Introduction
According to statistical data from 2012, 238,719 new
cases of ovarian cancer are diagnosed each year, while the
mortality rate exceeds 150,000 patients per year (1). Ovarian cancer is responsible for 4% of
all morbidities in females; however, it is associated with the
highest mortality rate of all gynecological morbidities in
developed countries (1,2). Due to poor clinical manifestations in
the early stages of the disease, it is usually diagnosed at a late
stage in which prognosis becomes extremely unfavorable. Of the
various prognostic factors that are associated with ovarian cancer,
clinical stage remains the most important (2). In recent years, it has been demonstrated
that the hospital at which the patient is diagnosed and treated is
of great importance; a correct and early diagnosis, appropriate
preparation for a major surgical procedure, and the procedure
itself being performed by an experienced gynecological surgeon who
operates on a sufficiently large number of ovarian tumors each year
are key factors (3). Therefore, there
is a requirement for diagnostic tools that can allow the primary
care physicians, including family doctors and gynecologists in
outpatient practices, to perform simple and rapid preliminary
diagnostic examinations on patients with suspected ovarian cancer
so as to refer these patients to appropriate referral centers.
At present, several algorithms are available for the
stratification of risk in patients with ovarian tumors. The most
popular of these include the Risk of Malignancy Index (RMI)
(4–6),
OVA1 (7–9), LR2 (a logistic regression model)
(10) and Risk of Ovarian Malignancy
Algorithm (ROMA) (11–15). As RMI and LR2 require expertise in
gynecological ultrasonography, they cannot be used by family
physicians or gynecologists with poor knowledge of ultrasonographic
diagnostic techniques. OVA1, which was recently approved by the
Food and Drug Administration (FDA), is relatively expensive as it
requires as many as five laboratory assays (10). ROMA appears to be the most simple
diagnostic tool, while also being appropriately sensitive and
specific. ROMA requires only the determination of carbohydrate
antigen 125 (CA125) and human epididymis protein 4 (HE4) marker
levels in the serum and knowledge of the patient's hormonal status
in order to calculate the percentage chance of a diagnosis of
ovarian cancer in a patient with an adnexal tumor (12). Levels of CA125 and of the recently
introduced HE4 may be assessed using various laboratory assays
(11–15). Currently, four main combinations of
the most popular laboratory assays are used in calculating ROMA
values: Chemiluminescent microparticle immunoassays (CMIAs) for
CA125 and for HE4; CMIA for CA125 and EIA for HE4; CanAg EIA for
CA125 and EIA for HE4; and finally, electrochemiluminescence
immunoassays (ECLIAs) for CA125 and for HE4.
The objective of the current study was to assess the
diagnostic usefulness of ROMA in patients with ovarian cancer as
compared with benign ovarian lesions and other gynecological
malignancies, making use of a novel combination of known laboratory
assays: CMIA for determination of CA125 levels and ECLIA for
determination of HE4 levels. In addition, the usefulness of ROMA in
patients subjected to prophylactic adnexectomy due to BRCA1
gene mutation carrier status was assessed.
Materials and methods
Patients
The study was conducted on 619 patients undergoing
surgical treatment in the Department of Gynecological Surgery and
Gynecological Oncology of Adults and Adolescents, Pomeranian
Medical University (Szczecin, Poland) between November 2012 and
December 2014. Of these, 354 women were premenopausal and the
remaining 265 were postmenopausal. Patients who qualified for the
study were those presenting at the clinic due to pathological
lesions (tumors or cysts) within the adnexa, suspected ovarian
cancer, other gynecological malignancies (except endometrial
cancer) or BRCA1 gene mutation. After signing the informed
consent form, all patients were subjected to blood collection
procedures on the day of their hospital admission. Determinations
of HE4 and CA125 marker levels were performed on the same day at
the hospital's central laboratory. Due to use of laboratory assays
that had not been previously tested for usefulness in the
calculation of ROMA values, ROMA values were not calculated
preoperatively and the respective results were not taken into
account in everyday clinical practice. Additional diagnostic value
was provided preoperatively only by the separate results of CA125
and HE4 assessments. Following histopathological assessment,
patients were assigned into individual groups and subgroups, and
ROMA values were calculated for each group and subgroup on the
basis of standard mathematical formulas.
The inclusion criteria were as follows: i) ≥18 years
of age; ii) presence of an ovarian cyst, ovarian tumor, tumor of
the pelvis minor, ascites or persistent elevated CA125 levels; iii)
consent to participate in the study; iv) availability of the final
histopathological result. The following exclusion criteria were
also applied: i) Renal diseases; ii) lung diseases; iii) creatinine
levels of >1.3 mg/dl; iv) no consent to participate in the
study.
Based on the inclusion and exclusion criteria, as
well as on the result of histopathological examination, the
patients were divided into the following groups: i) Ovarian cancer;
ii) benign gynecological disorders of the adnexa; iii) other
gynecological malignancies (excluding endometrial cancers) and
metastases into ovaries; iv) epithelial ovarian tumors of
borderline malignancy; v) BRCA1 mutation carriers. Detailed
information regarding the division of the study population into
groups and subgroups is presented in Table I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Parameter | Ovarian
cancers | Benign
diseases | Other cancers | Borderline
tumors | BRCA1
mutations |
|---|
| Total, n | 162 | 342 | 33 | 23 | 59 |
| Age, years |
|
|
Mean | 59.7 | 40.7 | 57.5 | 46.3 | 47.6 |
|
Range | 24–90 | 18–89 | 27–87 | 19–83 | 34–64 |
| Hormonal status,
n |
|
|
Premenopausal | 38 | 256 | 10 | 11 | 39 |
|
Postmenopausal | 124 | 86 | 23 | 12 | 20 |
| Ovarian cancer
histopathology, n (%) |
|
|
Serous | 132 (81.5) | – | – | 16 (69.6) | – |
|
Mucinous | 9
(5.6) | – | – | 6
(26.1) | – |
| Clear
cell | 8
(4.9) | – | – | 0 (0.0) | – |
|
Endometrioid | 13 (8.0) | – | – | 1 (4.3) | – |
| Ovarian cancer FIGO
stage, n (%) |
|
| I and
II | 54
(33.3) | – | – | 22 (95.7) | – |
| III and
IV | 108 (66.7) | – | – | 1 (4.3) | – |
| Ovarian cancer
grade, n (%) |
|
| 1 | 34 (21.0) | – | – | – | – |
| 2 | 54 (33.3) | – | – | – | – |
| 3 | 74 (45.7) | – | – | – | – |
| Benign tumor
histopathology, n (%) |
|
|
Endometriosis | – | 121 (35.4) | – | – | – |
|
Teratoma | – | 44
(12.9) | – | – | – |
| Serous
cystadenoma | – | 22 (6.4) | – | – | – |
|
Mucinous cystadenoma | – | 25 (7.3) | – | – | – |
|
Cystadenofibroma | – | 21 (6.1) | – | – | – |
|
Follicular cysts | – | 36
(10.5) | – | – | – |
|
Paraovarian cysts | – | 26 (7.6) | – | – | – |
|
Hemorrhagic cysts | – | 30 (8.8) | – | – | – |
|
Inflammatory tumors | – | 13 (3.8) | – | – | – |
|
Cirrhosis | – | 4
(1.2) | – | – | – |
Comparative analysis of groups and appropriate
subgroups was conducted with regard to the serum HE4 and CA125
levels as well as the calculated ROMA values. Sensitivity,
specificity, positive predictive value (PPV) and negative
predictive value (NPV) were calculated for each marker, with
cut-off values of 35 U/ml for CA125, and 70 or 140 pmol/l (for
premenopausal or postmenopausal patients, respectively) for HE4, as
previously determined (11,12). Cut-off points for ROMA values were
determined by the DeLong method (16). The diagnostic usefulness of each
marker was assessed by means of the area under curve the receiver
operating characteristic curve (ROC-AUC).
Laboratory methods
The HE4 serum levels of the marker were determined
using the Roche Elecsys® assay (Roche Diagnostics,
Basel, Switzerland) on a Cobas e601 apparatus. This is a one-step
sandwich ECLIA for quantitative determination of HE4. The detection
range for HE4 was 15.0–1,500 pmol/l; in case of values >1,500
pmol/l, the samples were diluted in a 1:20 ratio using Elecsys
Diluent.
The serum CA125 levels were determined using the
ARCHITECT CA125 II assay on an ARCHITECT 2200SR System (Abbott
Diagnostics, Abbott Park, IL, USA). This is a two-step immunoassay
to determine the presence of CA125 antigen using CMIA technology.
