Introduction
Squamous cell carcinoma is a major cause of cancer
morbidity and mortality, and is one of the most commonly occurring
malignancies worldwide. The incidence of head and neck squamous
cell carcinoma (HNSCC) is estimated at 500,000 per year in the
United States (1). Changes have been
made regarding the therapeutic strategies applied for such
patients, including different surgical approaches, radiotherapy and
chemotherapy either in alone or in various combinations; however,
despite this multi-modal treatment strategy, survival rates have
not improved significantly over the past several decades, with an
overall 5-year survival rate of 40–50% (2).
There is increasing evidence that tumor growth is
not only determined by malignant cancer cells, but also by the
tumor stroma (3). The tumor stroma is
partially composed of fibroblasts and the connective tissue they
produce. Physiologically, due to their close proximity, continuous
crosstalk between the stroma and epithelia controls tissue
differentiation (4). In the pathology
of a tissue wound, the stroma takes on the role of repair, while
paracrine signaling alters epithelial proliferation and
differentiation (4,5). As cancer functionally resembles a
chronic wound, these mechanisms of wound repair are useful to
examine from an oncological point of view. In this regard,
fibroblasts, which are the primary component of tumor stroma, have
been shown to be prominent modifiers of cancer progression
(6). It has also become increasingly
clear that there are different subpopulations of fibroblasts, as a
result of their continuous interaction with epithelial or cancer
cells. One of these subpopulations, termed cancer-associated
fibroblasts (CAFs), has been shown to be an important promoter of
tumor growth and progression (7).
CAFs induce epithelial-mesenchymal transition (EMT) in epithelial
tumor cells, which is a key factor in the invasion of squamous cell
carcinomas (8).
It is also known that cytokines are involved in
tumor progression. For example, interleukin-6 (IL-6) has been shown
to promote cancer resistance against chemotherapeutic agents, such
as cisplatin (9), while elevated IL-8
expression is associated with an enhanced metastatic potential in
different cancer entities (9,10). Numerous therapeutic strategies
concerning HNSCC involve the use of radiation, either combined with
chemotherapy as the primary therapy or via adjuvant radiation
following surgery. However, there have been few studies analyzing
the impact of radiation on the tumor stroma. Recent studies showed
no significant changes in the growth or proliferation of CAFs
themselves when exposed to radiation in vitro (11,12).
Instead, the application of low doses of radiation (<20 Gy)
appears to enhance the capability of fibroblasts to promote
survival of co-cultured cancer cells (13). However, in these aforementioned
studies, irradiation was delivered to cells as a monolayer culture
in vitro. Thus, the complex interactions of pre-irradiated
tissue in vivo and their influence on CAFs is still largely
unknown.
The primary focus of the present study was the
influence of pre-irradiation on the interactions between
fibroblasts and HNSCC. The aim of the present study was to analyze
the in vitro effects pre-irradiation of fibroblasts on HNSCC
cell lines compared with non-irradiated fibroblasts in terms of
morphological changes, viability and apoptosis.
Materials and methods
Culture of the FaDu cell line
The FaDu HNSCC cell line was established from a
human hypopharyngeal squamous cell carcinoma (14) and was obtained from DSMZ
(Braunschweig, Germany). Cells were cultivated in RPMI-1640 medium
with 10% fetal calf serum (FCS) (Biochrom GmbH, Berlin, Germany),
100 µg/ml streptomycin, 100 U/ml penicillin, 1% sodium pyruvate
(100 mM; Biochrom GmbH, Berlin, Germany) and 1% non-essential amino
acids [RPMI-expansion medium (EM); 100-fold concentration]. The
culture conditions included a temperature of 37°C with 5%
CO2 in culture flasks. Medium was replaced every other
day and passaging was performed by trypsinization (0.25% trypsin;
Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) once cells
reached 70–80% confluencey. Subsequently, cells were washed and
seeded in new flasks or treatment wells. Cells in the exponential
growth phase were used for performing experiments.
Acquisition and culture of
fibroblasts
Fibroblasts were obtained from skin samples of
voluntary patients undergoing neck surgery (n=20) at the University
Hospital of Würzburg (Würzburg, Germany) between August 2011 and
October 2012, with 10/20 patients having been previously irradiated
with intensity modulated irradiation with 60–70 Gy for 6 weeks
during head and neck cancer therapy 6–16 months previously.
