Introduction
Non-small cell lung cancer (NSCLC) accounts for the
majority of all lung cancers, and represents the leading cause of
cancer-related mortality worldwide. The pathogenesis of NCSLC is
complex and is believed to occur as a result of interactions
between environmental and genetic factors (1,2). Cigarette
smoking, environmental pollution, and radiation have been
identified as the main risk factors of NSCLC. However, not every
type of lung carcinoma is associated with these factors, and
additionally, oncogenic types of HPV have also been proposed as
potential causes of NSCLC (3,4).
HPVs are double-stranded, non-enveloped DNA viruses.
Recent studies suggest that HPV infection is an important cause of
cervical cancer (5,6). HPVs are commonly divided into high-risk
and low-risk types, with the high-risk type HPV 16 representing the
predominant type associated with cervical cancer (7). A number of research groups have reported
a high detection rate of HPV in lung cancer tissues, particularly
HPV 16 and 18, suggesting a role for HPV in promoting this disease
(8–10). Although HPV has been hypothesized to
be a possible contributory agent for lung cancer, its role in the
pathology of this disease remains controversial. This controversy
relates to regional and organizational differences in HPV
infection, as well as its unknown pathogenesis in lung cancer
(11–13). Identification of diagnostic markers
for HPV-associated NSCLC is necessary both to clarify the
relationship between HPV infection and NSCLC pathogenesis and to
improve therapeutic strategies.
TP53 is an important regulator of the cell cycle,
and alterations in the TP53 gene are frequently observed in
invasive tumors (14). A number of
previous studies have demonstrated that the HPV E6 protein binds
TP53 and triggers its degradation through the ubiquitin pathway,
promoting proliferation and inhibition of apoptosis (15,16). In
addition, a significant correlation between HPV infection and TP16
expression has been observed in a variety of HPV-associated tumor
specimens (17–19). Overexpression of TP16, a
cyclin-dependent kinase inhibitor, can prevent the phosphorylation
of the retinoblastoma protein, an important cellular tumor
suppressor, resulting in its activation and consequent inhibition
of cell cycle progression (20).
While there is accumulating evidence that the HPV 16/18 E6
oncoprotein is indeed expressed in lung tumors and is involved in
TP53 and TP16 signaling, the correlation between HPV infection and
the expression of these proteins remains controversial.
Consequently the expression of TP53 and TP16 in relation to that of
the E6 oncoprotein in NSCLC requires further clarification.
Although heterogeneity in the expression of TP53 and
TP16 has been demonstrated to correlate with similarly
heterogeneous clinical outcomes in other tumor types, these
relationships have been more difficult to evaluate for NSCLC.
Evaluation of TP53 and TP16 mutation by standard techniques
requires tissue quantities that are often only available from
resected tumors (21,22). For this reason, the majority of
studies of TP53 and TP16 status in NSCLC have been performed during
the early stages of disease. The small numbers of patients included
in some of these studies, differences in follow-up, and the various
criteria used to classify TP53 and TP16 mutation have led to
contradictory results. In comparison with TP53, the role of TP16 in
HPV-related NSCLC has received little attention.
In the present study, we have analyzed TP53 and TP16
expression in a training cohort of 83 patients with advanced NSCLC,
and evaluated the relationship between the expression of these
proteins and various clinical parameters. The findings were
evaluated in an independent cohort of 20 HPV-positive patients.
Materials and methods
Clinical specimens
The present study was a retrospective analysis of a
total of 83 patients with advanced NSCLC, grouped according to HPV
status. All patients were diagnosed with stage III–IV disease at
the Anhui Provincial Hospital between August 2011-August 2013.
Patient samples included 17 surgical specimens, 12 lung biopsy
specimens, 15 bronchoscopic biopsy specimens, 24 pleural effusion
specimens, 13 lymph node biopsy specimens and two bone biopsy
specimens. In all cases, cancer-containing tissues were obtained at
the time of diagnosis of metastatic disease. Written informed
consent was provided by all patients, and approval was obtained
from the Institutional Review Board of Anhui Provincial Hospital.
Clinical characteristics including age, sex, smoking history,
histology type, histological differentiation, lymph node
metastasis, distant metastasis, and expression of TP53 and TP16
were collected for subsequent analyses.