CA125 and HE4 assays were conducted according to the manufacturer's
instructions, with appropriate controls testing within the normal
ranges (http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4403b1-03%20ARCHITECT%20CA125II%20Package%20I
nsert%20.pdf).
The predictive index (PI) of ROMA was calculated
separately for premenopausal and postmenopausal patients using the
following formulas: Premenopausal PI = −12.0 + 2.38 × ln[HE4] +
0.0626 × ln[CA125]; Postmenopausal PI = −12.0 + 1.04 × ln[HE4] +
0.732 × ln[CA125]; where ln is the natural logarithm. The CA125
levels wer in IU/ml and the HE4 levels were in pmol/l. Final ROMA
values were calculated by inserting the obtained PI into the
following formula: % ROMA = exp(PI) / [1-exp(PI)] × 100.
Statistical analysis
Descriptive characteristics of the examined
population of patients were prepared, including the minimum,
maximum, mean and median values. Additionally, the scatter diagrams
of the empirical values of markers were plotted for individual
study groups. The mean/median values in individual groups and
subgroups were compared using the non-parametric Mann-Whitney U
test.
A contingency table was used in the assessment of
the diagnostic usefulness of CA125 and HE4 assays and ROMA values,
and subsequent calculation of the following parameters: Sensitivity
= TP/(TP+FN); specificity = TN/(FP+TN); PPV = TP/(TP+FP); and NPV =
TN/(FN+TN); where TP is the number of true positives, FN is the
number of false negatives, TN is the number of true negatives and
FP is the number of false positives.
Diagnostic performance was assessed using receiver
operating characteristic (ROC) curves based on continuous
variables. HE4, CA125, and ROMA represented diagnostic variables
acting as stimulants which increase the probability of ovarian
cancer proportionally to their rising value. The area under the
curve (AUC), standard error, and confidence interval values for AUC
were calculated according to the non-parametric method of DeLong
(16). This method was used to
compare AUCs considering the fact that measurements of HE4, CA125
and ROMA were performed for the same objects (groups of patients).
The level of significance was set as P<0.05.
Results
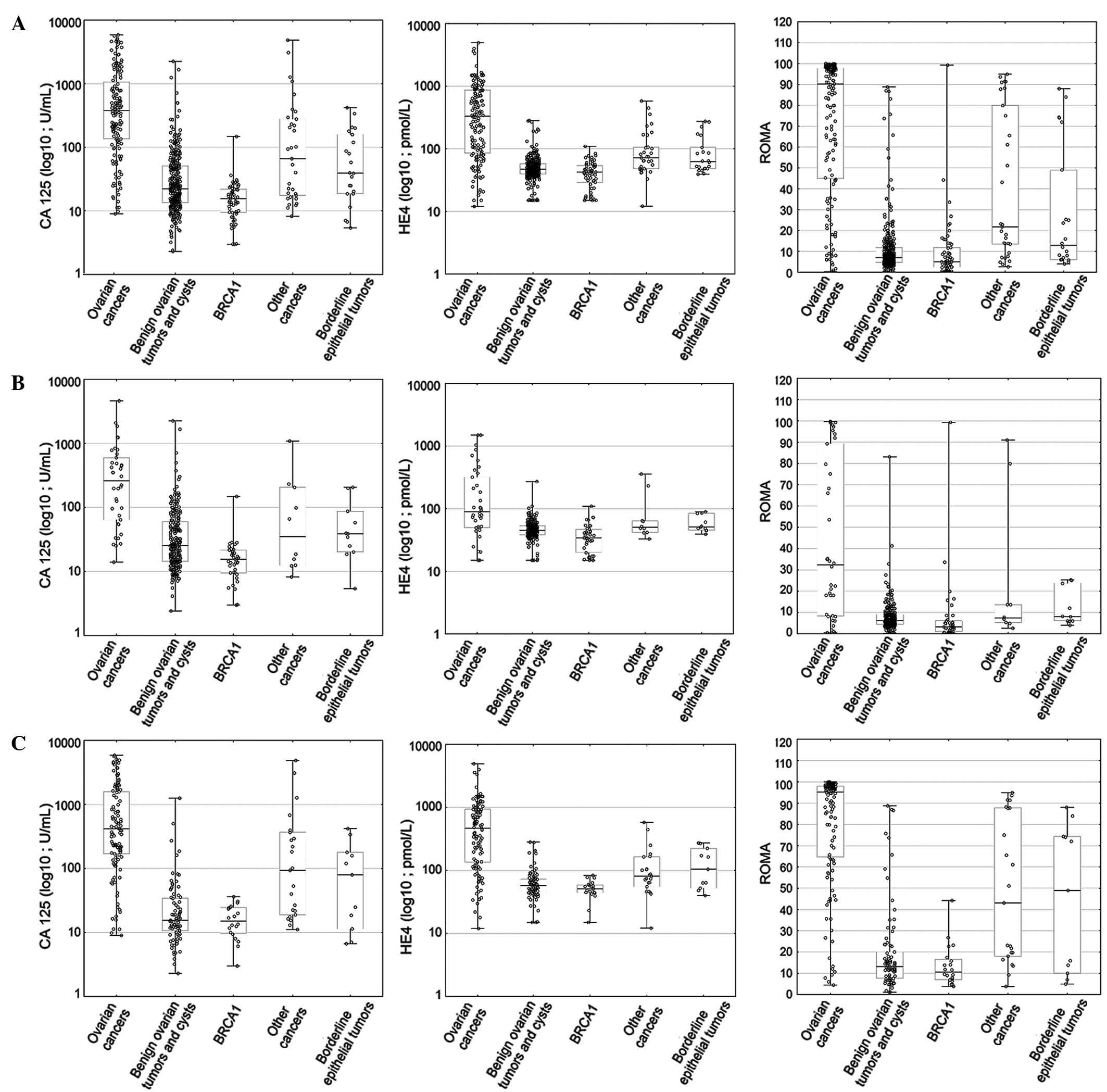
Serum levels and comparisons of
biomarkers (HE4 and CA125) and ROMA algorithm in different
gynecological conditions
Table II lists the
median values as well as the appropriate ranges for HE4, CA125 and
ROMA values in ovarian cancer patients (with consideration given to
the histopathological type, stage and differentiation of cancer),
epithelial borderline tumors, patients with other gynecological
cancers and patients with metastatic ovarian tumors, as well as in
patients with benign ovarian lesions according to different
histopathological diagnoses. Hormonal status of patients was taken
into consideration when presenting the results. When analyzing all
patients (regardless of their menopausal status), the median HE4,
CA125 and ROMA values in ovarian cancer patients (HE4, 333.35
pmol/l; CA125, 380.35 U/ml; ROMA, 90.15%) were found to be
significantly higher than in patients with benign ovarian lesions
and BRCA1 mutation (HE4, 46.8 pmol/l, P<0.001; CA125, 20.6 U/ml,
P<0.001; ROMA, 6.9%, P<0.001), patients with other
gynecological cancers/ovarian metastases (HE4, 71.8 pmol/l,
P<0.001; CA125, 66.2 U/ml, P<0.001; ROMA, 21.7%, P<0.001)
and patients with borderline ovarian tumors (HE4, 63.5 pmol/l,
P<0.001; CA125, 39.25 U/ml, P<0.001; ROMA, 12.9%,
P<0.001). All comparisons in postmenopausal patients were
similar to the above, with HE4, CA125 and ROMA values being
significantly higher in ovarian cancer patients than in patients
with benign gynecological lesions, epithelial borderline tumors,
other cancers and metastatic tumors. By contrast, the behavior of
serum levels of HE4 and CA125 and ROMA values in premenopausal
patients is somewhat different: When comparing the ovarian cancer
group with the benign lesion group, the marker levels and ROMA
values remain significantly higher in the former; however, when
comparing the ovarian cancer group with the group of other
gynecological cancers or metastatic tumors, significant differences
can be observed only with regard to CA125 (P=0.0153) and ROMA
(P=0.0496). Additionally, in cases of borderline tumors, CA125 and
ROMA values perform better as biomarkers than HE4 (P=0.0016,
P=0.0154 and P=0.0508, respectively).
 | Table II.Serum CA125, HE4 and ROMA levels
according to histology, FIGO stage and tumor grade. |
Table II.
