassichis et
al | 1/M | Masseter muscle | Synchronous AML | NR | Mortality during
chemotherapy | (16) |
| Au et al | 37/M | Conductive hearing
loss; nasopharyngeal mass | Solitary GS | Normal | IFRT+chemotherapy; CR
at 3 years | (17) |
| Nayak et
al | 24/F | Bilateral parotid and
nasopharyngeal mass | Solitary GS | NR | Mortality during
chemotherapy | (18) |
| Geisse et
al | 60/M | Waldeyer's ring
lymphadenopathy | Synchronous MDS | NR | Diagnosis made on
autopsy | (19) |
| Prades et
al | 20/F | Sinonasal
obstruction; right maxillary and sphenoid sinus mass | GS of the nasal
cavity and paranasal sinus | t(19;1) | Chemotherapy+AHSCT;
CR at 18 months | (20) |
| Ozcelik et
al | 37/M | Vocal cord paralysis;
involvement of 9th, 10th and 12th cranial nerves | AML 6 months earlier,
treated with chemotherapy to CR | NR | Mortality during
chemotherapy | (21) |
| Sugimoto et
al | 31/F | Nasopharyngeal
mass | AML 3 months earlier,
treated with chemotherapy to CR | t(8;21) | CR2 achieved with
IFRT, reinduction chemotherapy+AHSCT | (22) |
| Imamura et
al | 7/F | Waldeyer's ring
lymphadenopathy | Synchronous JMML | t(9;12) | Chemotherapy+AHSCT;
CR at 3 years | (23) |
| Ferri et
al | 72/F | Right facial swelling
and fever; maxillo-ethmoidal mass | AML 1 year earlier,
treated with hydroxyurea | NR | Best supportive care
only; mortality after 10 days of hospitalization | (24) |
| Teramoto et
al | 81/F | Nasopharyngeal
mass | Developed AML 1
year later | Complex genomic
defects on cDNA microarray | Radiation therapy
only for GS; chemotherapy for AML; mortality 6 months after
diagnosis of AML | (25) |
| Selvarajan et
al | 25/M | Dysphagia,
hoarseness, facial nerve palsy | AML 4 years
earlier, treated with chemotherapy to CR followed by AHSCT | t(8;21) | Chemotherapy;
systemic relapse 1 year later | (26) |
| Cho et
al | 18/M | Conductive hearing
loss; nasopharyngeal mass | Synchronous
AML | t(8;21) | Recurrence after 7
months of chemotherapy; CR2 achieved with reinduction
chemotherapy+AHSCT | (27) |
| Mei et
al | 56/F | Left maxillary
sinus | Solitary site of
GS | NR | Surgical resection
and chemotherapy; CR at 4 months | (28) |
| Current case | 53/F | Conductive hearing
loss; nasopharyngeal mass | AML 7 years
earlier, treated with chemotherapy to CR followed by AHSCT | Normal | Chemoradiaton
therapy; no sign of recurrence at 1 year |
GS has a high rate of misdiagnosis (46%) (12,13), and
its differential diagnosis may include non-Hodgkin's lymphoma
(lymphoblastic, Burkitt and diffuse large B-cell lymphomas),
lymphoblastic leukemia, melanoma, Ewing's sarcoma, primitive
neuroectodermal tumor, rhabdomyosarcoma, neuroblastoma,
medulloblastoma, undifferentiated carcinoma, blastic plasmacytoid
dendritic cell neoplasm, extramedullary hematopoiesis (13) and small undifferentiated round cell
tumors (6,14).
Previous studies have demonstrated that
immunophenotype is of utmost importance in determining the
diagnosis of GS (3–5). In particular, the literature has focused
on the CD13 and CD68 markers for granular monocytic and macrophagic
cells, MPO and CD117 markers for myeloid differentiation, lysozyme
marker for monocytic lineage, CD43 marker for myeloid cells as well
as T cells and B precursors, and CD34 and terminal deoxynucleotidyl
transferase (TdT) markers for immature cells (10). Immunohistochemical detection of
intracellular MPO, a major constituent of primary granules of
neutrophilic myeloid cells, confirms a diagnosis of GS. However,
while MPO is expressed in the majority of GSs, some minimally
differentiated and monocytic GSs do not express it (8).
CD68-KP1 is the most commonly expressed marker,
followed by MPO, CD117, CD99, CD68/PG M1, lysozyme, CD34, TdT,
CD56, CD61/linker of activated T lymphocyte/factor VIII-related
antigen, CD30, glycophorin A and CD4 (3). Rarely, aberrant antigenic expression is
observed (such as cytokeratins, B- or T-cell markers) (3).