Processing of tumor samples and DNA
extraction
All samples used in the present study were
formalin-fixed, paraffin-embedded tumor tissues that had previously
been evaluated by an expert pathologist. To deparaffinize and
extract DNA from tissue sections, the QIAamp DNA FFPE Tissue Kit
(cat. no 56404, QIAGEN, Hilden, Germany) was used and samples were
processed according to the manufacturer's recommendations. Briefly,
5–6 formalin-fixed, paraffin-embedded 8-µm sections were soaked in
xylene and vortexed vigorously. The extracted tissue was then
pelleted by centrifugation (20,000 × g) and the DNA subsequently
purified following the kit's protocol. All DNA samples were
quantified by spectrophotometric absorbance at 260 nm and aliquots
of equal concentration were then prepared for all samples.
Detection and typing of HPV-DNA
HPV testing of NSCLC samples was performed by PCR
amplification of a fragment of the HPV L1 gene, and the subsequent
screening of PCR products using a Tellgenplex™ HPV DNA Test kit
(Tellgen, Shanghai, China) in combination with the Luminex
technique, which allowed the detection of 26 HPV genotypes
including 19 high-risk types (HPV 16, 18, 26, 31, 33, 35, 39, 45,
51, 52, 53, 55, 56, 58, 59, 66, 68, 82, and 83) and seven low-risk
types (HPV 6, 11, 40, 42, 44, 61, and 73). The experiments were
performed using a Bio-Plex 200 system (Bio-Rad, Hercules, CA, USA)
according to the manufacturer's instructions. Briefly, 2 µg of the
extracted DNA was used as a template for PCR, and the reaction was
performed as follows. A denaturing step of 95°C for 30 sec,
followed by an annealing step of 58°C for 30 sec, and then an
extension step of 72°C for 30 sec were performed for the first five
cycles, and then an additional 35 cycles were performed as
described except that the annealing temperature was reduced to
55°C. The PCR products were then subjected to fast hybridization
and the data were analyzed with the software supplied by the
manufacturer (23).
Qualitative analysis of TP53 and TP16
protein expression
Paraffin-embedded tumor tissue sections were
deparaffinized and rehydrated through a series of graded alcohol
washes. Microwave-based antigen retrieval was conducted using 10 mM
citric acid buffer (pH 6.0). Sections were blocked in normal rabbit
serum (Dako North America, Inc., Carpinteria, CA, USA) containing
10% (v/v) stock avidin solution (Themo Fisher Scientific, Inc.,
Waltham, MA, USA) for 1 h followed by a 12-h incubation at 4°C with
mouse monoclonal antibodies against human TP53 or TP16 (Cell
Signaling, Beverly, MA, USA). Sections were then incubated with
biotinylated anti-mouse antibody (for monoclonal antibodies) for 1
h and finally treated with the chromogenic agent diaminobenzidine
(Dako) for 5 min. Sections were counterstained with Mayer's
hematoxylin. Samples were assessed by light microscopy, and TP16
and TP53 positivity was evaluated according to the intensity and
expected pattern of cellular staining.
Statistical analysis
All statistical analyses were performed using SPSS
software version 16.0 (SPSS Inc., Chicago, USA). Chi-square tests
were used to examine associations between HPV status in tumors and
clinical characteristics (age, sex, smoking history, histology
type, differentiation, lymph node metastasis, distant metastasis,
and TP53 and TP16 expression). Progression-free survival (PFS) was
defined as the time from diagnosis to recurrence or the date of
censorship (the last date of follow-up). We analyzed the combined
effects of HPV infection and over-expression of TP53 and TP16 on
PFS. Survival curves were estimated using the Kaplan-Meier method
and differences in survival distributions were evaluated using a
log-rank test. To evaluate mortality risk, the hazard ratio (HR)
and the corresponding confidence interval (CI) were estimated using
Cox proportional hazards models to identify potential prognostic
factors. All statistical tests were two-sided and P<0.05 was
considered to indicate a statistically significant difference.