Serum CA125, HE4 and ROMA levels
according to histology, FIGO stage and tumor grade.
|
| Premenopausal | Postmenopausal |
|---|
|
|
|
|
|---|
|
| CA125 (U/ml) | HE4 (pmol/l) | ROMA (%) | CA125 (U/ml) | HE4 (pmol/l) | ROMA (%) |
|---|
|
|
|
|
|
|
|
|
|---|
|
| Median | Range | Median | Range | Median | Range | Median | Range | Median | Range | Median | Range |
|---|
| Ovarian cancer
(all) |
265.4 |
14.0–4,638.8 |
89.5 |
15.0–1,500.0 | 32.3 | 3.2–99.7 | 416.9 |
9.0–5,887.0 | 470.3 |
15.0–4,940.0 | 95.2 |
4.5–100.0 |
|
Serous |
352.4 |
14.0–4,638.8 |
99.7 |
15.0–1,500.0 | 35.2 | 2.0–99.7 | 425.0 |
9.0–5,887.0 | 555.9 |
18.0–4,940.0 | 96.3 |
4.5–100.0 |
|
Mucinous |
27.0 | 26.4–37.9 |
51.9 | 45.4–75.6 |
8.4 | 6.2–18.0 |
87.2 | 11.3–600.0 |
76.4 |
15.0–538.0 | 52.9 |
7.8–95.8 |
| Clear
cell |
146.3 |
95.8–196.8 |
75.8 |
49.4–102.1 | 21.1 | 8.1–34.1 | 787.5 |
96.4–1,725.5 | 276.9 |
76.0–849.9 | 94.2 | 44.2–98.2 |
|
Endometrioid |
500.0 | 500.0 |
64.5 |
64.5 | 21.0 | 21.0 | 448.8 |
41.5–2,996.9 | 390.0 |
46.1–1,235.0 | 94.8 | 26.7–99.3 |
| FIGO stage |
|
| I and
II |
95.8 |
14.0–1,252.0 |
64.5 |
15.0–211.6 | 18.0 | 2.5–75.1 | 174.9 |
9.0–2,347.0 | 108.8 |
15.0–1,235.0 | 56.8 |
4.5–99.3 |
| III and
IV |
543.5 |
64.3–4,638.8 | 343.3 |
20.7–1,500.0 | 90.7 | 9.0–99.7 | 540.0 |
18.0–5,887.0 | 639.0 |
62.8–4,940.0 | 96.9 |
25.0–100.0 |
| Grade |
|
| 1 |
69.7 |
25.3–459.7 |
47.7 |
15.0–414.3 |
7.2 | 2.5–93.9 |
66.5 |
9.0–245.0 |
86.4 |
18.0–283.5 | 47.1 |
4.5–85.9 |
| 2 |
389.1 |
14.0–2,096.0 |
94.1 |
20.7–1,500.0 | 44.5 | 8.4–99.7 | 415.9 |
41.5–5,659.0 | 385.3 |
35.0–3,608.0 | 94.6 | 26.7–99.0 |
| 3 |
350.8 |
54.3–4,638.8 | 211.6 |
20.4–1,500.0 | 75.1 | 2.0–99.7 | 559.1 |
11.2–5,109.8 | 639.0 |
30.0–4,001.0 | 97.0 | 12.0–100 |
| Borderline
tumors |
38.9 |
5.4–206.3 |
51.0 | 39.2–89.0 |
8.0 | 4.0–25.3 |
79.9 |
6.7–421.2 | 103.8 |
40.1–274.2 | 49.0 |
5.0–88.0 |
| Other cancers and
metastatic ovarian tumors |
42.4 |
8.2–1,090.7 |
50.4 |
32.9–356.6 |
7.4 | 2.6–91.0 |
93.7 |
11.1–4,855.4 |
81.6 |
15.0–580.1 | 43.1 |
8.0–94.9 |
| Benign diseases
(all) |
22.7 |
2.4–2,257.0 |
44.2 |
15.0–269.8 |
5.8 | 2.0–99.3 |
15.6 |
2.3–1,255.0 |
56.4 |
15.0–282.6 | 12.9 |
1.1–88.8 |
|
Endometriosis |
46.8 |
8.8–377.0 |
45.2 | 17.8–86.7 |
6.4 | 1.8–24.1 |
18.9 | 6.7–82.1 |
47.2 | 27.5–73.0 | 11.4 |
1.8–35.3 |
|
Teratoma tumors |
15.9 |
7.7–51.9 |
44.4 | 26.3–69.1 |
5.8 | 1.7–14.7 |
15.3 | 6.3–18.2 |
49.1 | 45.7–72.4 | 11.2 |
6.7–17.9 |
|
Cystadenoma tumors |
22.3 |
8.6–125.9 |
41.4 |
15.0–101.6 |
5.2 | 2.2–32.8 |
19.2 |
2.3–272.0 |
56.9 |
15.0–282.6 | 14.9 |
1.1–86.4 |
|
Follicular cysts |
4.6 |
9.1–88.0 |
43.5 | 24.2–84.7 |
5.5 | 1.6–22.1 |
11.7 | 3.2–79.8 |
65.7 |
41.5–206.5 | 13.1 |
5.3–65.7 |
|
Paraovarian cysts |
15.3 |
4.1–111.4 |
48.2 | 36.4–78.0 |
7.1 | 3.7–18.5 |
9.3 |
4.9–502.7 |
52.0 |
40.5–186.8 |
8.7 |
4.8–87.0 |
|
Hemorrhagic cysts |
14.6 |
5.5–274.8 |
44.2 | 24.8–88.6 |
5.7 | 1.7–23.3 | – | – | – | – | – | – |
|
Inflammatory tumors |
101.4 |
2.4–1,670.6 |
54.3 |
39.0–269.8 |
9.3 | 3.8–83.1 | – | – | – | – | – | – |
|
Cirrhosis | 2,257.0 | 2,257.0 | 109.2 | 109.2 | 41.3 | 41.3 | 254.9 |
161.3–1,256.0 | 167.8 | 115.8–199.2 | 83.4 | 75.7–88.8 |
| BRCA1
mutations |
15.5 |
3.0–148.1 |
34.2 |
15.0–109.1 |
3.2 | 2.4–33.6 |
15.2 | 3.0–36.3 |
51.3 | 15.0–83.9 | 10.6 |
3.9–26.8 |
The comparison of the group of patients with benign
ovarian lesions and patients with borderline ovarian tumors also
appears to be clinically significant. In all study patients,
regardless of their menopausal status, the values of HE4, CA125 and
ROMA were observed to be significantly higher in borderline tumors
(P<0.001, P=0.0152, and P<0.001 for HE4, CA125, and ROMA,
respectively). In postmenopausal women, HE4, CA125 and ROMA values
were significantly higher in patients with borderline tumors than
in patients with benign tumors (P=0.0058, P=0.0394 and P=0.0316,
respectively). In younger, premenopausal women, the situation is
different, with only HE4 and ROMA being significantly higher in the
group of borderline tumor patients than in patients with benign
lesions (P=0.0169 and P=0.0441 for HE4 and ROMA, respectively;
P=0.1212 for CA125). The data obtained regarding CA125, HE4 and
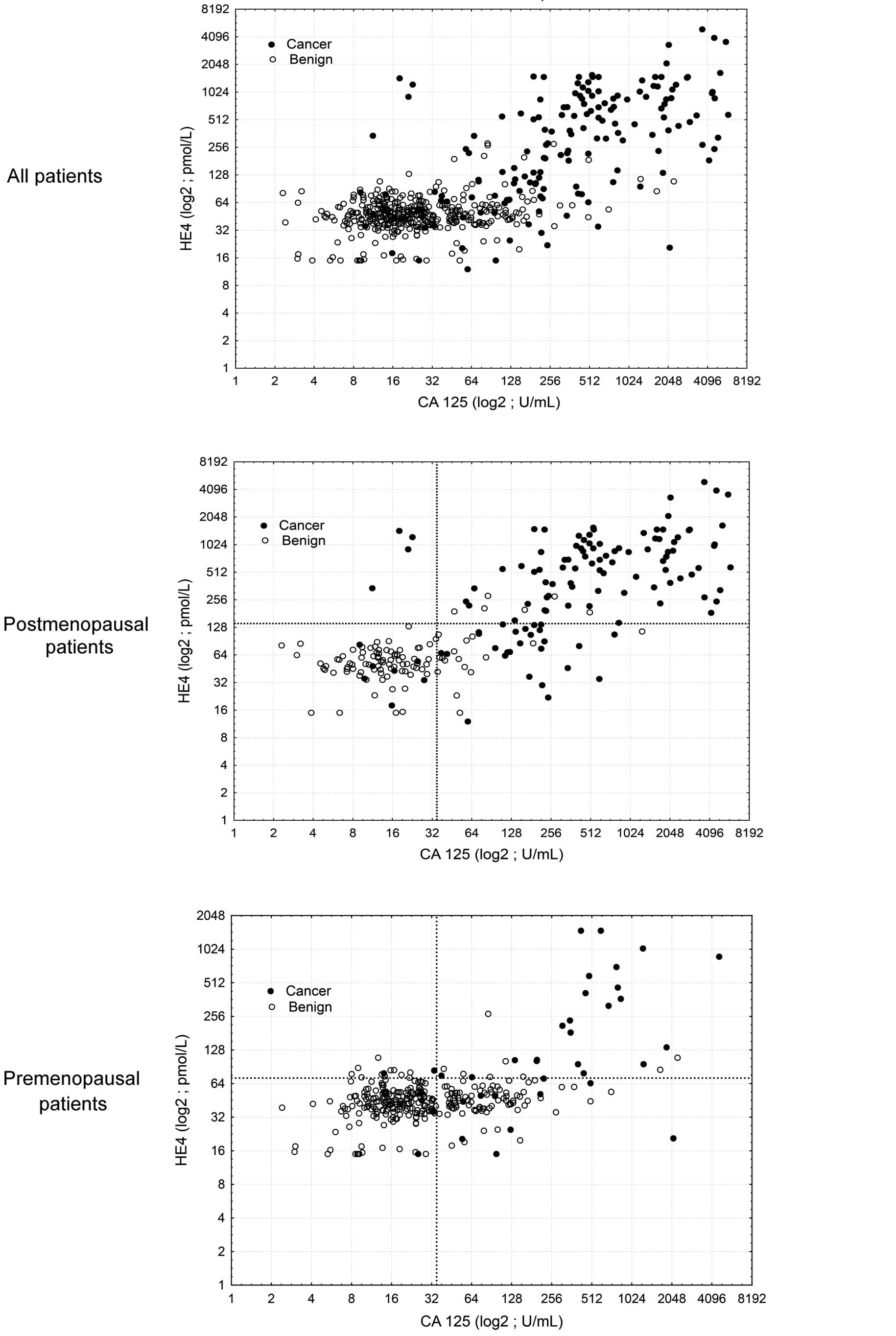
ROMA values are graphically presented in Figs. 1 and 2.
