Hepatocellular carcinoma (HCC) is the sixth most
common neoplasm and the third most frequent cause of
cancer-associated mortality worldwide (1). Despite advances in the diagnosis
(2–4)
and treatment (5–11) of this disease, its prognosis remains
poor (12,13). Therefore, clarification of the
mechanisms underlying its development is critically important
(14). Numerous studies have
investigated gene alterations in tumor tissues of HCC and their
functions in hepatocarcinogenesis (14–16).
Various oncogenic and tumor-suppressor genes have been detected in
HCC cell lines and resection specimens. However, HCC usually
develops from an established background of chronic liver disease
(13). Therefore, the elucidation of
the mechanisms underlying HCC carcinogenesis requires the study of
the background liver parenchyma as the potential foundation of this
malignancy, in addition to the biology of the tumor. The present
study reviews previous studies of molecular alterations identified
in HCC, with a focus on the tumor factors and background
factors.
HCC tumor specimens offer important information
regarding certain prognostic factors, including vascular invasion
(17–21), tumor size (21–25) and
pathological grade (26,27), which are robust predictors of clinical
outcomes following curative resection surgeries for HCC.
α-fetoprotein levels are also a prognostic indicator (21,25). These
factors may affect postoperative prognosis and, thus, HCC tumor
tissues require further studies and molecular examinations.
Epigenetic alterations have also been identified to
promote hepatocarcinogenesis; changes in microRNAs (miRNAs) and the
expression of their target genes may provide tools and
opportunities to detect and treat HCC (46–48).
Numerous studies have identified specific miRNAs and their target
genes, and their possible roles in hepatocarcinogenesis (49–54).
Previous studies observed that miRNA-101 (49), −122 (50) and −195 (51) levels are downregulated, whereas
miRNA-21 (52), −221 (53) and −224 (54) levels are upregulated in HCC. The
epigenetic inactivation of tumor-suppressor genes by promoter
hypermethylation has been identified as an important mechanism
underlying tumorigenesis (55).
Hypermethylation has been detected in numerous HCC-associated
genes, including cyclin dependent kinase inhibitor 2A
(p16INK4a) (56,57), runt related transcription factor 3
(RUNX3) (58,59), suppressor of cytokine signaling 1
(SOCS1) (60,61), secreted frizzled related protein 1
(SFRP1) (62,63), O-6-methylguanine-DNA methyltransferase
(MGMT) (64–66), Ras association domain family member 1
(RASSF1A) (67–69) and glutathione S-transferase pi 1
(GSTP1) (66,70) by investigations using various kinds of
HCC cell lines and various stage of clinical HCC samples.
Expression array analysis is frequently used to
detect novel cancer-associated genes (71–75) by
comparing gene expression levels between cancerous and
non-cancerous tissues. Okabe et al (72) and Shirota et al (73) extracted mRNA from the cancerous and
noncancerous tissues of a number of patients with HCC, in order to
evaluate the variations in gene expression levels, and detected
certain genes that characterize HCC. Nomoto et al (74) performed a double-combination array
analysis consisting of an expression array and single nucleotide
polymorphism (SNP) array, which facilitated the identification of
novel genes associated with hepatocarcinogenesis using expression
profiling and chromosomal copy number analysis (74,76–80).
Microarray analysis was also used to reveal cancer-associated
miRNAs (52,81). Wong et al (81) detected the downregulation of miRNA-223
expression levels in HCC tissues and investigated stathmin 1
(STMN1) as a downstream target. Methylation array analysis
has been used to detect cancer-specific epigenetic alterations in
HCC (82–91). Shen et al (82) analyzed tumor and adjacent non-tumor
tissue samples from 62 Taiwanese patients with HCC, using
Illumina® methylation arrays, and identified several
genes that were hyper-or hypo-methylated in tumor tissues, as
compared with adjacent non-tumor tissues (82). The candidate genes were pyrosequenced
and the array data was validated; analysis of plasma DNA suggested
those genes may be used as biomarkers for early-stage HCC. Okamura
et al (83) created a
triple-combination array, consisting of an SNP array, gene
expression microarray and methylation array analysis (83–89). The
tumor-suppressor gene candidates were efficiently identified by
detecting downregulation and methylation in tumor tissues, and
without chromosomal deletion of the loci of the targeted genes.