Approval was obtained from the Ethics Committee of the Medical
Faculty, University of Würzburg (approval no., 12/06) and evidence
of informed consent was received from all individuals included in
the present study. The skin samples were cleared of fat and cut
into small pieces (2–3 mm), which were then seeded on 6-plate
wells. After 60 min of culture without medium, tissue pieces were
attached to the bottom of the plates strongly enough not to be
washed away by the addition of Dulbecco's modified Eagle medium
(DMEM; Gibco, Thermo Fisher Scientific, Inc.) with 10% FCS, 100
U/ml penicillin and 100 µg/ml streptomycin [DMEM-expansion medium
(DMEM-EM)] at 37°C in 5% CO2. Fibroblasts grew out from
these tissue pieces into the periphery. Medium was replaced every
other day and passaging was performed by trypsinization (0.25%
trypsin; Gibco, Thermo Fisher Scientific, Inc.) once cells reached
70–80% confluencey. Subsequently, cells were washed with phosphate
buffered saline (PBS) and seeded in new flasks or treatment
wells.
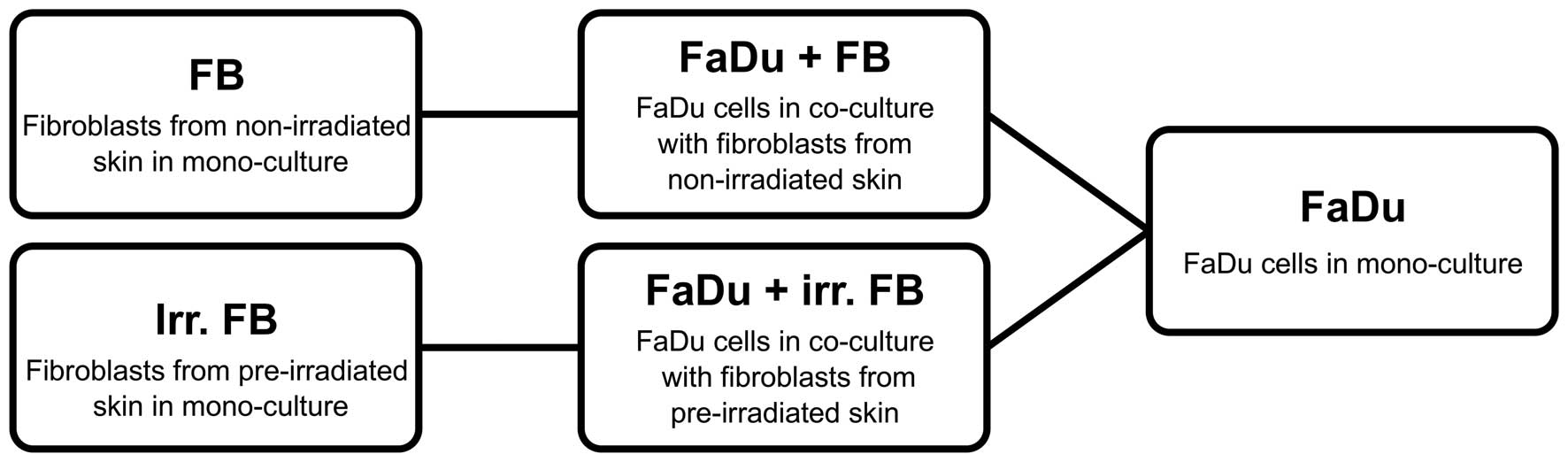
Group composition
The experiment included 10 fibroblast cultures from
pre-irradiated tissue and 10 from non-irradiated samples.
Simultaneously, FaDu cells were cultivated alone in parallel to
serve as the control group. A schematic of all groups are shown in
Fig. 1.
Transwell culture
A Transwell system (Costar; Corning, Inc., Corning,
NY, USA) was used to analyze the effects of fibroblasts on the FaDu
HNSCC cell line. First, a co-culture was generated, as follows:
FaDu cells (5×104) were added to 1 ml RPMI-EM on the
bottom well of the Transwell 12-well plate; subsequently,
fibroblasts (5×104) with 0.5 ml RPMI-EM were added to
the upper wells of the Transwell system and transferred to the
wells containing FaDu cells. Following co-culture for 3 days at
37°C, the fibroblasts were discarded, and the co-culture of FaDu
cells was used in additional analysis.
MTT assay
After 3 days of co-culture, the MTT (Sigma-Aldrich)
colorimetric staining method was performed, according to Mosmann
(15), to determine cell viability.
Cells were seeded at 10,000 cells per well in a 12-well plate. All
wells were incubated with 1 ml MTT (1 mg/ml) for 5 h at 37°C and 5%
CO2. MTT was then removed and 1 ml isopropanol was
added, followed by another incubation period of 1 h at 37°C and 5%
CO2. Measurement of the color conversion of the blue
formazan dye was performed using a multi-plate reader (Titertek
Multiskan PLUS MK II; Thermo Labsystems, Thermo Fisher Scientific,
Inc.) at a wavelength of 570 nm.
Annexin V-propidium iodide
analysis
A BD Pharmingen Annexin V-APC kit from (BD
Biosciences, Heidelberg, Germany) was used to evaluate apoptosis.
After 3 days of co-culture, cells in suspension and adherent cells
were harvested then washed twice with PBS, followed by resuspension
in 1:10 binding buffer [0.1 M HEPES (pH 7.4), 1.4 M NaCl, 25 mM
CaCl2) at a concentration of 1×106 cells/ml.