Assessment of the patterns of HE4, CA125, and ROMA
values within the ovarian cancer group indicated that the analyzed
marker levels and the ROMA values were significantly lower in
mucinous tumors compared with serous or endometrial tumors
(P=0.0026, P=0.0004 and P=0.0024 for ROMA, HE4 and CA125,
respectively). No significant differences were observed between
mucinous and clear cell carcinomas with regard to HE4 and ROMA
values; however, significantly higher CA125 levels were observed in
clear cell carcinomas compared with mucinous tumors (P=0.0183).
When restricting the analysis to the postmenopausal group, similar
significantly lower concentrations of HE4 and CA125 and ROMA values
were observed in the subgroup of mucinous tumors as compared with
serous tumors (P=0.0121, P=0.0281 and P=0.0093, respectively) and
endometrial tumors (P=0.0495, P=0.035 and P=0.0277, respectively).
No comparisons were made in the postmenopausal group due to the
insufficient number of cases, with respective values presented in
Table II. In the entire study
population as well as in the groups of pre- and postmenopausal
patients, significantly higher concentrations of markers and ROMA
values were observed in patients with advanced ovarian cancer
[International Federation of Gynecology and Obstetrics (FIGO)
stages III and IV] as compared with less advanced ovarian cancer
(FIGO stages I and II) (P<0.0001 for ROMA, HE4 and CA125).
Cancer differentiation grade (G) also affected the
values of the tested markers. Analysis of all cases (regardless of
hormonal status) revealed similar results as those for
postmenopausal patients: HE4, CA125, and ROMA values were
significantly higher in G3 patients as compared with G1 patients
and with G2 patients (P<0.0001); for G1 vs. G2 groups,
statistical significance was observed only for HE4 and ROMA values
(P<0.0001). In premenopausal women, significant differences were
observed only between the highly differentiated (G1) and poorly
differentiated (G3) tumors, with all analyzed parameters being
higher in the G3 group (P=0.0132, P=0.0154 and P=0.0030 for ROMA,
HE4 and CA125, respectively). When comparing G2 and G3 tumors,
there were no significant differences in any parameters between the
two groups. Analysis of HE4, CA125 and ROMA values between G1 and
G2 tumors revealed statistically significant differences only for
serum HE4 levels and ROMA values (P=0.0075 and P=0.0408 for ROMA
and HE4, respectively).
Table II shows the
median values of HE4, CA125 and ROMA in individual subgroups of
benign ovarian lesions. In all patients, as well as in
premenopausal patients only, the median CA125 levels were higher in
the endometrial cyst subgroup as compared to the subgroups with
teratomas, cystadenomas, follicular cysts, paraovarian cysts or
hemorrhagic cysts. There were no significant differences between
both groups with regard to HE4 and ROMA values. Significantly
higher values of CA125, HE4 and ROMA were observed in patients with
endometrial tumors as compared with patients carrying BRCA1
gene mutations (P=0.0437, P=0.0336 and P<0.0001 for ROMA, HE4
and CA125, respectively). Higher values of all tested parameters
were also found in patients with inflammatory ovarian tumors as
compared with patients with endometrial cysts (P=0.0032, P=0.0033
and P=0.0039 for ROMA, HE4 and CA125, respectively). No significant
differences were observed in the marker levels or the ROMA value
between the compared subgroups of postmenopausal women. In all
analyzed BRCA1 mutation carriers, the mean marker levels and
ROMA values were not significantly different from those observed in
the remaining patients in the group of benign ovarian lesions. In
younger, premenopausal patients, statistically significant
differences between BRCA1 mutation carriers and the
remaining patients were observed in each subgroup: HE4 and ROMA
values were significantly higher in patients with endometriosis,
teratomas, follicular cysts, hemorrhagic cysts, paraovarian cysts,
and inflammatory tumors than in BRCA1 mutation carriers
(P<0.0001, P=0.0035, P=0.0348, P=0.0005, P=0.0036 and P=0.0021
for HE4, and P<0.0001, P=0.0027, P=0.0433, P=0.0005, P=0.0023
and P=0.0033 for ROMA, in endometriosis, teratomas, follicular
cysts, inflammatory tumors, hemorrhagic cysts and paraovarian
cysts, respectively). Serum levels of CA125 were significantly
higher in premenopausal women with endometriosis, follicular cysts
and inflammatory tumors than in BRCA1 mutation carriers
(P<0.0001, P<0.0001 and P=0.0489 for CA125 in endometriosis,
follicular cysts and inflammatory tumors, respectively). Latent
ovarian cancer was diagnosed in histopathological material
collected during prophylactic surgery in three BRCA1
mutation carriers. ROMA values measured in those patients were 19
and 3% for two premenopausal women and 25% in one postmenopausal
patient.
ROC-AUC analysis of ROMA, HE4 and
CA125
Table III shows
ROC-AUC values for the examined markers and ROMA values with
consideration of hormonal status and comparison of results for
different statuses. All parameters evaluated in a separate manner
meet the criteria of useful diagnostic tests. No statistical
superiority of CA125 was observed in any comparison in Table III, while ROMA value was found to be
significantly statistically superior to CA125 in three comparisons:
Patients with advanced ovarian cancers vs. benign ovarian lesions,
P=0.0071 (all) and P=0.0235 (postmenopausal); and postmenopausal
ovarian cancer patients vs. other gynecological cancers and
metastatic ovarian tumors, P=0.016. The diagnostic value of ROMA
was also, in many cases, better than that of HE4 when analyzed
separately; superiority of ROMA (P=0.0012) and CA125 (P=0.0236)
over HE4 is significant, particularly in patients with early-stage
ovarian cancer vs. benign disease.
 | Table III.Values and comparisons of ROC-AUC for
ROMA, CA125 and HE4 in the studied groups. |
Table III.