Revill et al (90) performed a
genome-wide methylation array analysis of HCC samples and a
microarray analysis of gene re-expression in HCC cell lines, and
then combined the data to locate an epigenetically-silenced
tumor-suppressor gene. These studies demonstrate that the majority
of hepatocarcinogenesis studies focus on the changes in tumor
tissues and the comparison of HCC cell lines or tumor tissues with
adjacent normal liver tissues.
Typically, HCC develops within an established
background of chronic liver disease (13,92,93).
Oncogenic agents, including aflatoxin, are common (94) and the majority of patients with HCC
already have background liver disorders, including infections with
hepatitis B (HBV) or C (HBC) virus (95–98),
alcohol intake (99) or non-alcoholic
fatty liver disease (100,101). Following hepatic resection, the
principal HCC recurrence site is the residual liver (102). Senthilnathan et al (103) demonstrated that the majority of
patients treated with loco-regional therapies for HCC succumbed to
the disease prior to developing extra-hepatic metastasis,
regardless of age; this is unique to HCC, as compared with other
types of malignancies. Therefore, previous studies reported that
background liver status was an effective prognostic factor
following tumor resection in patients with HCC (102,104).
Zhou et al (104) performed a
multivariate analysis of significant survival predictors in 1,000
patients with HCC (tumor diameter <5 cm) and determined liver
cirrhosis to be an independent risk factor.
Multinodular HCCs are classified as multicentric
(MC) occurrence (MO) or intrahepatic metastasis (IM) (105). Wang et al (106) investigated 15 high-frequency
loss-of-heterozygosity DNA microsatellites in 100 tumor nodules in
60 matched pairs of recurrent HCC tissue samples from 40 patients
who underwent liver tumor re-resections. The data demonstrated that
IM-type recurrences were more common and had a poorer prognosis,
compared with MO-type recurrences. By contrast, Nomoto et al
(107) reported clonal analyses of
tumor cells that demonstrated recurrent HCC genotypes,
mitochondrial gene mutations (107)
or patterns of promoter hypermethylation in various
tumor-suppressor genes (108), and
identified MO to be a more common type of recurrence, compared with
IM, in Japanese patients. HCC patients with an HBV infection, which
is the principal underlying factor of this malignancy in Eastern
Asia and sub-Saharan Africa, were compared with patients with an
HCV infection (primarily endemic in North America, Europe and
Japan) (13). The patients with
HCV-based HCC exhibited poorer liver function and had shorter
overall survival and disease-free survival times (109). Therefore, background liver status is
crucial in predicting prognosis following HCC surgical treatment,
particularly in patients who are likely to have MO-type
recurrences. Detailed studies regarding the background liver may be
essential for the improved understanding of the carcinogenesis and
progression of this malignancy.
A number of previous studies have reported the
detection of molecular changes in the HCC background liver
(110–116). Okamoto et al (112) compared gene-expression patterns in
noncancerous liver tissue specimens from HCV-positive patients with
HCC, between those patients with single-node HCC and those with MC
HCC. The study selected 36 genes commonly associated with MC HCC
and MC recurrence, and created a scoring system to determine the
risk for MC hepatocarcinogenesis; the prediction score was higher
in multicentric recurrence group than in that without (112). Utsunomiya et al (113) performed an miRNA microarray to
examine the variations in miRNA expression patterns in
non-cancerous liver tissue samples between the MC recurrence group
and non-MC recurrence group, in order to identify miRNAs associated
with MC recurrence. The study detected 20 differentially expressed
miRNAs, of which 18 were downregulated and 2 were upregulated in
the MC group. The same research group reviewed the concept of field
cancerization, in which tumorigenesis may be viewed as cancer
initiated by numerous cumulative epigenetic and genetic changes
that transform a cell, or a group of cells, in a particular organ
(110–113). Hoshida et al (114) revealed that the gene expression
profiles in non-tumor liver tissue, but not in primary HCC tissues,
were highly associated with late recurrence (114). Nomoto et al (115) detected novel candidates for
cancer-associated genes from array analyses, which compared
supernormal liver tissue samples from resected secondary metastatic
liver malignancies without HCC and corresponding normal tissue
samples of HCC with chronic HCV (115,116).