Aliquots of this cell suspension (100 µl; 1×105 cells)
were then transferred to a 5 ml culture tube. Propidium iodide (5
µl) and Annexin V-APC (5 µl) were added to each aliquot. After 15
min of incubation at room temperature in the dark, the cells were
resuspended with 400 µl 1:10 binding buffer. A FACSCanto flow
cytometer was used to analyze the samples with BD FACSDiva version
5.0.3 software (BD Biosciences). Only cells with damaged membranes
were stained by propidium iodide.
IL-6/8 enzyme-linked immunosorbent
assay (ELISA)
For measurement of the secretion of IL-6 and IL-8,
the supernatants were collected (centrifugation, 150 × g for 5 min
at 37°C) after 3 days of co-culture and stored at −20°C in sterile
tubes until further use. DMEM-EM served as control. Human IL-6 and
IL-8 kits (catalog nos., 950.030.192 and 950.050.192, respectively;
Diaclone SAS, Besançon, France) were used and the experiments were
performed in duplicate. The ELISA plate was read at 450 nm
(Titertek Multiskan PLUS MK II; Thermo Labsystems, Thermo Fisher
Scientific, Inc.). The concentration of IL-6 and IL-8 were
determined by constructing a standard curve using recombinant IL-6
and IL-8. For statistical analysis, mono-cultures [FaDu or
fibroblasts (non-irradiated or pre-irradiated)] were compared with
co-cultures [FaDu and fibroblasts (non-irradiated or
pre-irradiated)].
Statistical analysis
The data collected was transferred to standard
spreadsheets and statistically analyzed using GraphPad Prism
software (version 4.0; GraphPad Software, Inc., San Diego, CA,
USA). Data are presented as the mean ± standard deviation of three
experiments, unless otherwise stated. The Gaussian distribution was
tested via first column analysis. In cases of Gaussian
distribution, one-way analysis of variance followed by Tukey's
multiple comparison test was used, and in cases of a non-Gaussian
distribution, the Kruskal-Wallis test was performed. P<0.05 was
used to indicate a statistically significant difference.
Results
MTT assay
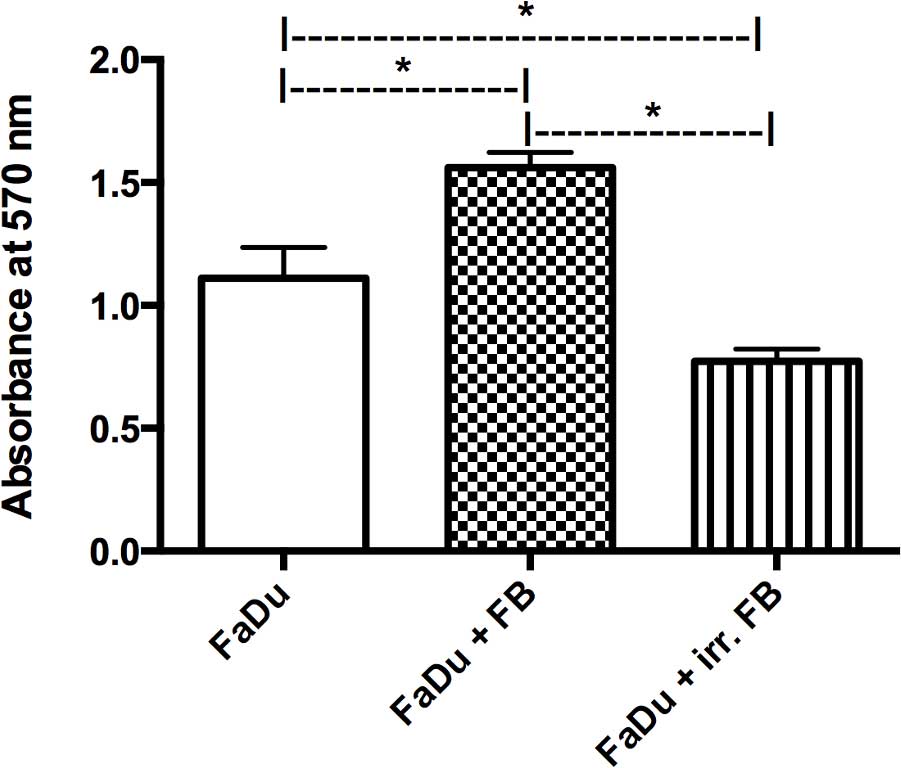
A co-culture of FaDu cells with fibroblasts from
non-irradiated skin samples showed a significant increase in FaDu
cell viability compared with the mono-culture of FaDu cancer cells
alone (P=0.0001). By contrast, when co-cultured with fibroblasts
from pre-irradiated tissue, there was a significant decrease in
FaDu cell viability compared with the non-irradiated co-culture and
the mono-culture (P=0.0001; Fig.