Values and comparisons of ROC-AUC for
ROMA, CA125 and HE4 in the studied groups.
| A, All ovarian
cancers vs. benign ovarian diseases and BRCA1 mutation
patients |
|---|
|
|---|
|
|
| Comparison of
ROC-AUC, P-value |
|---|
|
|
|
|
|---|
| Menopausal
status | Tumor marker
ROC-AUC (95% CI) | ROMA vs. CA125 | ROMA vs. HE4 | CA125 vs. HE4 |
|---|
| All |
| 0.3692 | 0.0004 | 0.1285 |
|
ROMA | 0.926
(0.894–0.957) |
|
|
HE4 | 0.879
(0.838–0.921) |
|
|
CA125 | 0.911
(0.881–0.941) |
|
| Premenopausal |
| 0.1619 | 0.2064 | 0.072 |
|
ROMA | 0.813
(0.712–0.916) |
|
|
HE4 | 0.783
(0.673–0.892) |
|
|
CA125 | 0.879
(0.822–0.938) |
|
| Postmenopausal |
| 0.6677 | 0.0014 | 0.0453 |
|
ROMA | 0.939
(0.907–0.971) |
|
|
HE4 | 0.889
(0.843–0.935) |
|
|
CA125 | 0.934
(0.901–0.968) |
|
|
| B, Advanced ovarian
cancers vs. benign ovarian diseases and BRCA1 mutation
patients |
|
|
|
| Comparison of
ROC-AUC, P-value |
|
|
|
|
| Menopausal
status | Tumor marker
ROC-AUC (95% CI) | ROMA vs. CA125 | ROMA vs. HE4 | CA125 vs. HE4 |
|
| All |
| 0.0071 | 0.1249 | 0.3607 |
|
ROMA | 0.992
(0.984–0.999) |
|
|
HE4 | 0.977
(0.957–0.996) |
|
|
CA125 | 0.965
(0.946–0.9840 |
|
| Premenopausal |
| 0.2646 | 0.2850 | 0.3506 |
|
ROMA | 0.982
(0.955–1.000) |
|
|
HE4 | 0.926
(0.821–1.000) |
|
|
CA125 | 0.977
(0.954–1.000) |
|
| Postmenopausal |
| 0.0235 | 0.0406 | 0.5872 |
|
ROMA | 0.989
(0.981–0.999) |
|
|
HE4 | 0.975
(0.958–0.991) |
|
|
CA125 | 0.971
(0.949–0.992) |
|
|
| C, Non-advanced
ovarian cancers vs. benign ovarian diseases and BRCA1
mutation patients |
|
|
|
| Comparison of
ROC-AUC, P-value |
|
|
|
|
| Menopausal
status | Tumor marker
ROC-AUC (95% CI) | ROMA vs. CA125 | ROMA vs. HE4 | CA125 vs. HE4 |
|
| All |
| 0.7657 | 0.0012 | 0.0236 |
|
ROMA | 0.803
(0.721–0.883) |
|
|
HE4 | 0.690
(0.588–0.793) |
|
|
CA125 | 0.816
(0.746–0.884) |
|
| Premenopausal |
| 0.1201 | 0.0355 | 0.1004 |
|
ROMA | 0.660
(0.484–0.836) |
|
|
HE4 | 0.650
(0.709–0.896) |
|
|
CA125 | 0.803
(0.709–0.896) |
|
| Postmenopausal |
| 0.3741 | 0.0048 | 0.0123 |
|
ROMA | 0.819
(0.723–0.914) |
|
|
HE4 | 0.678
(0.546–0.809) |
|
|
CA125 | 0.848
(0.761–0.935) |
|
|
| D, Ovarian cancers
vs. other malignant neoplasms and metastatic ovarian tumors |
|
|
|
| Comparison of
ROC-AUC, P-value |
|
|
|
|
| Menopausal
status | Tumor marker
ROC-AUC (95% CI) | ROMA vs. CA125 | ROMA vs. HE4 | CA125 vs. HE4 |
|
| All |
| 0.3116 | 0.851 | 0.4518 |
|
ROMA | 0.765
(0.688–0.840) |
|
|
HE4 | 0.767
(0.630–0.839) |
|
|
CA125 | 0.733
(0.630–0.835) |
|
| Premenopausal |
| 0.4531 | 0.2582 | 0.3000 |
|
ROMA | 0.700
(0.518–0.882) |
|
|
HE4 | 0.673
(0.495–0.849) |
|
|
CA125 | 0.753
(0.559–0.945) |
|
| Postmenopausal |
| 0.016 | 0.7613 | 0.1253 |
|
ROMA | 0.801
(0.719–0.884) |
|
|
HE4 | 0.807
(0.731–0.884) |
|
|
CA125 | 0.716
(0.591–0.841) |
|
|
| E, Ovarian cancers
vs. borderline tumors |
|
|
|
| Comparison of
ROC-AUC, P-value |
|
|
|
|
| Menopausal
status | Tumor marker
ROC-AUC (95% CI) | ROMA vs. CA125 | ROMA vs. HE4 | CA125 vs. HE4 |
|
| All |
| 0.941 | 0.0058 | 0.1419 |
|
ROMA | 0.835
(0.762–0.907) |
|
|
HE4 | 0.784
(0.713–0.855) |
|
|
CA125 | 0.836
(0.767–0.909) |
|
| Premenopausal |
| 0.2816 | 0.1057 | 0.1007 |
|
ROMA | 0.743
(0.602–0.883) |
|
|
HE4 | 0.696
(0.551–0.842) |
|
|
CA125 | 0.815
(0.689–0.942) |
|
| Postmenopausal |
| 0.942 | 0.1494 | 0.5279 |
|
ROMA | 0.827
(0.730–0.924) |
|
|
HE4 | 0.794
(0.707–0.882) |
|
|
CA125 | 0.823
(0.715–0.932) |
|
Sensitivity, specificity, PPV and NPV
of ROMA, HE4 and CA125 in the analyzed groups of patients
Table IV presents the
values of sensitivity, specificity, PPV and NPV for CA125, HE4, and
ROMA in different inter-group comparisons. Based on the DeLong
method we calculated new cut-off values for ROMA algorithm based on
new combination of CA125 and HE4 kits: 14.1% for premenopausal and
25% for postmenopausal women. The sensitivities of CA125 and ROMA
were comparable for all patients in the present study at 88.4 vs.
88.3%; however, the sensitivity of ROMA measurement was lower than
that of CA125 in premenopausal women (71.1 vs. 84.2%) and higher
than that of HE4 in postmenopausal women (93.6 vs. 73.0%). In terms
of specificity, ROMA and HE4 were markedly superior to CA125 in
virtually all comparisons. In the majority of comparisons, the PPV
and NPV were also more favorable for ROMA and HE4 values than for
CA125. CA125 was only slightly superior in differential diagnostics
of ovarian cancer and hemorrhagic, follicular and paraovarian
cysts, as well as in differential diagnostics of borderline tumors
and benign ovarian lesions.
 | Table IV.Sensitivity, specificity, PPV and NPV
of ROMA, HE4 and CA125 between compared groups. |
Table IV.
Sensitivity, specificity, PPV and NPV
of ROMA, HE4 and CA125 between compared groups.
|
| Sensitivity
(%) | Specificity
(%) | PPV (%) | NPV (%) |
|---|
|
|
|
|
|
|
|---|
| Groups
compared | All | PM | M | All | PM | M | All | PM | M | All | PM | M |
|---|
| OC vs. benign
group |
|
|
ROMA | 88.3 | 71.1 | 93.6 | 88.2 | 90.1 | 82.9 | 75.3 | 48.2 | 86.6 | 94.9 | 96.1 | 91.6 |
|
HE4 | – | 65.8 | 73.0 | – | 93.8 | 94.3 | – | 58.1 | 93.9 | – | 95.4 | 74.4 |
|
CA125 | 88.4 | 84.2 | 89.7 | 70.2 | 67.0 | 79.1 | 54.9 | 25.0 | 83.7 | 93.6 | 97.0 | 86.5 |
| OC vs.