Certain studies have investigated the epigenetic
status of the HCC background liver and reported that molecular
alterations, including DNA methylation, may change gradually during
the transition from normal liver tissues to non-tumor liver tissue,
and then to malignant HCC tissues (110,117–119).
Ammerpohl et al (117)
performed a methylation array analysis using 17 HCC, 17 cirrhosis
and 12 normal control tissues; they detected certain genes with
significantly differences in hyper-or hypo-methylation between
normal liver controls and cirrhosis as well as between cirrhosis
and HCC (117). Um et al
(118) evaluated the methylation
status in CpG islands of the promoter regions of nine genes in
normal liver tissues, cirrhosis, low-grade dysplastic nodules,
high-grade dysplastic nodules, early-stage HCC tissues and
late-stage HCC tissues (118). The
study identified an increased methylation frequency along the
sequence of multi-step hepatocarcinogenesis in some of these genes.
Arai et al (119)
investigated genome-wide DNA methylation profiles in normal liver
tissue samples obtained from patients without HCC and cancerous and
noncancerous liver tissue samples from patients with HCC; DNA
methylation status was revealed to be correlated with the
cancer-free and overall survival rates of patients with HCC
(119).
Previous studies have also investigated the
background tissues of numerous other malignancies (120–122).
Yoshida et al (120) examined
the methylation status of CpG islands in the promoter regions of
six genes and two repetitive DNA elements in the gastric mucosae,
and revealed that the methylation levels were consistently
increased in a stepwise manner with activity of gastric
inflammation, among patients who were H. pylori–negative, or
had low-or high-titers (120), and
concluded that evaluating DNA methylation levels in the gastric
mucosae may predict the risk of gastric cancer. Lee et al
(121) evaluated promoter region
methylation of the cadherin 1 (CDH1) gene in the
non-neoplastic mucosae of diffuse gastric cancer and revealed there
was a significantly higher methylation ratio, compared with normal
non-cancerous gastric mucosae. Kadara et al (122) compared the gene expression profiles
in non-small cell lung cancer airway tissues at various distances
from the tumor tissues and normal lung tissues (122); significantly higher levels of
lysosomal protein transmembrane 4β (LAPTM4B) mRNA