2).
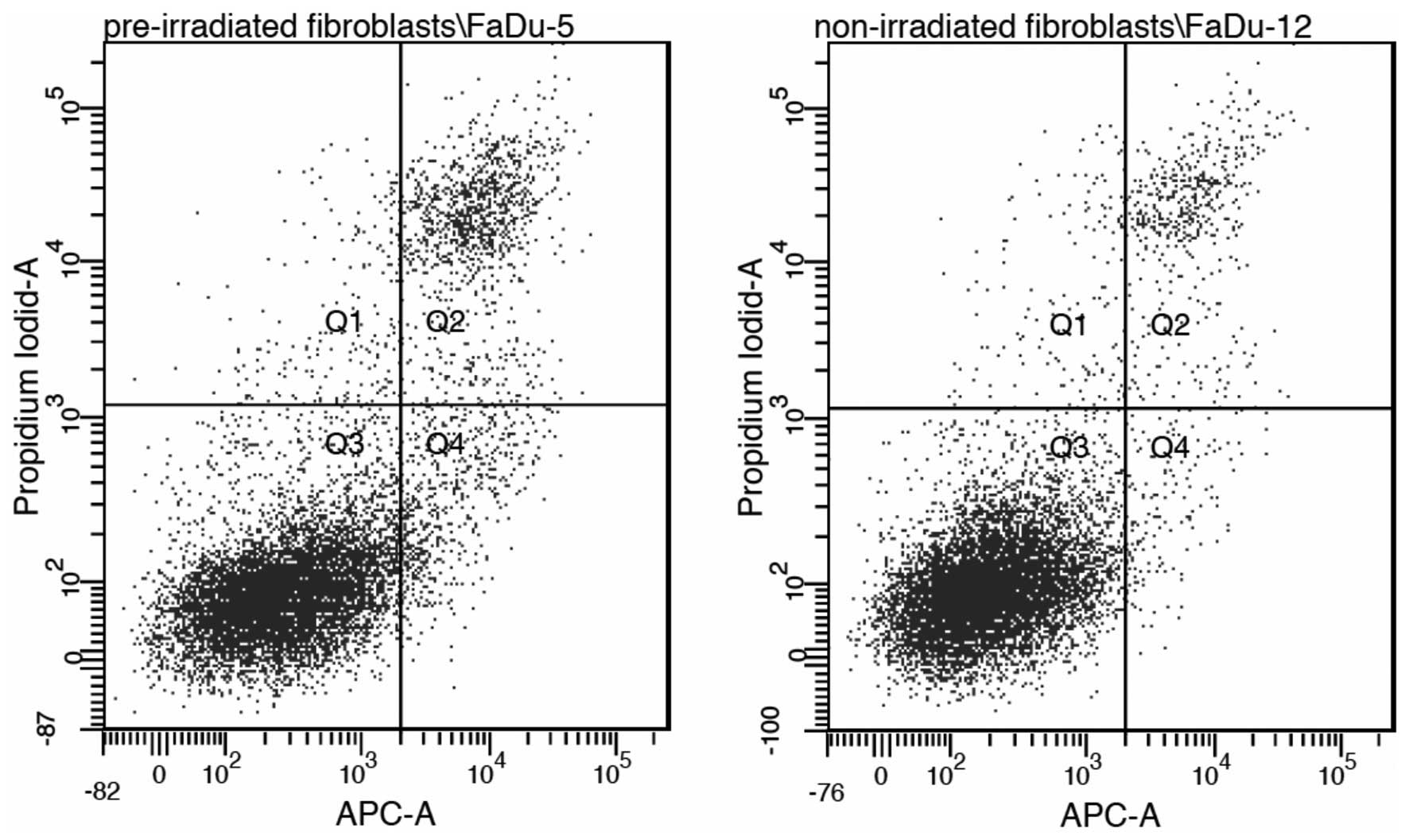
Annexin V-propidium iodide
Annexin V-propidium iodide analysis revealed no
statistically significant differences between a co-culture with
non-irradiated fibroblasts and pre-irradiated fibroblasts regarding
the rate of apoptosis in FaDu cells (P=0.21). This indicates that
apoptosis may not be the dominant mechanism involved (Fig. 3).