endometriosis |
|
|
ROMA | 88.3 | 71.1 | 93.5 | 95.0 | 95.5 | 88.9 | 95.9 | 84.4 | 99.2 | 85.8 | 90.7 | 50.0 |
|
HE4 | – | 65.8 | 73.0 | – | 99.1 | 100.0 | – | 96.2 | 100.0 | – | 89.4 | 20.9 |
|
CA125 | 88.4 | 84.2 | 89.7 | 41.3 | 38.7 | 77.8 | 67.1 | 32.0 | 98.3 | 72.5 | 87.7 | 35.0 |
| OC vs. teratoma
tumors |
|
|
ROMA | 88.3 | 71.1 | 93.5 | 97.7 | 97.4 | 100.0 | 99.3 | 96.4 | 100.0 | 69.4 | 77.1 | 42.8 |
|
HE4 | – | 65.8 | 73.0 | – | 100.0 | 100.0 | – | 100.0 | 100.0 | – | 74.1 | 15.0 |
|
CA125 | 88.4 | 84.2 | 89.7 | 90.9 | 89.5 | 100.0 | 97.3 | 88.9 | 100.0 | 67.8 | 85.0 | 31.6 |
| OC vs. cystadenoma
tumors |
|
|
ROMA | 88.3 | 71.1 | 93.6 | 80.6 | 88.0 | 76.2 | 91.6 | 90 | 92.1 | 73.9 | 66.7 | 80.0 |
|
HE4 | – | 65.8 | 73.0 | – | 92.0 | 92.9 | – | 92.6 | 96.8 | – | 63.9 | 53.4 |
|
CA125 | 88.4 | 84.2 | 89.7 | 68.7 | 76.0 | 64.3 | 87.4 | 84.2 | 88.3 | 70.8 | 76.0 | 67.5 |
| OC vs. follicular,
hemorrhagic and paraovarian cysts |
|
|
ROMA | 88.3 | 71.1 | 93.6 | 85.9 | 84.9 | 88.5 | 91.7 | 72.9 | 97.5 | 80.6 | 83.6 | 74.2 |
|
HE4 | – | 65.8 | 73.0 | – | 86.1 | 92.3 | – | 73.5 | 97.9 | – | 81.2 | 41.4 |
|
CA125 | 88.4 | 84.2 | 89.7 | 90.2 | 84.9 | 88.5 | 94.2 | 76.2 | 97.4 | 81.4 | 90.3 | 63.9 |
| A-OC vs. benign
group |
|
|
ROMA | 99.1 | 94.4 | 100.0 | 88.2 | 90.1 | 82.9 | 68.9 | 36.9 | 82.9 | 99.7 | 99.6 | 100.0 |
|
HE4 | – | 88.9 | 89.9 | – | 93.8 | 94.3 | – | 47.1 | 93.0 | – | 99.3 | 91.7 |
|
CA125 | 95.3 | 100.0 | 94.4 | 70.2 | 67.0 | 79.1 | 46.2 | 15.8 | 79.3 | 98.3 | 100.0 | 94.3 |
| NA-OC vs. benign
group |
|
|
ROMA | 70.4 | 52.6 | 80.0 | 88.2 | 90.1 | 82.9 | 44.8 | 25.6 | 60.9 | 95.7 | 96.7 | 92.6 |
|
HE4 | – | 47.4 | 31.4 | – | 93.4 | 94.3 | – | 33.3 | 64.7 | – | 96.5 | 80.5 |
|
CA125 | 77.8 | 73.7 | 80.0 | 70.2 | 67.0 | 79.1 | 26.1 | 12.7 | 56.0 | 95.9 | 97.5 | 92.2 |
| OC vs. other
malignant neoplasms |
|
|
ROMA | 88.3 | – | – | 57.6 | – | – | 91.1 | – | – | 50.0 | – | – |
|
HE4 | – | – | – | – | – | – | – | – | – | – | – | – |
|
CA125 | 88.4 | – | – | 42.2 | – | – | 88.4 | – |
| 42.2 | – | – |
| OC vs. borderline
ovarian tumors |
|
|
ROMA | 88.3 | 71.1 | 93.6 | 59.1 | 72.7 | 45.5 | 94.1 | 90.0 | 95.1 | 40.6 | 42.1 | 38.5 |
|
HE4 | – | 65.8 | 73.0 | – | 72.7 | 58.3 | – | 89.3 | 94.9 | – | 38.1 | 17.1 |
|
CA125 | 88.4 | 84.2 | 89.7 | 45.5 | 40.0 | 50.0 | 92.4 | 84.2 | 94.9 | 34.5 | 40.0 | 31.6 |
| Borderline ovarian
tumors vs. benign group |
|
|
ROMA | 11.8 | 9.9 | 17.1 | 59.1 | 72.7 | 45.5 | 83.9 | 90.6 | 75.0 | 3.6 | 2.9 | 5.4 |
|
HE4 | – | 6.2 | 5.7 | – | 72.7 | 58.3 | – | 85.7 | 54.6 | – | 2.9 | 6.6 |
|
CA125 | 29.8 | 32.3 | 20.1 | 45.5 | 40.0 | 50.0 | 90.8 | 94.1 | 78.6 | 3.5 | 2.0 | 6.7 |
Discussion
Numerous studies have demonstrated that combined
analysis of CA125 and HE4 allows for significant improvements of
the sensitivity and specificity of prediction of pathological
lesions within the ovaries (11,12,17–23).
For Moore et al (11), this
became the basis for the development of a new diagnostic algorithm
based on the levels of these two serum proteins. As reported by the
authors, a logistic regression model, developed from the
examination of >500 patients, was characterized by sensitivities
and specificities of 76.5 and 74.8% in premenopausal patients, and
92.3 and 74.7% in postmenopausal patients, respectively (12). Their first study, presenting the
diagnostic importance and basic principles of ROMA, made use of the
EIA assay (Fujirebio Diagnostics, Inc., Malvern, PA, USA) to
determine HA4 and the ARCHITECT CA125 II assay (Abbott Diagnostics,
Abbott Park, IL, USA) for determination of CA125. Finally, the FDA
approved the algorithm for the prediction of pathological lesions
within the ovaries on the basis of the study conducted by Moore
et al (12) in 2011.
Subsequently, the era of examinations conducted with the use of
this algorithm began (17–24). The diagnostic usefulness of the
algorithm in conjunction with various diagnostic tests was
examined, with the four pairs of assays listed in the introduction
making their way into routine clinical practice. The usefulness of
the algorithm was also assessed in relation to different control
groups (healthy individuals and patients with various pathological
lesions within the adnexa) (24–30). The
algorithm was rapidly established in the context of diagnosis of
pathological adnexal lesions due to its simplicity, relatively low
price and wide availability.
The present study, comprising a sample of >600
patients, demonstrated that our novel combination of laboratory
assays may be routinely used in clinical practice, as the obtained
values of sensitivity, specificity, PPV and NPV for ROMA, and the
results of the ROC-AUC assessment, are very similar or superior to
those obtained by other researchers who conducted studies using the
widely available combinations of HE4 and CA125 assays. The current
study was conducted in patients with ovarian cancer and other
adnexal pathologies, benign or malignant, which are commonly
included in differential diagnostics of ovarian cancer.
Deliberately excluded from the study were patients with adnexitis
and liver cirrhosis, who pose significant difficulties in
differential diagnostics due to the extremely high CA125 levels and
the accompanying ascites.
When comparing the group of ovarian cancers with all
benign diseases included in the study, in postmenopausal women,
ROMA was characterized by the highest sensitivity (93.6%),
specificity (82.9%), PPV (86.6%) and NPV (91.6%) as compared with
CA125 and HE4 analyzed separately. In premenopausal women, ROMA was
characterized by the highest specificity (90.1%) and PPV (48.2%),
but the selectivity (71.1%) and NPV (96.1%) of the algorithm were
marginally inferior to those of CA125 (84.2 and 97%, respectively).
The highest ROC-AUCs were observed for ROMA for the entire study
population (0.926) and in postmenopausal patients (0.939). In
premenopausal patients, better results were obtained for CA125
(0.874) than for ROMA (0.813). The differences, however, were not
statistically significant.
The literature on this topic contains varying
reports on the usefulness of ROMA in the differential diagnosis of
adnexal pathologies in groups of patients similar to those analyzed
in the present study. The majority of results are consistent with
those obtained in the current study (12–15,25–30).
In one of the earlier studies, highly similar results were obtained
by Moore et al (11), who
examined samples from 476 patients (including 89 cases of ovarian
cancer); however, the sensitivity, specificity, PPV and NPV results
in the current study were somewhat better. It must be considered
that these authors did not compare the ROMA results with CA125 and
HE4 levels as analyzed separately. Lenhardt et al (27) assessed the diagnostic efficacy of ROMA
by comparing two standard combinations of assays (CA125 II with HE4
ARCHITECT; and CA125 II ARCHITECT with HE4 EIA). Analyzing the
ROC-AUCs, the authors obtained results that were nearly identical
to those obtained in the present study: 0.831 (HE4 and CA125 II
ARCHITECT) and 0.820 (CA125 II ARCHITECT and HE4 EIA) for
premenopausal patients; and 0.939 and 0.932, respectively, for
postmenopausal patients.
In their ROMA calculations, Anton et al
(24) used a combination of CA125
Elecsys and HE4 EIA assays, with different cut-off points: One set
equal to these proposed to Moore et al (12) and another resulting from their own
statistical analyses. ROC-AUC values of 0.791 and 0.840 were
obtained for premenopausal and postmenopausal women, respectively;
these results were inferior to those obtained in the current study.
The sensitivity and specificity values using the standard cut-off
points were 77.8 and 69%, respectively, for premenopausal women,
and 72.2 and 81%, respectively, for postmenopausal women. Using
their own optimum cut-off points (24), the authors arrived at different
results, particularly improving the specificity parameter. However,
in the conclusions, the authors highlighted that similar results
had been obtained for ROMA and CA125 and HE4 levels, as analyzed
separately.