Results
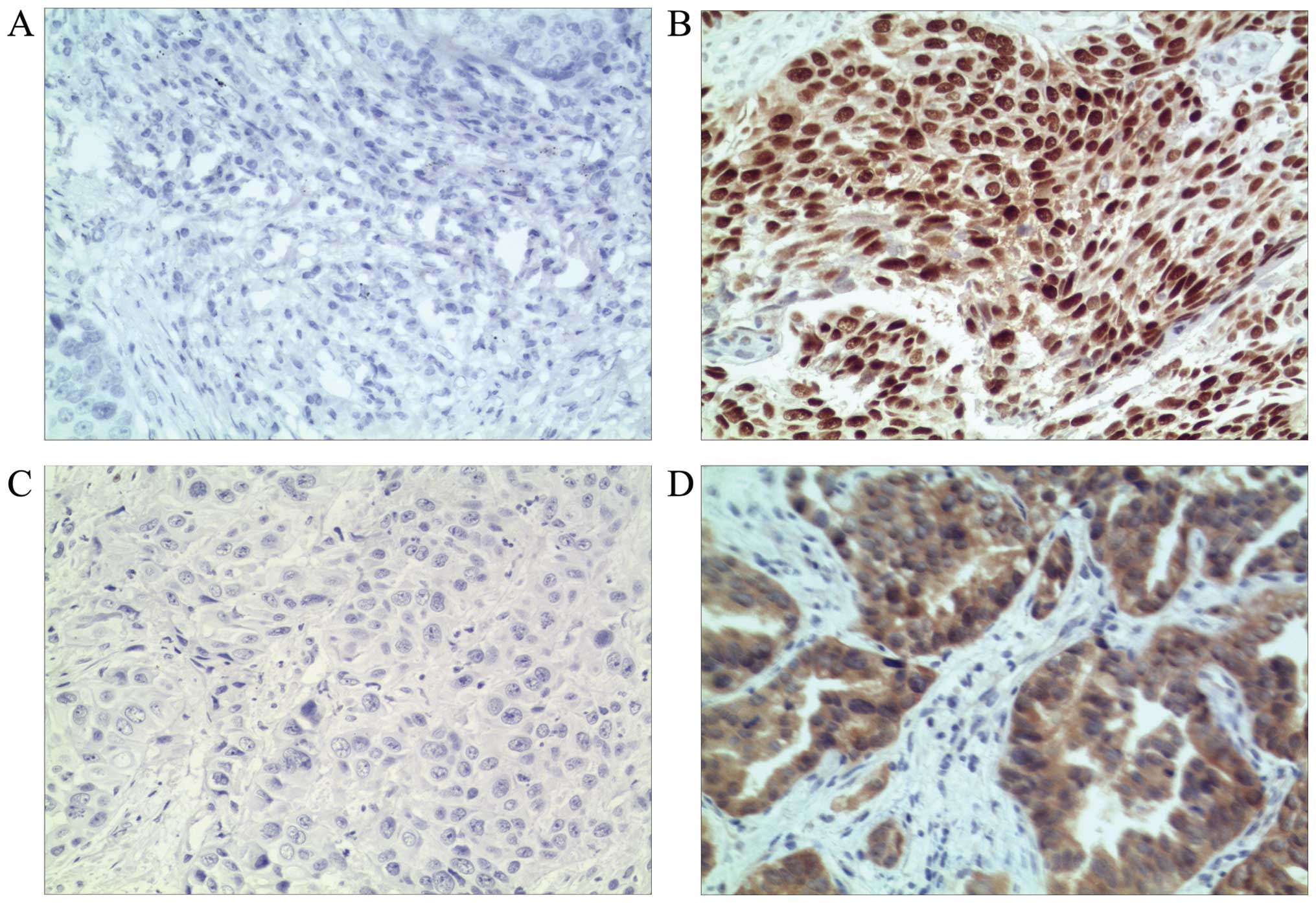
TP53 and TP16 expression in NSCLC
To evaluate the expression of TP53 and TP16 in NSCLC
tissues, qualitative, intensity-based analysis of tissue staining
was performed. Histological samples were assigned one of four
possible scores, as follows: No detectable expression, detectable
nuclear staining, easily visible nuclear staining, and strong
staining. Samples that were scored as ‘no detectable expression’
were defined as negative; samples scored as ‘strong staining’ were
defined as positive for TP53 or TP16 expression. Representative
examples of NSCLC patient tissue samples stained for TP53 or TP16
that scored as either positive or negative are shown in Fig. 1.
HPV status and TP53 and TP16
expression scores
Table I summarizes the
clinical characteristics of the 83 patients with advanced NSCLC
included in this study. Patients were assigned to one of two groups
according to their HPV status, as determined by PCR-based HPV DNA
screening of tissue specimens. Of the 83 specimens examined, 20
were HPV-positive and 63 were HPV-negative. HPV 16 was the most
common subtype detected, although HPV 18, 33, 58, 6 and 11 were
also detected. HPV infection was significantly associated with poor
differentiation (P=0.024), never smoking (P=0.048), and lymph node
metastasis (P=0.044). A total of 28 (33.7%) patients were found to
be TP16-positive and 48 (57.8%) were found to be TP53-positive.
However, the presence of HPV DNA did not significantly correlate
with positive TP53 or TP16 expression status (Table I). Multivariate analysis revealed that
poor differentiation (OR=0.163) was an independent predictive
factor of HPV infection in advanced NSCLC (Table II). This phenomenon was not found in
the equation of logistic regression analysis for HPV infection
(Table III).
 | Table I.Clinical characteristics of patients,
grouped according to HPV status. |
Table I.