transcripts were identified in the airway tissues near tumors.
These attempts to clarify the molecular changes in non-cancerous
tissues in several kinds of cancers are similar in concept to the
evaluation of molecules in background liver of HCC.
Although investigations of non-cancerous tissues
adjacent to HCC are required to clarify the mechanisms underlying
hepatocarcinogenesis, few studies have previously evaluated the
microenvironment of the HCC background liver. The concept of
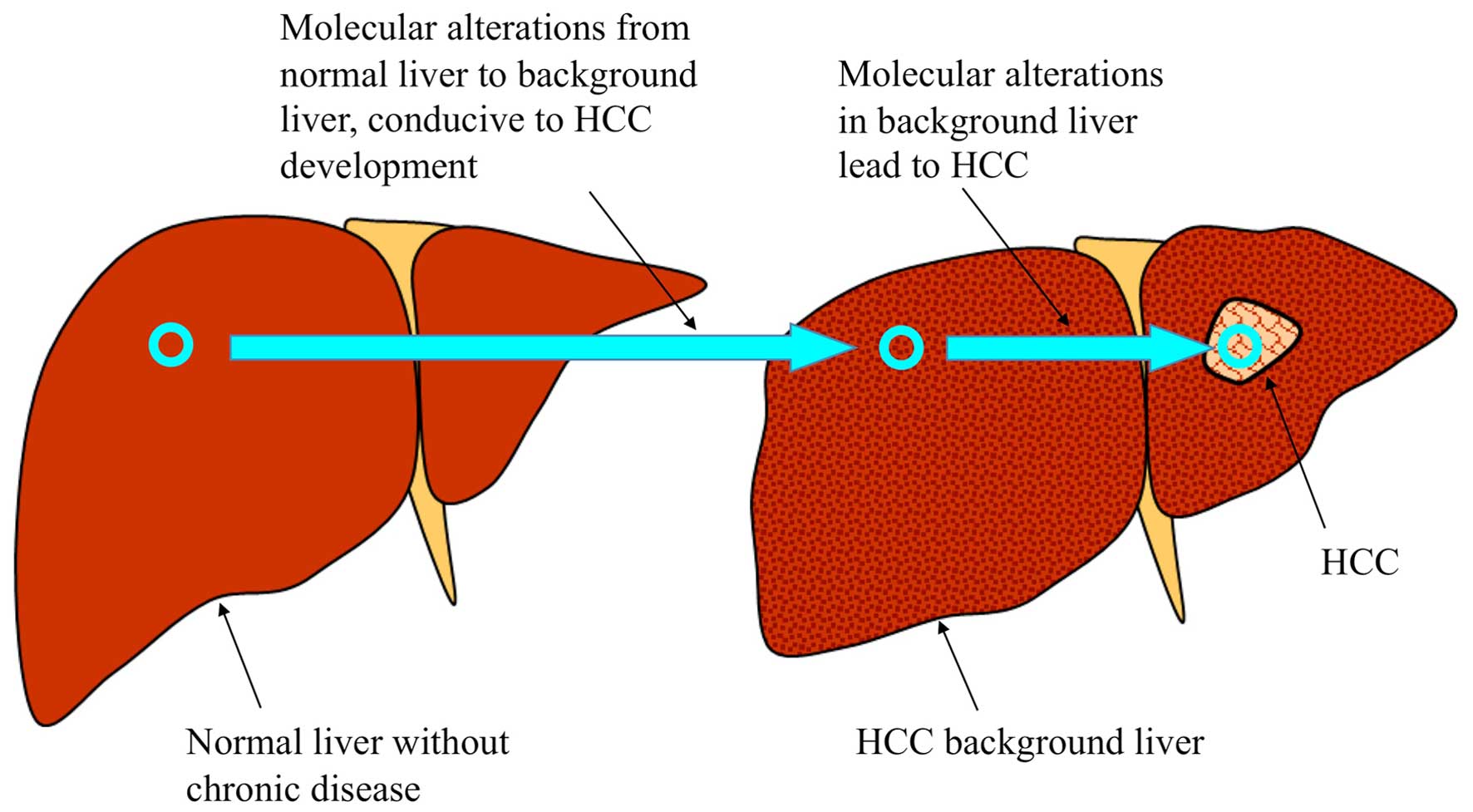
molecular changes in hepatocarcinogenesis is presented in Fig. 1. Although molecular alterations in the
background liver tissues and in HCC tissues require further
elucidation, the importance of background liver is particularly
characteristic of HCC.
Identification of genes associated with
hepatocarcinogenesis and their roles in transforming the background
liver of HCC may facilitate the early detection of HCC in patients
with liver damage, and liver biopsy specimens may provide
information regarding early-stage HCC detection for certain
patients. Furthermore, analysis of the adjacent liver parenchymal
tissues in resected tumor samples from patients with HCC may assist
the prediction of recurrence in the remaining liver tissues.
Sorafenib is a molecular-targeted agent for HCC, which has been
approved as a standard treatment for patients with advanced HCC
that may not be treated with surgical resection (123); however, its therapeutic value has
yet to be clinically established in patients with HCC who have
undergone tumor resection surgery (124). The efficacies of adjuvant
chemotherapies are currently limited, although certain
postoperative antiviral treatments, including nucleotide analogs
administered following surgical resection for HBV-associated HCC,
or interferon for HCV-associated HCC, have been previously reported
to indirectly reduce the recurrence of HCC following surgery in
some studies (125). The elucidation
of the mechanisms underlying hepatocarcinogenesis in the background
liver may facilitate the development of novel adjuvant
chemotherapies, which may be administered to patients following the
resection of primary lesions.
In summary, although the mechanisms underlying
hepatocarcinogenesis require further study, the status of the
background liver tissues in HCC may have a crucial role in the
development and progression of this type of malignancy.