In the current case, immunohistochemical evaluation
revealed strong reactivity for CD43, CD34 and CD99, whereas MPO was
negative. In particular, the presence of CD99 made it difficult to
differentiate GS from other CD99-positive round cell tumors
(14), whereas the positivity for
CD34 (15) and the negativity for MPO
indicated the presence of a mass of immature cells. In the present
case, the clinical history of previous AML was the key to the
specific diagnosis of GS.
From a therapeutic perspective, data from the
literature suggest that GSs are extremely sensitive to focal
irradiation or chemotherapy; however, their role is not well
defined (13,16–28). The
optimal treatment for the GS-AML association remains uncertain.
However, high-dose chemotherapy and stem cell transplantation may
be the treatment of choice (6).
The risk of metachronous AML in non-leukemic
patients with GS is very high, with a median delay of 5 months; the
majority of patients may develop AML within 1 year. Therefore,
early intensive (induction/intensification) chemotherapy similar to
that used to treat AML must be administered, even in GS patients
without AML upon initial diagnosis (6).
Byrd et al stated that 97% of all primary GS
patients who did not receive systemic chemotherapy developed AML
(12). Furthermore, 66% of patients
who received chemotherapy for the primary GS did not develop AML,
suggesting that early systemic therapy is helpful in preventing
AML, which increases the overall survival time (6).
Literature data demonstrated that clinical behavior
and response to therapy are not influenced by any of the following
factors: Age, gender, anatomic site, de novo presentation,
histotype, phenotype or cytogenetic findings (6).
In the current case, given the previous history of
AML and according to data in the literature, the patient was
treated with conventional induction AML therapy, followed by
radiotherapy. This choice, so far, has proved to be a successful
strategy, since the patient has shown no signs of relapse after
3-year follow-up.
In conclusion, the current study highlights certain
interesting features of GS. Firstly, the negative reactivity for
MPO in the current case suggested the diagnosis of a poorly
differentiated GS with a poor prognosis. Thus, the patient was
assigned to a multidisciplinary protocol of close follow-up for the
high risk of relapse. Secondly, since the rhinopharynx is involved
in a variety of malignant neoplasms, immunohistochemistry is
required for the diagnosis of GS, particularly for the
undifferentiated forms, as in the present case. Indeed, it is not
uncommon for GS to be misdiagnosed as lymphoma. Finally, a
combination of detailed clinical, radiographic and serological
work-ups, in association with a thorough histological assessment,
is essential to establish the correct diagnosis (29).
References
|
1
|
Guermazi A, Feger C, Rousselot P, Merad M,
Benchaib N, Bourrie P, Mariette X, Frija J and de Kerviler E:
Granulocytic sarcoma (chloroma): Imaging findings in adults and
children. AJR Am J Roentgenol. 178:319–325. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vardiman JW, Thiele J, Arber DA, Brunning
RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM,
Hellström-Lindberg E, Tefferi A and Bloomfield CD: The 2008
revision of the World Health Organization (WHO) classification of
myeloid neoplasms and acute leukemia: Rationale and important
changes. Blood. 114:937–951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Campidelli C, Agostinelli C, Stitson R and
Pileri SA: Myeloid sarcoma: Extramedullary manifestation of myeloid
disorders. Am J Clin Pathol. 132:426–437. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vishnu P, Chuda RR, Hwang DG and Aboulafia
DM: Isolated granulocytic sarcoma of the nasopharynx: A case report
and review of the literature. Int Med Case Rep J. 7:1–6. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alrumaih R, Saleem M, Velagapudi S and
Dababo MA: Lateral pharyngeal wall myeloid sarcoma as a relapse of
acute biphenotypic leukemia: A case report and review of the
literature. J Med Case Rep. 7:2922013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Messager M, Amielh D, Chevallier C and
Mariette C: Isolated granulocytic sarcoma of the pancreas: A tricky
diagnostic for primary pancreatic extramedullary acute myeloid
leukemia. World J Surg Oncol. 10:132012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Noh BW, Park SW, Chun JE, Kim JH, Kim HJ
and Lim MK: Granulocytic sarcoma in the head and neck: CT and MR
imaging findings. Clin Exp Otorhinolaryngol. 2:66–71. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Papamanthos MK, Kolokotronis AE, Skulakis
HE, Fericean AA, Zorba MT and Matiakis AT: Acute myeloid leukaemia
diagnosed by intra-oral myeloid sarcoma. A Case Report. Head Neck
Pathol. 4:132–135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hwang JI and Kim TY: Primary granulocytic
sarcoma of the face. Ann Dermatol. 23(Suppl 2): S214–S217. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raphael J, Valent A, Hanna C, Auger N,
Casiraghi O, Ribrag V, De Botton S and Saada V: Myeloid Sarcoma of
the nasopharynx mimicking an aggressive lymphoma. Head Neck Pathol.
8:234–238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yilmaz AF, Saydam G, Sahin F and Baran Y:
Granulocytic sarcoma: A systematic review. Am J Blood Res.