Clinical characteristics of patients,
grouped according to HPV status.
|
| HPV |
|
|---|
|
|
|
|
|---|
| Characteristic | Positive | Negative | P-value |
|---|
| Age |
|
| 0.965 |
| ≤Median
age | 11 (13.3) | 35 (42.2) |
|
|
>Median age | 9 (10.8) | 28 (33.7) |
|
| Sex |
|
| 0.344 |
|
Female | 9 (10.8) | 21 (25.3) |
|
| Male | 11 (13.3) | 42 (50.6) |
|
| Differentiation |
|
| 0.024 |
| Well | 2 (2.4) | 23 (27.7) |
|
| Poor | 18 (21.7) | 40 (48.2) |
|
| Smoking status |
|
| 0.048 |
| Never
smokers | 13 (15.7) | 25 (30.1) |
|
| Current
and former smokers | 7 (8.4) | 38 (45.8) |
|
| Histology |
|
| 0.224 |
|
Squamous | 17 (20.5) | 45 (54.2) |
|
|
Adenocarcinoma | 3 (3.6) | 18 (21.7) |
|
| Lymph node
metastasis |
|
| 0.044 |
| Yes | 9 (10.8) | 44 (53) |
|
| No | 11 (13.3) | 19 (22.9) |
|
| Distant
metastasis |
|
| 0.282 |
| Yes | 9 (10.8) | 37 (44.6) |
|
| No | 11 (13.3) | 26 (31.3) |
|
| Expression of
TP16 |
|
| 0.496 |
|
Negative | 12 (14.5) | 43 (51.8) |
|
|
Positive | 8 (9.6) | 20 (24.1) |
|
| Expression of
TP53 |
|
| 0.456 |
|
Negative | 7 (8.4) | 28 (33.7) |
|
|
Positive | 13 (15.7) | 35 (42.2) |
 | Table II.Multivariate analysis of predictive
factors for HPV infection. |
Table II.
Multivariate analysis of predictive
factors for HPV infection.
| Characteristic | OR | 95% CI | P-value |
|---|
| Age (≤median age
vs. >median age) | 1.129 | 0.337–3.781 | 0.844 |
| Sex (female vs.
male) | 0.639 | 0.135–3.029 | 0.572 |
| Differentiation
(well vs. poor) | 0.163 | 0.030–0.896 | 0.037 |
| Smoking status
(never smokers vs. current and former smokers) | 0.348 | 0.075–1.610 | 0.177 |
| Histology (squamous
vs. adenocarcinoma) | 0.282 | 0.050–1.600 | 0.153 |
| Lymph node
metastasis (yes vs. no) | 0.364 | 0.099–1.347 | 0.130 |
| Distant metastasis
(yes vs. no) | 0.638 | 0.168–2.416 | 0.508 |
| Expression of P16
(low vs. high) | 1.087 | 0.324–3.653 | 0.893 |
| Expression of P53
(low vs. high) | 2.649 | 0.749–9.367 | 0.131 |
 | Table III.Variables in the equation of logistic
regression analysis for HPV infection. |
Table III.
Variables in the equation of logistic
regression analysis for HPV infection.