Using the combination of CA125 CanAg and HE4 EIA
assays, Montagnana et al (31)
were able to obtain excellent results for ROMA, particularly in
postmenopausal women, with ROC-AUC values (0.77 in premenopausal
patients and 0.92 for postmenopausal patients) that were nearly
identical to those obtained in the current study.
However, it must be considered that even the
‘negative’ studies never questioned the fact that the algorithm in
question met all the criteria for a diagnostic test; said studies
challenged only its superiority to other diagnostic methods
(10,16,32). The
usefulness and widespread use of the test, shown not only by the
ROC-AUC values or the sensitivity and specificity parameters, but
also by the availability, objectivity and cost, favor ROMA-based
assessments in the majority of cases.
Endometriosis is a common ovarian pathology observed
in young premenopausal women. In many cases, patients are childless
women in whom any surgical intervention should consist of the best
fertility-sparing procedure, characterized by the lowest possible
degree of invasiveness so as to reduce the risk of adhesions. To
date, it has been fairly common for high CA125 values in
endometriosis patients (sometimes even close to those observed in
ovarian cancer patients) to lead to an excessively aggressive
surgical treatment, particularly at centers with less clinical
experience. Inclusion of HE4 and, later, ROMA, into preoperative
diagnosis (12) significantly
increased the accuracy of preoperative diagnoses of endometriosis.
The results of the current study confirm the excellent specificity
of ROMA, which allows correct diagnoses to be made with >90%
accuracy. By comparison, the specificity of CA125 in diagnosing
endometrial lesions determined in the current study was only 38.7%.
Additionally, as revealed by the comparative analysis between the
study groups, of all the study parameters, only CA125 was
statistically significantly elevated in patients with endometriosis
compared with patients with other benign adnexal pathologies; this
was responsible for the high number of false positive results. HE4
and ROMA performed much better in this comparative analysis. The
usefulness of HE4 and ROMA in preoperative diagnosis of
endometriosis was also confirmed in other studies (14,33,34).
Borderline tumors are a significant challenge for
gynecologists and gynecological oncologists. It is known that, in
premenopausal women, these tumors may be successfully treated in a
sparing fashion, particularly at earlier clinical stages. However,
para-aortic lymphadenectomy should be performed in patients with
suspected ovarian cancer; this burdensome procedure is unnecessary
in cases of borderline tumors. Therefore, preoperative suspicion of
borderline tumor is a highly important issue; similar to ovarian
cancers, surgery for these tumors should be performed at high
volume hospitals, where a large number of patients with ovarian
cancer are operated, such as teaching hospitals. In the current
study, the sensitivity, specificity, PPV and NPV were assessed with
regard to the comparison of cancers vs. borderline tumors, and
borderline tumors vs. benign, non-neoplastic lesions within the
ovaries. The superiority of ROMA over CA125 and HE4 was
demonstrated in the differential diagnosis of borderline tumors vs.
cancers. The results of the use of ROMA as well as CA125 and HE4
levels in the differential diagnosis of endothelial tumors of
borderline malignancy and non-neoplastic ovarian cysts were most
favorable for separately determined CA125; however, good results
were obtained for none of the examined parameters with the
exception of PPV. A comparative analysis of the groups demonstrated
that borderline tumors were characterized by significantly lower
levels of CA125 and values of ROMA than ovarian cancers, and also
that significantly higher serum levels of CA125 and HE4 were
observed as compared to benign ovarian pathologies. The median ROMA
values for borderline tumors in the current study are similar to
those obtained by Partheen et al (35), equal to 8.0% (4–25.3%) in the present
study and 14.4% (3.6–44.6%) in the reference study. Analogously,
median results for postmenopausal women were 44.8% (5–88%) and
34.2% (9.5–83.7%), respectively. Unfortunately, none of the
analyzed parameters was ideal in this pathology. Interesting
results on the use of ROMA in patients with borderline ovarian
tumors were presented by Gizzo et al (36), who showed that knowledge of the ROMA
value reduced the risk of under-diagnosis (36).
BRCA1 mutation carriers undergo screening
tests until the decision to perform prophylactic adnexectomy is
made. In some cases, occult ovarian cancers are detected following
histopathological assessment of ovaries and fallopian tubes
resected during surgery (37–39). The reported incidence of diagnosing
occult ovarian cancer varies in the literature from 1.9% (36) to 16% (39). To date, no reports have been published
regarding the usefulness of HE4 or ROMA in preoperative diagnosis
aimed at possible detection of occult ovarian cancers in this group
of patients. In the group of 59 BRCA1 mutation carriers in
the present study, occult cancer was detected in 3 patients. Of
these, 2 patients had elevated ROMA values and each of them
presented with elevated levels of HE4 or CA125. It appears that
CA125, HE4 and ROMA values should be determined in female patients
subjected to prophylactic surgeries; in cases with elevated values,
this would be associated with intraoperative examination. In
addition, particular attention should be paid to the fact that HE4
and ROMA values are significantly lower in the group of
premenopausal BRCA1 mutation carriers, and therefore even
marginal increases in these values should trigger oncological
alertness. This, however, requires further investigation.
In conclusion, the novel combination of laboratory
tests (ECLIA for determination of HE4 levels and CMIA for
determination of CA125 levels) for use in calculating ROMA values
and stratifying ovarian tumor patients into groups of high or low
risk of ovarian cancer, meets the criteria of a very good
diagnostic test. Similarly to other assays associated with ROMA
calculations, the highest diagnostic precision was demonstrated in
postmenopausal women. The algorithm is also useful in diagnosing
ovarian endometriosis changes in younger women. Further studies are
required to assess the usefulness of ROMA in the group of
BRCA1 mutation carriers.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Angelis R, Sant M, Coleman MP,
Francisci S, Baili P, Pierannunzio D, Trama A, Visser O, Brenner H,
Ardanaz E, et al: Cancer survival in Europe 1999–2007 by country
and age: Results of EUROCARE-5-a population-based study. Lancet
Oncol. 15:23–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cliby WA, Powell MA, Al- Hammadi N, Chen
L, Miller J Philip, Roland PY, Mutch DG and Bristow RE: Ovarian
cancer in the United States: Contemporary patterns of care
associated with improved survival. Gynecol Oncol. 136:11–17. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Macucs R, Baidekalna I and Donina S:
Comparison of different ovarian cancer detection algorithms. Eur J
Gynecol Oncol. 32:408–410. 2011.
|
|
5
|
Morgante G, Ia Marca A, Ditto A and De Leo
V: Comparison of two malignancy risk indices based on serum Ca125,
ultrasound score and menopausal status in the diagnosis of ovarian
masses. Br J Obstet Gynaecol. 106:524–527. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tinglustad S, Hagen B, Skjeldestad FE,
Halvorsen T, Nustad K and Onsrud M: The risk-of-malignancy index to
evaluate potential ovarian cancers in local hospitals. Obstet
Gynecol. 93:448–452. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Z and Chan DW: The road from
discovery to clinical diagnostics: Lessons learned from the first
FDA-cleared in vitro diagnostic multivariate index assay of
proteomic biomarkers. Cancer Epidemiol Biomarkers Prev.
19:2995–2999. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miller R Ware, Smith A, Desimone CP,
Seamon L, Giidrich S, Podzieliński I, Sokoll L, van Nagell JR Jr,
Zhang Z and Ueland FR: Performance of the American collage of
obstetricians and gynecologists' ovarian tumor referral guidelines
with multivariate index assay. Obstet Gynecol. 117:1298–1306. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ueland FR, Desimone CP, Seamon LG, Miller
RA, Goodrich S, Podzieliński I, Sokoll L, Smith A, van Nagell JR Jr
and Zhang Z: Effectiveness of multivariate index assay in the
preoperative assessment of ovarian tumors. Obstet Gynecol.
117:1289–1297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaijser J, Van Gorp T, Van Hoorde K,
Holsbeke C, Sayasneh A, Vergote I, Bourne T, Timmerman D and Van
Calster B: A comparison between an ultrasound based prediction
model (LR2) and the risk of ovarian malignancy algorithm (ROMA) to
assess the risk of malignancy in women with adnexal mass. Gynecol
Oncol. 129:377–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moore RG, McMeekin DS, Brown AK,
DiSilvestro P, Miller MC, Allard WJ, Gajewski W, Kurman R, Bast RC
Jr and Skates SJ: A novel multiple marker bioassay utilizing HE4
and CA125 for the prediction of ovarian cancer in patients with a
pelvic mass. Gynecol Oncol. 112:40–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moore RG, Miller MC, Disilvestro P,
Landrum LM, Gajewski W, Ball JJ and Skates SJ: Evaluation of the
diagnostic accuracy of the risk of ovarian malignancy algorithm in
women with a pelvic mass. Obstet Gynecol. 118:280–288. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bandiera E, Romani C, Specchla C, Zanotti
L, Galli C, Ruggieri G, Tognon G, Bignotti E, Tassi RA, Odicino F,
et al: Serum human epididymis protein 4 and risk for ovarian
malignancy algorithm as new diagnostic and prognostic tools for
epithelial ovarian cancer management. Cancer Epidemiol Biomarkers
Prev. 20:2496–2506. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kadija S, Stefanovic A, Jeremic K,
Radojevic MM, Nikolic L, Markovic I and Atanackovic J: The utility
of human epididymal protein 4, cancer antigen 125, and risk form
malignancy algorithm in ovarian cancer and endometriosis. Int J
Gynecol Cancer. 22:238–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Gao J, Yao H, Wu Z, Wang M and Qi
J: Diagnostic accuracy of serum HE4, CA125 and ROMA in patients
with ovarian cancer: A meta analysis. Tumor Biol. 35:6127–6138.