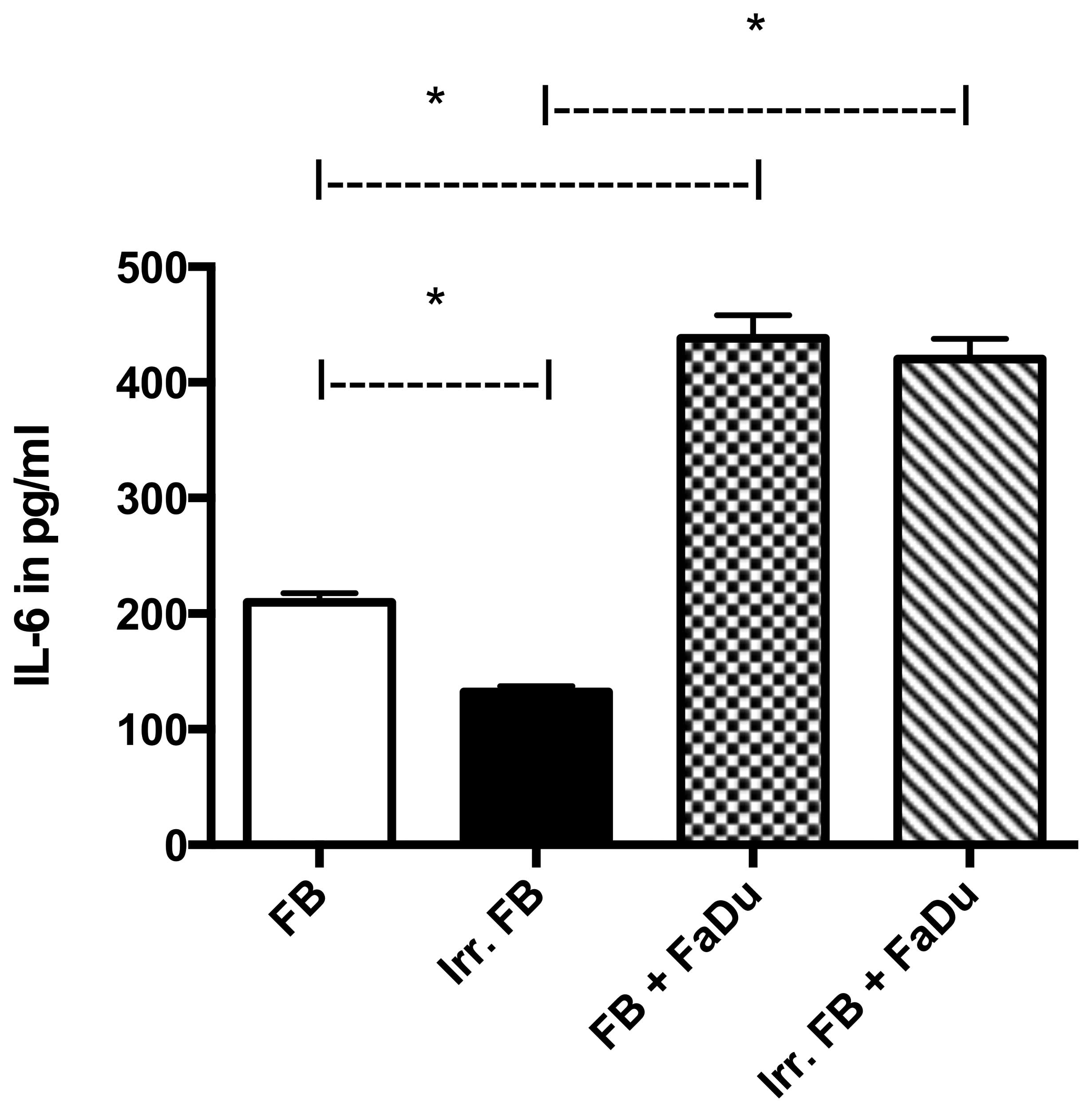
Quantitative analysis of IL-6 and IL-8
expression
In mono-culture, IL-6-expression was significantly
higher in normal fibroblasts than in fibroblasts from
pre-irradiated tissue (P=0.0001). FaDu cells in mono-culture showed
negligible secretion of IL-6 (data not shown). When in co-culture,
the level of secretion in both groups (pre-irradiated or
non-irradiated) was significantly higher (P=0.001) compared with
mono-culture, yet there was no significant difference between the
two groups (P=0.0787) (Fig. 4).