| Variable | β | Sample mean | Wald value | RR | 95% CI | P-value |
|---|
| HPV infection | −0.382 | 0.282 | 1.841 | 0.682 | 0.393–1.185 | 0.175 |
| TP16 | −0.514 | 0.259 | 3.946 | 0.598 | 0.772–2.006 | 1.245 |
| TP53 | 0.219 | 0.244 | 0.809 | 1.245 | 0.360–0.993 | 0.598 |
|
Differentiation | −0.220 | 0.265 | 0.691 | 0.802 | 0.477–1.348 | 0.406 |
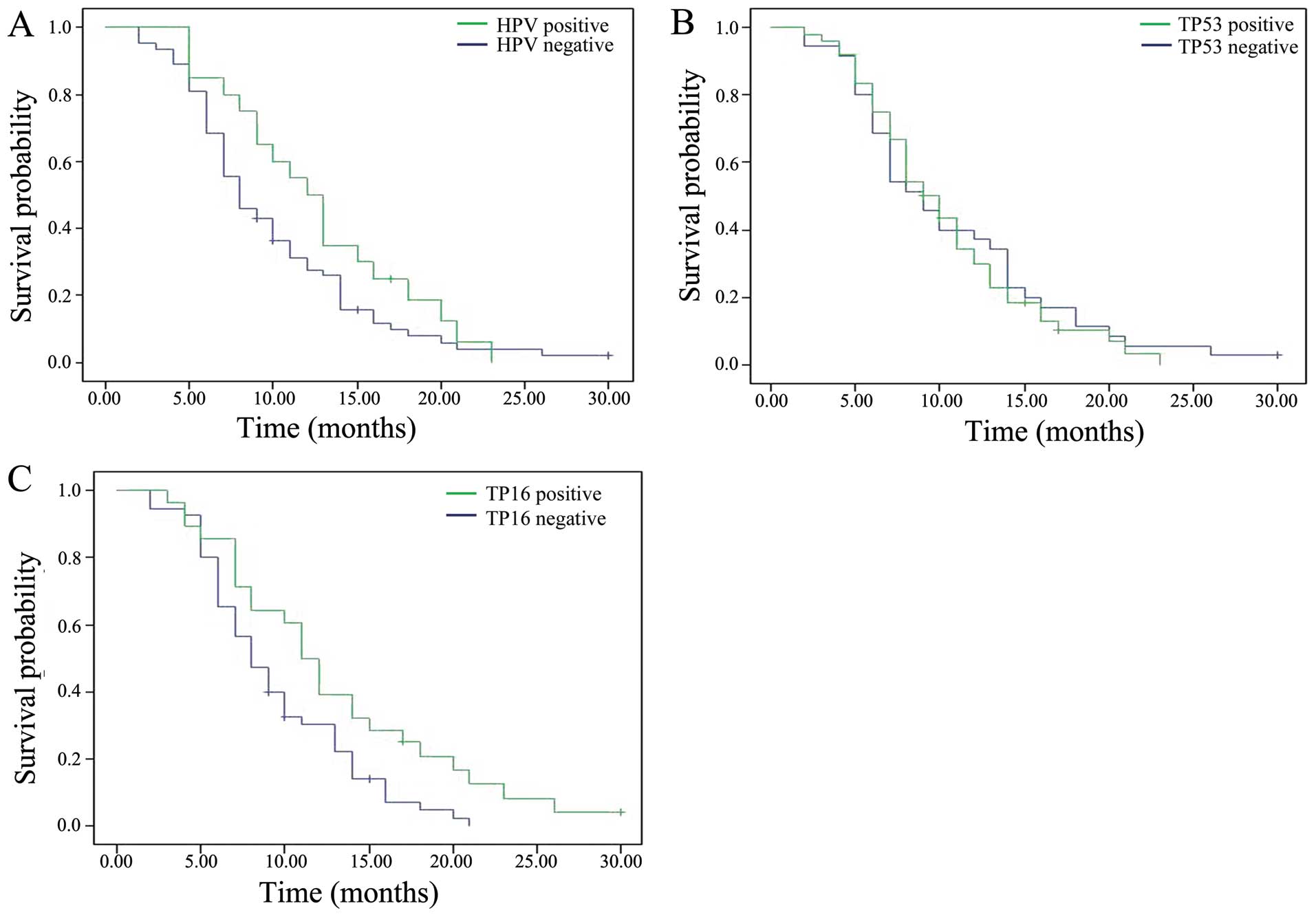
Effects of HPV status and positive
TP16 expression on progression-free survival (PFS)
To examine the clinical significance of HPV
infection as well as TP16 and TP53 expression status in advanced
NSCLC, we evaluated their impact on PFS. Survival curves were
generated using Kaplan-Meier estimates (Fig. 2). HPV-positive patients had a median
PFS of 12 months compared with 8 months for HPV-negative patients.
However, this difference was not statistically significant (HR,
0.68; 95% CI, 0.409–1.161; P=0.162).
As shown in Table IV,
TP53 expression status had no significant effect on patient
survival (HR, 1.102; 95% CI 0.698–1.738; P=0.678). However, when we
analyzed the impact of TP16 expression status on survival we found
that TP16-positive patients had a median PFS of 11 months compared
with 8 months for TP16-negative patients, and this difference was
statistically significant (HR, 0.562; 95% CI, 0.341–0.924;
P=0.023).
 | Table IV.Results of the univariate and
multivariate analyses of selected factors for progression-free
survival in all patients. |
Table IV.
Results of the univariate and
multivariate analyses of selected factors for progression-free
survival in all patients.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender (male vs.
female) | 0.624 | 0.383–1.017 | 0.059 |
|
|
|
| Age (<64 vs.