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forner A, Vilana R, Ayuso C, Bianchi L,
Solé M, Ayuso JR, Boix L, Sala M, Varela M, Llovet JM, et al:
Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis:
Prospective validation of the noninvasive diagnostic criteria for
hepatocellular carcinoma. Hepatology. 47:97–104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bolondi L, Sofia S, Siringo S, Gaiani S,
Casali A, Zironi G, Piscaglia F, Gramantieri L, Zanetti M and
Sherman M: Surveillance programme of cirrhotic patients for early
diagnosis and treatment of hepatocellular carcinoma: A cost
effectiveness analysis. Gut. 48:251–259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Meer S, de Man RA, Siersema PD and van
Erpecum KJ: Surveillance for hepatocellular carcinoma in chronic
liver disease: Evidence and controversies. World J Gastroenterol.
19:6744–6756. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tateishi R, Shiina S, Teratani T, Obi S,
Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T and Omata M:
Percutaneous radiofrequency ablation for hepatocellular
carcinoma-An analysis of 1000 cases. Cancer. 103:1201–1209. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
Chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abou-Alfa GK, Schwartz L, Ricci S, Amadori
D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz
B, et al: Phase II study of sorafenib in patients with advanced
hepatocellular carcinoma. J Clin Oncol. 24:4293–4300. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takayasu K, Arii S, Ikai I, Omata M, Okita
K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M, et al:
Prospective cohort study of transarterial chemoembolization for
unresectable hepatocellular carcinoma in 8510 patients.
Gastroenterology. 131:461–469. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Llovet JM and Bruix J: Novel advancements
in the management of hepatocellular carcinoma in 2008. J Hepatol.
48(Suppl 1): S20–S37. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morise Z, Kawabe N, Tomishige H, Nagata H,
Kawase J, Arakawa S, Yoshida R and Isetani M: Recent advances in
the surgical treatment of hepatocellular carcinoma. World J
Gastroenterol. 20:14381–14392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen X, Liu HP, Li M and Qiao L: Advances
in non-surgical management of primary liver cancer. World J
Gastroenterol. 20:16630–16638. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
El-Serag HB and Rudolph L: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai
P, Chu PW, Lam CT, Poon RT and Fan ST: Significance of CD90+ cancer
stem cells in human liver cancer. Cancer Cell. 13:153–166. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ladeiro Y, Couchy G, Balabaud C,
Bioulac-Sage P, Pelletier L, Rebouissou S and Zucman-Rossi J:
MicroRNA profiling in hepatocellular tumors is associated with
clinical features and oncogene/tumor suppressor gene mutations.
Hepatology. 47:1955–1963. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eguchi S, Takatsuki M, Hidaka M, Soyama A,
Tomonaga T, Muraoka I and Kanematsu T: Predictor for histological
microvascular invasion of hepatocellular carcinoma: A lesson from
229 consecutive cases of curative liver resection. World J Surg.
34:1034–1038. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roayaie S, Jibara G, Taouli B and Schwartz
M: Resection of hepatocellular carcinoma with macroscopic vascular
invasion. Ann Surg Oncol. 20:3754–3760. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Faber W, Stockmann M, Kruschke JE, Denecke
T, Bahra M and Seehofer D: Implication of microscopic and
macroscopic vascular invasion for liver resection in patients with
hepatocellular carcinoma. Dig Surg. 31:204–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sumie S, Nakashima O, Okuda K, Kuromatsu
R, Kawaguchi A, Nakano M, Satani M, Yamada S, Okamura S, Hori M, et
al: The significance of classifying microvascular invasion in
patients with hepatocellular carcinoma. Ann Surg Oncol.
21:1002–1009. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tandon P and Garcia-Tsao G: Prognostic
indicators in hepatocellular carcinoma: A systematic review of 72
studies. Liver Int. 29:502–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Poon RT and Fan ST: Hepatectomy for
hepatocellular carcinoma: Patient selection and postoperative
outcome. Liver Transpl. 10(2): Suppl 1. S39–S45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pandey D, Lee KH, Wai CT, Wagholikar G and
Tan KC: Long term outcome and prognostic factors for large
hepatocellular carcinoma (10 cm or more) after surgical resection.
Ann Surg Oncol. 14:2817–2823. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ishii T, Hatano E, Yasuchika K, Taura K,
Seo S and Uemoto S: High risk of lung metastasis after resection of
hepatocellular carcinoma more than 7 cm in diameter. Surg Today.