2014. View Article : Google Scholar
|
|
16
|
Kaijser J, Van Gorp T, Smet ME, Van
Holsbeke C, Sayasneh A, Epstein E, Bourne T, Vergote I, Van Calster
B and Timmerman D: Are serum HE4 or ROMA scores useful to
experienced examiners for improving characterization of adnexal
masses after transvaginal ultrasonography? Ultrasound Obstet
Gynecol. 43:89–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Macus R, Baidekalna I and Donina S: An
ovarian cancer malignancy risk index composed of HE4, CA125,
ultrasonographic score, and menopausal status: Use in
differentiation of ovarian cancers and benign lesions. Tumor Biol.
33:1811–1817. 2012. View Article : Google Scholar
|
|
18
|
Moore RG, Jabre-Raughley M, Brown AK,
Robison KM, Miller MC, Allard WJ, Kurman RJ, Bast RC and Skates SJ:
Comparison of a novel multiple marker assay vs. the risk of
malignancy index for the prediction of epithelial ovarian cancer in
patients with a pelvic mass. Am J Obstet Gynecol. 203:e1–e6. 2010.
View Article : Google Scholar
|
|
19
|
Moore RG, Miller MC, Steinhoff MM, Skates
SJ, Lu KH, Lambert-Messerlian G and Bast RC Jr: Serum HE4 levels
are less frequently elevated than CA125 in women with benign
gynecological disorders. Am J Obstet Gynecol. 206:e1–e8. 2012.
View Article : Google Scholar
|
|
20
|
Azzam AZ, Hashad DI and Kamel NA:
Evaluation of HE4 as an extra biomarker to CA125 to improve
detection of ovarian carcinoma: Is it time for step forward? Arch
Gynecol Obstet. 288:167–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park Y, Lee JH, Hong DJ, Lee EY and Kim
HS: Diagnostic performance of HE4 and CA125 for the detection of
ovarian cancer from patients with various gynecologic and
non-gynecologic diseases. Clin Biochem. 44:884–888. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin J, Qin J and Sangvatanakul V: Human
epididymis protein 4 for differential diagnosis between benign
gynaecologic disease and ovarian cancer: A systematic review and
meta-analysis. Eur J Obstet Gynecol Reprod Biol. 167:81–85. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferraro S, Bragna F, Lanzoni M, Boracchi
P, Bignazoli EM and Panteghini M: Serum human epididymis protein 4
vs. carbohydrate antigen 125 for ovarian cancer diagnosis: A
systematic review. J Clin Pathol. 66:273–281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anton C, Carvalho FM, Oliveira EI, Maciel
GA, Baracat EC and Carvalho JP: A comparison of CA125, HE4, risk
ovarian malignancy algorithm (ROMA), and risk malignancy index
(RMI) for the classification of ovarian masses. Clinics (Sao
Paulo). 67:437–441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim YM, Whang DH, Park J, Kim SH, Lee SW,
Park HA, Ha M and Choi KH: Evaluation of accuracy of serum human
epididymis protein 4 in combination with CA125 for detecting
ovarian cancer: A prospective case control study in Korean
population. Clin Chem Lab Med. 49:527–534. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ortiz-Muñoz B, Aznar-Oroval E, Garcia A
Garcia, Peris A Covisa, Ballestero P Perez, Yepes M Sanchez, Lozano
T Garcia, Ballester C Illueca and García Garcia E: HE4, CA125 and
ROMA algorithm for differential diagnosis between benign
gynaecological diseases and ovarian cancer. Tumor Biol.
35:7249–7258. 2014. View Article : Google Scholar
|
|
27
|
Lenhardt M, Stieber P, Hertlein L,
Kirschenhofer A, Fürst S, Mayr D, Nagel D, Hofmann K, Krocker K and
Burges A: The diagnostic accuracy of two human epididymis protein 4
(HE4) testing system in combination with CA125 in the differential
diagnosis of ovarian masses. Clin Chem Lab Med. 49:2081–2088.
2011.PubMed/NCBI
|
|
28
|
Kalapotharakos G, Asciutto C, Henic E,
Casslén B and Borgfeldt C: High preoperative blood levels of HE4
predicts poor prognosis in patients with ovarian cancer. J Ovarian
Res. 5:202012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ruggieri G, Bandiera E, Zanotti L, Belloli
S, Ravaggi A, Romani C, Bignotti E, Tassi RA, Tognon G, Galli C, et
al: HE4 and epithelial ovarian cancer: Comparison and clinical
evaluation of two immunoassays and a combination algorithm. Clin
Chim Acta. 412:1447–1453. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang J, Sa M, Huang M, Yang J, Xiang Z,
Liu B and Tang A: The reference intervals for HE4, CA125 and ROMA
in healthy female with electrochemiluminescence immunoassay. Clin
Biochem. 46:1705–1708. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Montagnana M, Danese E, Ruzzenente O,
Bresciani V, Nuzzo T, Gelati M, Salvagno GL, Franchi M, Lippi G and
Guidi GC: The ROMA (Risk of Ovarian Malignancy Algorithm) for
estimating the risk of epithelial ovarian cancer in women
presenting with pelvic mass: Is it really useful? Clin Chem Lab
Med. 49:521–525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Van Gorp T, Veldman J, Van Calster B,
Cadron I, Leunen K, Amant F, Timmerman D and Vergote I: Subjective
assessment by ultrasound is superior to the risk of malignancy
index (RMI) or the risk of ovarian malignancy algorithm (ROMA) in
discriminating benign from malignant adnexal masses. Eur J Cancer.
48:1649–1656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Janas L, Głowacka E, Wilczyński JR,
Malinowski A and Nowak M: Evaluation of applicability of HE4 and
ROMA in the preoperative diagnosis of adnexal masses. Ginekol Pol.
86:193–197. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Molina R, Escudero JM, Augé JM, Filella X,
Foj L, Torné A, Lejarcegui J and Pahisa J: HE4 a novel tumor marker
for ovarian cancer: Comparison with CA 125 and ROMA algorithm in
patients with gynaecological diseases. Tumor Biol. 32:1087–1095.
2011. View Article : Google Scholar
|
|
35
|
Partheen K, Kristjansdottir B and
Sundfeldt K: Evaluation of ovarian cancer biomarkers HE4 and CA-125
in women presenting with a suspicious cystic ovarian mass. J
Gynecol Oncol. 22:244–252. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gizzo S, Berretta R, Di Gangi S, Guido M,
Zanni GC, Franseschetti I, Quaranta M, Plebani M, Nardelli GB and
Patrelli TS: Borderline ovarian tumors and diagnostic dilemma of
intraoperative diagnosis: Could preoperative He4 assay and ROMA
score assessment increase the frozen section accuracy? A
multicentre case-control study. Biomed Res Int. 2014:8035982014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Olivier RI, van Burden M, Lubsen MA,
Rookus MA, Mooij TM, van de Vijver MJ and van't Veer LJ: Clinical
outcome of prophylactic oophorectomy in BRCA1/BRCA2 mutation
carriers and events during follow-up. Br J Cancer. 90:1492–1497.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Evans DG, Clayton R, Donnai P, Shenton A
and Lallo P: Risk-reducucing surgery for ovarian cancer: Outcomes
in 300 surgeries suggest a low peritoneal primary risk. Eur J Hum
Genet. 17:1381–1385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Carcangiu ML, Peissel B, Pasini B, Spatti
G, Radice P and Monoukian S: Incidental carcinomas in prophylactic
specimens in BRCA1 and BRCA2 germ-line mutations carriers, with
emphasis on fallopian tube lesions: Report of 6 cases and review of
the literature. Am J Surg Pathol. 30:1222–1230. 2006. View Article : Google Scholar : PubMed/NCBI
|