≥64) | 0.929 | 0.593–1.456 | 0.749 |
|
|
|
| Smoking (never vs.
ever) | 2.012 | 1.254–3.229 | 0.004 | 0.274 | 0.822–2.408 | 0.213 |
| Histology
(adenocarcinoma vs. squamous cell carcinoma) | 2.168 | 1.273–3.695 | 0.004 | 0.306 | 1.105–3.662 | 0.022 |
| Histological
differentiation (well vs. poor) | 0.873 | 0.535–1.424 | 0.586 |
|
|
|
| Lymph node
metastasis (yes vs. no) | 2.576 | 1.493–4.443 | 0.001 | 0.292 | 1.357–4.256 | 0.003 |
| Distant metastasis
(yes vs. no) | 1.294 | 0.824–2.032 | 0.264 |
|
|
|
| HPV (positive vs.
negative) | 0.689 | 0.409–1.161 | 0.162 |
|
|
|
| TP16 (positive vs.
negative) | 0.562 | 0.341–0.924 | 0.023 | 0.261 | 0.404–1.125 | 0.132 |
| TP53 (positive vs.
negative) | 1.102 | 0.698–1.738 | 0.678 |
|
|
|
In addition, our data confirmed that patients who
never smoked had a longer PFS than smokers, while patients with
adenocarcinoma had a longer PFS than those with squamous cell
carcinoma. Multivariate analysis also demonstrated that histology
type and lymph node metastasis were significant determinants of
PFS.
Discussion
The present study demonstrated that, of the 83
Chinese patients with advanced NSCLC, the overall frequency of HPV
detection was 24%, and that HPV 16 was the mostly commonly detected
type. Our data indicate that HPV infections were associated with
non-smokers, patients with lymph node metastasis and patients with
poorly differentiated tumors. In our multiple biomarker assessment
(namely, HPV, TP53 and TP16 status) we identified that
TP16-positive patients exhibited longer survival times when
compared with other subgroups. However, we did not find this trend
for the TP53-positive subgroups.
Although the most common cause of lung cancer is
long-term exposure to tobacco smoke, 15% of men and 53% of women
with lung cancer (worldwide) have cancers that are not attributable
to tobacco smoking (24). The
possibility of HPV involvement in bronchial squamous cell carcinoma
was first suggested in 1979, and since then, several studies have
provided strong support for a role for HPV as an etiological agent
of lung cancer (25). A recent study
found that the prevalence of HPV in NSCLC was 28.1% in Asian
countries (N=2337 NSCLC cases), 8.4% in European countries (n=1553)
and 21.3% in North and South America (n=160) (26). The frequency of HPV infection in Asian
NSCLC samples was higher when compared with lung cancer samples
from Europe and the US. To date, >100 subtypes of HPV have been
identified. HPV l6 and HPV 18 are the most common subtypes
associated with cancer, although numerous other types (HPV 6, 11,
31, 33, 39, 52, 58, and 82) have also been detected (27,28). In
the present study, we detected HPV 16, 18, 33, 58, 6, and 11 in
patient samples, although HPV 16 was detected in the majority of
cases. The overall frequency of HPV-DNA detection in NSCLC
patients' samples was 24% less than the mean number of HPV cases in
Asian NSCLC samples. Given that the 83 patients in our study were
diagnosed with stage III–IV disease, we hypothesize that HPV
infection may be associated with disease stage and metastasis in
lung cancer.
Recent studies have shown that for HPV-related
NSCLC, patients are more likely to be female, non-smoking, have
poorly differentiated tumors, present at a late clinical stage, and
exhibit more lymph node metastasis (29). In the present study, we also
identified that HPV infection was significantly associated with
poor differentiation, never-smokers and lymph node metastasis.
Therefore, our findings are consistent with those reported by
others.
The majorities of patients diagnosed with NSCLC
present with advanced stage disease and have extremely poor
prognoses. Currently there are no widely accepted independent
prognostic factors to evaluate prognosis and survival of advanced
NSCLC. Molina-Vial et al have demonstrated that
nondisruptive mutations of TP53 are an independent prognostic
factor of shorter survival in advanced NSCLC (30). In this study, TP53-positive patients
with HPV-related NSCLC were more numerous than TP53-negative
patients. Although the difference was not statistically
significant, the average survival of TP53-positive patients implies
that poor prognosis is associated with this clinical phenotype.
However, more clinical samples are needed to confirm this
hypothesis.
The present study has demonstrated that there is no
significant correlation between TP16 expression and HPV-positivity
in NSCLC patients. In a recent study, Gatta et al (31). Analyzed TP16 protein expression on an
83-NSCLC tissue sample microarray by immunohistochemistry and also
found no association between the presence of HPV DNA and TP16
protein expression. However, our survival analysis indicates that
TP16 positivity is associated with longer survival in NSCLC.