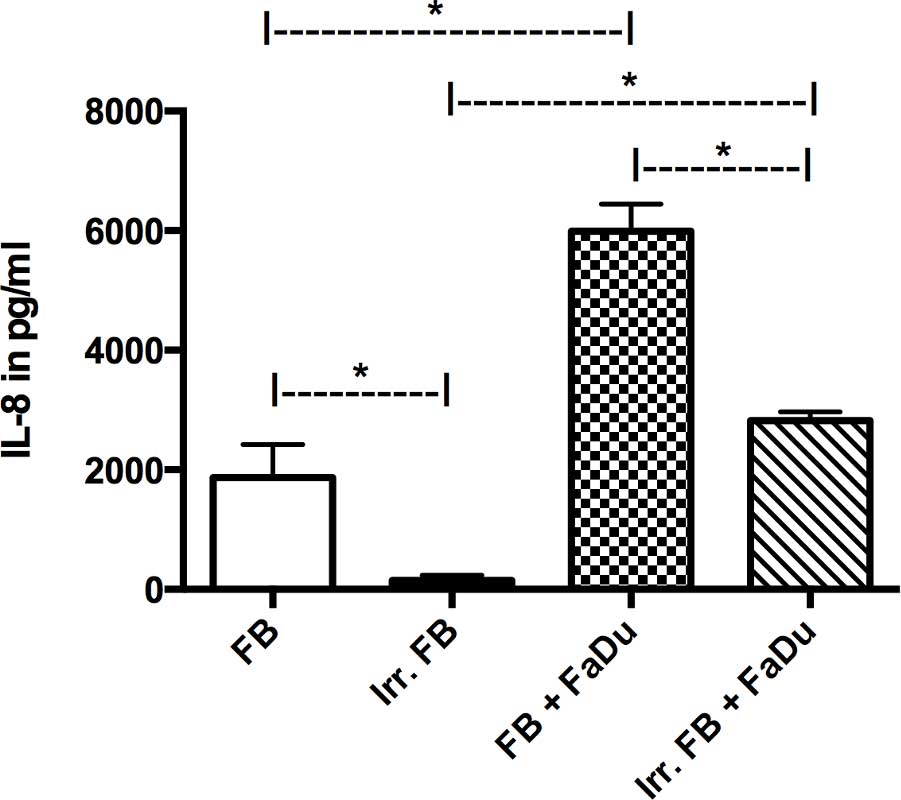
IL-8 expression in mono-culture was only markedly
present in non-irradiated fibroblasts, while FaDu cells (data not
shown) and pre-irradiated fibroblasts showed negligible expression.
When in co-culture, both groups demonstrated an increase in IL-8
secretion compared with their respective mono-cultures (P=0.0015),
although the non-irradiated fibroblasts still exhibited a
significantly higher level of expression compared with the
pre-irradiated fibroblasts (P=0.001; Fig.
5).
Discussion
The present study focused on the effects of prior
radiation on the properties of fibroblasts in co-culture with FaDu
HNSCC cells. Over the past few decades, the effects of the tumor
stroma on cancer cells has been thoroughly investigated, and CAFs
have been shown to promote the growth of cancer cells, many of
which have already been extracted from a number of types of
invasive human carcinoma (16). CAFs
have been induced from regular fibroblasts, and even from
mesenchymal stem cells, via treatment with carcinoma cell-derived
medium (17) or a co-culture model
(18). In addition, previous studies
have demonstrated that co-culture with CAFs changes the level of
secretion of numerous cytokines from carcinoma cells (19,20), thus,
giving them a higher potential for invasion and metastasis. The
possibility of the two cell types mutually influencing each other
is one of the advantages of the Transwell co-culture model, and the
background for selecting it for use in the present study.
The results of the co-culture of FaDu cells with
fibroblasts from non-irradiated skin samples in the present study
revealed a significant increase in FaDu cell viability when
compared with a mono-culture of FaDu cells. This is consistent with
the data available in the literature regarding various other
carcinoma cell types, such as prostate carcinoma (20) or breast cancer (21). Thus, although data regarding the
effect of co-cultured fibroblasts on the proliferation of HNSCC is
scarce, particularly on a quantitative basis, the current results
were as anticipated.
In a co-culture of FaDu cells with fibroblasts
derived from irradiated tissue, a significant decrease in tumor
cell viability was found compared with the non-irradiated
fibroblast co-culture and compared with the mono-culture of FaDu
cells. According to the actual literature, there is no data
available regarding a co-culture of irradiated fibroblasts with
HNSCC cells. However, such data in regard to other carcinoma cell
types differs from the present study. For example, a study by
Ohuchida et al demonstrated an increase in cancer invasion
and progression for pancreatic cancer cells co-cultivated with
irradiated fibroblasts (22). This
result was predominantly explained by tumor-stromal interactions,
particularly via increased phosphorylation of c-Met, although no
increase in hepatocyte growth factor, a mediator of tumor-stromal
interactions, was found (22). In
addition, Tsai et al showed that a low-dose radiation of 20
Gy could promote the invasiveness of co-cultivated breast cancer
cells, and even render the cancer cells radioresistant (13). Both studies concluded that irradiation
of stromal fibroblasts is a promoting factor for cancer cell
growth. This is in accordance with the clinical findings of
increased cases of metastasis in previously irradiated tissue
following definitive radiotherapy, as described for nasopharyngeal
carcinoma (23), as well as in an
animal model of squamous cell carcinoma (24). However, this has yet to be
investigated for HNSCC cells. Furthermore, the radiation in the
aforementioned studies was applied at a dose of only 5–10 Gy in
vitro immediately prior to the experiments, whereas the
fibroblasts in the present study were cultivated from skin that had
been radiated with 60–70 Gy a number of months prior to the
experiment. Therefore, a prolonged effect of radiation on the
tissue samples in vivo may explain the differing results. A
comparative study on fibroblasts cultivated from pre-irradiated
skin and fibroblasts that received radiation in vitro in a
co-culture should be conducted in the future for further
clarification.