3:265–270. 2013.PubMed/NCBI
|
|
12
|
Byrd JC, Edenfield WJ, Shields DJ and
Dawson NA: Extramedullary myeloid cell tumors in acute
nonlymphocytic leukemia: A clinical review. J Clin Oncol.
13:1800–1816. 1995.PubMed/NCBI
|
|
13
|
Vachhani P and Bose P: Isolated gastric
myeloid sarcoma: A case report and review of the literature. Case
Rep Hematol. 2014:5418072014.PubMed/NCBI
|
|
14
|
Casiraghi O and Lefèvre M:
Undifferentiated malignant round cell tumors of the sinonasal tract
and nasopharynx. Ann Pathol. 29:296–312. 2009.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng Y, Jia M, Chen Y, Zhao H, Luo Z and
Tang Y: Re-evaluation of various molecular targets located on
CD34+CD38-Lin- leukemia stem cells and other cell subsets in
pediatric acute myeloid leukemia. Oncol Lett. 11:891–897.
2016.PubMed/NCBI
|
|
16
|
Bassichis B, McClay J and Wiatrak B:
Chloroma of the masseteric muscle. Int J Pediatr Otorhinolaryngol.
53:57–61. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Au WY, Kwong YL, Ho WK and Shek TW:
Primary granulocytic sarcoma of the nasopharynx. Am J Hematol.
67:273–274. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nayak DR, Balakrishnan R, Raj G, Pillai S,
Rao L and Manohar C: Granulocytic sarcoma of the head and neck: A
case report. Am J Otolaryngol. 22:80–83. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Geisse M, Mall G, Fritze D and
Gartenschläger M: Granulocytic sarcoma of the tonsils associated
with myelodysplastic syndrome. Dtsch Med Wochenschr. 127:2673–2676.
2002.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Prades JM, Alaani A, Mosnier JF, Dumollard
JM and Martin C: Granulocytic sarcoma of the nasal cavity.
Rhinology. 40:159–161. 2002.PubMed/NCBI
|
|
21
|
Ozçelik T, Ali R, Ozkalemkaş F, Ozkocaman
V, Coşkun H, Erişen L and Filiz G: A case of granulocytic sarcoma
during complete remission of acute myeloid leukemia with multiple
masses involving the larynx and nasopharynx. Kulak Burun Bogaz
Ihtis Derg. 11:183–188. 2003.PubMed/NCBI
|
|
22
|
Sugimoto Y, Nishii K, Sakakura M, Araki H,
Usui E, Lorenzo VF, Hoshino N, Miyashita H, Ohishi K, Katayama N
and Shiku H: Acute myeloid leukemia with t(8;21)(q22;q22)
manifesting as granulocytic sarcomas in the rhinopharynx and
external acoustic meatus at relapse after high-dose cytarabine:
Case report and review of the literature. Hematol J. 5:84–89. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Imamura T, Matsuo S, Yoshihara T,
Chiyonobu T, Mori K, Ishida H, Nishimura Y, Kasubuchi Y, Naya M,
Morimoto A, et al: Granulocytic sarcoma presenting with severe
adenopathy (cervical lymph nodes, tonsils, and adenoids) in a child
with juvenile myelomonocytic leukemia and successful treatment with
allogeneic bone marrow transplantation. Int J Hematol. 80:186–189.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferri E, Minotto C, Ianniello F, Cavaleri
S, Armato E and Capuzzo P: Maxillo-ethmoidal chloroma in acute
myeloid leukaemia: Case report. Acta Otorhinolaryngol Ital.
25:195–199. 2005.PubMed/NCBI
|
|
25
|
Teramoto H, Miwa H, Patel V, Letwin N,
Castellone MD, Imai N, Shikami M, Imamura A, Gutkind JS, Nitta M
and Lee NH: Gene expression changes in a patient presenting
nonleukaemic nasal granulocytic sarcoma to acute myelogenous
leukaemia using 40 K cDNA microarray. Clin Lab Haematol.
28:262–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Selvarajan S, Subramanian S, Thulkar S and
Kumar L: Granulocytic sarcoma of nasopharynx with perineural spread
along the trigeminal nerve. Neurol India. 56:210–212. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cho SF, Liu YC, Tsai HJ and Lin SF:
Myeloid sarcoma mimicking nasopharyngeal carcinoma. J Clin Oncol.
29:e706–e708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mei KD, Lin YS and Chang SL: Myeloid
sarcoma of the cheek and the maxillary sinus regions. J Chin Med
Assoc. 76:235–238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cantone E, Marino A, Ferranti I and Iengo
M: Nonallergic rhinitis in the elderly: A reliable and safe
therapeutic approach. ORL J Otorhinolaryngol Relat Spec. 77:117–22.
2015. View Article : Google Scholar : PubMed/NCBI
|