Although TP16 may be a prognostic indicator in NSCLC patients,
controversy regarding geographical differences as well as
differences in tissue types still exist (32). The present study provides new
possibilities in the prognosis and diagnosis of advanced NSCLC. The
role of TP16 in NSCLC disease progression will be investigated in
subsequent studies.
In conclusion, we have demonstrated that TP53 and
TP16 protein expression is not associated with the expression of
HPV DNA, but that TP16 positivity may be an independent prognostic
factor of longer survival in advanced NSCLC. Clinical trials are
now warranted to determine whether TP16-negative patients would
benefit from drugs that improve the expression of TP16.
Acknowledgements
The current study was supported by the National
Natural Science Foundation of China (grant no. 81172172).
References
|
1
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the Human
Development Index (2008–2030): A population-based study. Lancet
Oncol. 13:790–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murray CJ and Lopez AD: Alternative
projections of mortality and disability by cause 1990–2020: Global
burden of disease study. Lancet. 349:1498–1504. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lam WK, White NW and Chan-Yeung MM: Lung
cancer epidemiology and risk factors in Asia and Africa. Int J
Tuberc Lung Dis. 8:1045–1057. 2004.PubMed/NCBI
|
|
4
|
Rezazadeh A, Laber DA, Ghim SJ, Jenson AB
and Kloecker G: The role of human papilloma virus in lung cancer: A
review of the evidence. Am J Med Sci. 338:64–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Castle PE, Cuzick J, Stoler MH, Wright TC
Jr, Reid JL, Dockter J, Giachetti C and Getman D: Detection of
human papillomavirus 16, 18, and 45 in women with ASC-US cytology
and the risk of cervical precancer: Results from the CLEAR HPV
study. Am J Clin Pathol. 143:160–167. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slama J, Sehnal B, Dusek L, Zima T and
Cibula D: Impact of risk factors on prevalance of anal HPV
infection in women with simultaneous cervical lesion. Neoplasma.
62:308–314. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zur Hausen H: Papillomaviruses in the
causation of human cancers-a brief historical account. Virology.
384:260–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park MS, Chang YS, Shin JH, Kim DJ, Chung
KY, Shin DH, Moon JW, Kang SM, Hahn CH, Kim YS, et al: The
prevalence of human papillomavirus infection in Korean non-small
cell lung cancer patients. Yonsei Med J. 48:69–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Isa SI, Kurahara Y, Yamamoto S, Tamiya A,
Omachi N, Asami K, Okishio K, Utsumi T, Ito N, Yoon HE, et al:
Molecular analysis of human papillomavirus in never-smokers with
non-small cell lung cancer. Oncol Lett. 9:927–929. 2015.PubMed/NCBI
|
|
10
|
Ragin C, Obikoya-Malomo M, Kim S, Chen Z,
Flores-Obando R, Gibbs D, Koriyama C, Aguayo F, Koshiol J, Caporaso
NE, et al: HPV-associated lung cancers: An international pooled
analysis. Carcinogenesis. 35:1267–1275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng YW, Chiou HL, Sheu GT, Hsieh LL,
Chen JT, Chen CY, Su JM and Lee H: The association of human
papillomavirus 16/18 infection with lung cancer among nonsmoking
Taiwanese women. Cancer Res. 61:2799–2803. 2001.PubMed/NCBI
|
|
12
|
Srinivasan M, Taioli E and Ragin CC: Human
papillomavirus type 16 and 18 in primary lung cancers-a
meta-analysis. Carcinogenesis. 30:1722–1728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koshiol J, Rotunno M, Gillison ML, Van
Doorn LJ, Chaturvedi AK, Tarantini L, Song H, Quint WG, Struijk L,
Goldstein AM, et al: Assessment of human papillomavirus in lung
tumor tissue. J Natl Cancer Inst. 103:501–507. 2001. View Article : Google Scholar
|
|
14
|
Proctor I, Stoeber K and Williams GH:
Biomarkers in bladder cancer. Histopathology. 57:1–13. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Helt AM, Funk JO and Galloway DA:
Inactivation of both the retinoblastoma tumor suppressor and p21 by
the human papillomavirus type 16 E7 oncoprotein is necessary to
inhibit cell cycle arrest in human epithelial cells. J Virol.