Regarding the levels of cytokine secretion,
significantly higher levels of IL-6 and IL-8 were observed in a
mono-culture of non-irradiated fibroblasts compared with in a
mono-culture of pre-irradiated fibroblasts. When in co-culture with
FaDu cells, IL-6 secretion levels were similar in both groups,
while the levels of IL-8 remained significantly lower in the
pre-irradiated group compared with the non-irradiated group.
Various studies have shown that patients with HNSCC commonly
overexpress cytokines, including IL-1, IL-6, tumor necrosis factor
(TNF)-α and TNF-β (25,26). Among others, IL-6 has been shown to be
elevated in HNSCC cell lines of metastatic origin compared with
those cultivated from the primary tumor lesion (19). Therefore, an IL-6 assay was integrated
into the present study, as increased levels of IL-6 indicate a
higher potential for tumor proliferation and metastasis. IL-8 was
also included due to its known tumor-promoting properties (27), primarily through an increase in
angiogenesis. The increased secretion observed in both co-cultured
groups correlates well with previous clinical studies that describe
increased levels of IL-6 and IL-8 in HNSCC tumor patients compared
with healthy controls (25,26). The lower levels of IL-6 and IL-8 in
the pre-irradiated mono-culture group compared with the
non-irradiated mono-culture group may be explained by the
irradiation damage caused to the tissue from which the fibroblasts
were cultivated, indicating a long-term effect on the stromal
tissue. However, when co-cultured with FaDu cells, the
pre-irradiated fibroblasts appeared to increase cytokine
production; increasing to the same level as the non-irradiated
group for IL-6 and at least partially increasing for IL-8. This
raises the question of whether the differences in the co-cultures
are time-dependent, or if there is a limit on how much of the
productive capabilities the pre-irradiated fibroblasts can
regain.
It should be noted that there are certain drawbacks
to the present study design, as the setup is not completely
physiological. The fibroblasts used were not CAFs, which define the
tumor stroma, as they only develop in the vicinity of cancer cells.
Thus, we can only postulate the effects of regular fibroblasts with
and without previous irradiation on cancer cells rather than
changes in the actual tumor stroma caused by irradiation. To
evaluate changes in the tumor stroma caused by irradiation,
irradiation of tumor cells as well as with the surrounding
fibroblasts would be necessary. Thus, an in vitro model
would not be possible; instead, such experiments would have to be
conducted, for example, in an animal model with injection of tumor
cells, and subsequent irradiation of the tumor cells and the
surrounding skin. Therefore, the present study provides good
preliminary data, although further research into the aforementioned
mechanisms is of much interest.
Another interesting question raised by this setup
is, if a pre-irradiation of tissues has an effect on the efficacy
of cytostatic agents on co-cultured tumor cells. As a cytostatic
therapy often is the only treatment available for already
irradiated and inoperable tumors, more information concerning the
interactions of irradiated tissue and cytostatic agents would be of
much interest and is the aim of a study being undertaken at the
University Hospital of Würzburg.
In conclusion, pre-irradiation of tissue changes the
secretory abilities of the fibroblasts cultured from it as well as
the way these fibroblasts affect the growth of co-cultured tumor
cells in vitro. While major differences in the secretion of
IL-6 and IL-8 were observed, the exact mechanisms of the changes
caused by previous irradiation are still largely unknown. Thus,
irradiated tumor stroma should be examined more closely to gain
insight into the long-term effects of irradiation that may not yet
have become obvious. Future studies should be conducted to
specifically examine differences between irradiated fibroblast cell
lines in vitro and fibroblasts cultured from pre-irradiated
skin, as well as the effect of irradiated fibroblasts on the
efficiency of cytostatic agents in a co-culture and the involvement
of EMT in pre-irradiated tissue. Furthermore, to evaluate these
findings in a more clinical setting, an animal study with
irradiation of cancer cells and the surrounding tissue should be
conducted.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics,2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan GG, Tai BC, Liang S, Lim DT and Soo
KC: Squamous cell carcinoma of the head and neck
(HNSCC)-multi-modality treatment and impact on survival. Asian J
Surg. 25:35–40. 2002.PubMed/NCBI
|
|
3
|
Kalluri R: Basement membranes: Structure,
assembly and role in tumour angiogenesis. Nat Rev Cancer.
3:422–433. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tripathi M, Billet S and Bhowmick NA:
Understanding the role of stromal fibroblasts in cancer
progression. Cell Adh Migr. 6:231–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Joyce JA and Pollard JW:
Microenvironmental regulation of metastasis. Nat Rev Cancer.