76:10559–10568. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murao K, Yoshioka R and Kubo Y: Human
papillomavirus infection in Bowen disease: Negative p53 expression,
not p16INK 4a overexpression, is correlated with human
papillomavirus- associated Bowen disease. J Dermatol. 41:878–884.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vainshtein J, McHugh JB, Spector ME,
Walline HM, Komarck CM, Stenmark MH, Prince ME, Worden FP, Wolf GT,
Bradford CR, et al: Human papillomavirus-related oropharyngeal
cancer: HPV and p16 status in the recurrent versus parent tumor.
Head Neck. 37:8–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maniakas A, Moubayed SP, Ayad T, Guertin
L, Nguyen-Tan PF, Gologan O, Soulieres D and Christopoulos A:
North-American survey on HPV-DNA and p16 testing for head and neck
squamous cell carcinoma. Oral Oncol. 50:942–946. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Umbreit C, Aderhold C, Faber A, Sauter A,
Hofheinz RD, Stern-Straeter J, Hoermann K and Schultz JD:
Imatinib-associated matrix metalloproteinase suppression in
p16-positive squamous cell carcinoma compared to HPV-negative HNSCC
cells in vitro. Oncol Rep. 32:668–676. 2014.PubMed/NCBI
|
|
20
|
Zhang EY and Tang XD: Human papillomavirus
type 16/18 oncoproteins: Potential therapeutic targets in
non-smoking associated lung cancer. Asian Pac J Cancer Prev.
13:5363–5369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suzuki Y, Oonishi T, Kudo T and Doi H:
LKB1, TP16, EGFR and KRAS somatic mutations in lung adenocarcinomas
from a Chiba Prefecture, Japan cohort. Drug Discov Ther. 6:24–30.
2012.PubMed/NCBI
|
|
22
|
Trbusek M, Smardova J, Malcikova J,
Sebejova L, Dobes P, Svitakova M, Vranova V, Mraz M, Francova HS,
Doubek M, et al: Missense mutations located in structural p53
DNA-binding motifs are associated with extremely poor survival in
chronic lymphocytic leukemia. J Clin Oncol. 29:2703–2708. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vainshtein J, McHugh JB, Spector ME,
Walline HM, Komarck CM, Stenmark MH, Prince ME, Worden FP, Wolf GT,
Bradford CR, et al: Human papillomavirus-related oropharyngeal
cancer: HPV and p16 status in the recurrent versus parent tumor.
Head Neck. 37:8–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reck M, Popat S, Reinmuth N, De Ruysscher
D, Kerr KM and Peters S: Metastatic non-small-cell lung cancer
(NSCLC): ESMO clinical practice guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 25(Suppl 3): iii27–iii39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Syrjanen KJ: Condylomatous changes in
neoplastic bronchial epithelium. Respiration. 38:299–304. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hasegawa Y, Ando M, Kubo A, Isa S,
Yamamoto S, Tsujino K, Kurata T, Ou SH, Takada M and Kawaguchi T:
Human papilloma virus in non-small cell lung cancer in never
smokers: A systematic review of the literature. Lung Cancer.
83:8–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gillison ML, Koch WM, Capone RB, Spafford
M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, et
al: Evidence for acausal association between human papillomavirus
and a subset of head and neck cancers. J Natl Cancer Inst.
92:709–720. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kreimer AR, Clifford GM, Boyle P and
Franceschi S: Human papillomavirus types in head and neck squamous
cell carcinomas worldwide: A systematic review. Cancer Epidemiol
Biomarkers Prey. 14:467–475. 2005. View Article : Google Scholar
|
|
29
|
Subramanian J and Govindan R: Molecular
genetics of lung cancer in people who have never smoked. Lancet
Oncol. 9:676–682. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Molina-Vila MA, Bertran-Alamillo J, Gascó
A, Mayo-de-las-Casas C, Sánchez-Ronco M, Pujantell-Pastor L,
Bonanno L, Favaretto AG, Cardona AF, Vergnenègre A, et al:
Nondisruptive p53 mutations are associated with shorter survival in
patients with advanced non-small cell lung cancer. Clin Cancer Res.
20:4647–4659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gatta LB, Balzarini P, Tironi P, Berenzi
A, Benetti A, Angiero F, Grigolato P and Dessy E: Human
papillomavirus DNA and p16 gene in squamous cell lung carcinoma.
Anticancer Res. 32:3085–3089. 2012.PubMed/NCBI
|
|
32
|
Su CY, Chang YC, Chan YC, Lin TC, Huang
MS, Yang CJ and Hsiao M: MTAP is an independent prognosis marker
and the concordant loss of MTAP and p16 expression predicts short
survival in non-small cell lung cancer patients. Eur J Surg Oncol.
40:1143–1150. 2014. View Article : Google Scholar : PubMed/NCBI
|