9:239–252. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tlsty TD and Hein PW: Know thy neighbor:
Stromal cells can contribute oncogenic signals. Curr Opin Genet
Dev. 11:54–59. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mueller MM and Fusenig NE: Friends or
foes-bipolar effects of the tumour stroma in cancer. Nat Rev
Cancer. 4:839–849. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Higashikawa K, Yoneda S, Taki M, Shigeishi
H, Ono S, Tobiume K and Kamata N: Gene expression profiling to
identify genes associated with high-invasiveness in human squamous
cell carcinoma with epithelial-to-mesenchymal transition. Cancer
Lett. 264:256–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamada H, Kobune M, Nakamura K, Kawano Y,
Kato K, Honmou O, Houkin K, Matsunaga T and Niitsu Y: Mesenchymal
stem cells (MSC) as therapeutic cytoreagents for gene therapy.
Cancer Sci. 96:149–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang F, Cho SW, Son SM, Bogatyrev SR,
Singh D, Green JJ, Mei Y, Park S, Bhang SH, Kim BS, et al: Genetic
engineering of human stem cells for enhanced angiogenesis using
biodegradable polymeric nanoparticles. Proc Natl Acad Sci USA.
107:3317–3322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Affolter A, Schmidtmann I, Mann J and
Brieger J: Cancer-associated fibroblasts do not respond to combined
irradiation and kinase inhibitor treatment. Oncol Rep. 29:785–790.
2013.PubMed/NCBI
|
|
12
|
Grey B, Coppey J and Little JB: Modulation
of clonogenicity, growth, and radiosensitivity of three human
epidermoid tumor cell lines by a fibroblastic environment. J Rad
Onc Biol Phys. 34:1061–1071. 1996. View Article : Google Scholar
|
|
13
|
Tsai KK, Chuang EY, Little JB and Yuan ZM:
Cellular mechanisms for low-dose ionizing radiation-induced
perturbation of the breast tissue microenvironment. Cancer Res.
65:6734–6744. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rangan SR: A new human cell line (FaDu)
from a hypopharyngeal carcinoma. Cancer. 29:117–121. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Orimo A and Weinberg RA: Stromal
fibroblasts in cancer: A novel tumor-promoting cell type. Cell
Cycle. 5:1597–1601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mishra PJ, Mishra PJ, Humeniuk R, Medina
DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW and Banerjee D:
Carcinoma-associated fibroblast-like differentiation of human
mesenchymal stem cells. Cancer Res. 68:4331–4339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dudás J, Bitsche M, Schartinger V, Falkeis
C, Sprinzl GM and Riechelmann H: Fibroblasts produce brain-derived
neurotrophic factor and induce mesenchymal transition of oral tumor
cells. Oral Oncol. 47:98–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koontongkaew S, Amornphimoltham P and
Yapong B: Tumor-stroma interactions influence cytokine expression
and matrix metalloproteinase activities in paired primary and
metastatic head and neck cancer cells. Cell Biol Int. 33:165–173.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Olumi AF, Grossfeld GD, Hayward SW,
Carroll PR, Tlsty TD and Cunha GR: Carcinoma-associated fibroblasts
direct tumor progression of initiated human prostatic epithelium.
Cancer Res. 59:5002–5011. 1999.PubMed/NCBI
|
|
21
|
van Roozendaal KE, Klijn JG, van Ooijen B,
Claassen C, Eggermont AM, Henzen-Logmans SC and Foekens JA:
Differential regulation of breast tumor cell proliferation by
stromal fibroblasts of various breast tissue sources. Int J Cancer.
65:120–125. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohuchida K, Mizumoto K, Murakami M, Qian
LW, Sato N, Nagai E, Matsumoto K, Nakamura T and Tanaka M:
Radiation to stromal fibroblasts increases invasiveness of
pancreatic cancer cells through tumor-stromal interactions. Cancer
Res. 64:3215–3222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meltzer J, Ahmed SA and Archambeau JO: The
development of metastases within a field of previous irradiation: A
case report. Cancer. 48:717–720. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baker DG, Morgan EP and Persing JA:
Squamous cell carcinoma growth in irradiated tissue: A murine model
for quantitative assessment of treatment. Ann Plast Surg.
35:171–177. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Z, Malhotra PS, Thomas GR, Ondrey FG,
Duffey DC, Smith CW, Enamorado I, Yeh NT, Kroog GS, Rudy S, et al:
Expression of proinflammatory and proangiogenic cytokines in
patients with head and neck cancer. Clin Cancer Res. 5:1369–1379.
1999.PubMed/NCBI
|
|
26
|
Lathers DM and Young MR: Increased
aberrance of cytokine expression in plasma of patients with more
advanced squamous cell carcinoma of the head and neck. Cytokine.
25:220–228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Todorović-Raković N and Milovanović J:
Interleukin-8 in breast cancer progression. J Interferon Cytokine
Res. 33:563–570. 2013. View Article : Google Scholar : PubMed/NCBI
|