44:1900–1905. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han JH, Kim DG, Na GH, Kim EY, Lee SH,
Hong TH and You YK: Evaluation of prognostic factors on recurrence
after curative resections for hepatocellular carcinoma. World J
Gastroenterol. 20:17132–17140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jonas S, Bechstein WO, Steinmüller T,
Herrmann M, Radke C, Berg T, Settmacher U and Neuhaus P: Vascular
invasion and histopathologic grading determine outcome after liver
transplantation for hepatocellular carcinoma in cirrhosis.
Hepatology. 33:1080–1086. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han DH, Choi GH, Kim KS, Choi JS, Park YN,
Kim SU, Park JY, Ahn SH and Han KH: Prognostic significance of the
worst grade in hepatocellular carcinoma with heterogeneous
histologic grades of differentiation. J Gastroenterol Hepatol.
28:1384–1390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scaggiante B, Kazemi M, Pozzato G, Dapas
B, Farra R, Grassi M, Zanconati F and Grassi G: Novel
hepatocellular carcinoma molecules with prognostic and therapeutic
potentials. World J Gastroenterol. 20:1268–1288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giles RH, van Es JH and Clevers H: Caught
up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta.
1653:1–24. 2003.PubMed/NCBI
|
|
30
|
Tsao CM, Yan MD, Shih YL, Yu PN, Kuo CC,
Lin WC, Li HJ and Lin YW: SOX1 functions as a tumor suppressor by
antagonizing the WNT/β-catenin signaling pathway in hepatocellular
carcinoma. Hepatology. 56:2277–2287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim M, Lee HC, Tsedensodnom O, Hartley R,
Lim YS, Yu E, Merle P and Wands JR: Functional interaction between
Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin
signaling pathway in hepatocellular carcinoma cells. J Hepatol.
48:780–791. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wong CM, Fan ST and Ng IO: Beta-Catenin
mutation and overexpression in hepatocellular carcinoma:
Clinicopathologic and prognostic significance. Cancer. 92:136–145.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fujimoto A, Totoki Y, Abe T, Boroevich KA,
Hosoda F, Nguyen HH, Aoki M, Hosono N, Kubo M, Miya F, et al:
Whole-genome sequencing of liver cancers identifies etiological
influences on mutation patterns and recurrent mutations in
chromatin regulators. Nat Genet. 44:760–764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guichard C, Amaddeo G, Imbeaud S, Ladeiro
Y, Pelletier L, Maad IB, Calderaro J, Bioulac-Sage P, Letexier M,
Degos F, et al: Integrated analysis of somatic mutations and focal
copy-number changes identifies key genes and pathways in
hepatocellular carcinoma. Nat Genet. 44:694–698. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guan YS, La Z, Yang L, He Q and Li P: p53
gene in treatment of hepatic carcinoma: Status quo. World J
Gastroenterol. 13:985–992. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Staib F, Hussain SP, Hofseth LJ, Wang XW
and Harris CC: TP53 and liver carcinogenesis. Hum Mutat.
21:201–216. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hussain SP, Schwank J, Staib F, Wang XW
and Harris CC: TP53 mutations and hepatocellular carcinoma:
Insights into the etiology and pathogenesis of liver cancer.
Oncogene. 26:2166–2176. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bressac B, Kew M, Wands J and Ozturk M:
Selective G to T Mutation Of p53 gene in hepatocellular carcinoma
from southern Africa. Nature. 350:429–431. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hsu IC, Metcalf RA, Sun T, Welsh JA, Wang
NJ and Harris CC: Mutational hotspot in the p53 gene in human
hepatocellular carcinomas. Nature. 350:427–428. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oda T, Tsuda H, Scarpa A, Sakamoto M and
Hirohashi S: p53 gene mutation spectrum in hepatocellular
carcinoma. Cancer Res. 52:6358–6364. 1992.PubMed/NCBI
|
|
42
|
Zhou JD, Shen F, Ji JS, Zheng K, Huang M
and Wu JC: FAM9C plays an anti-apoptotic role through activation of
the PI3K/Akt pathway in human hepatocellular carcinoma. Oncol Rep.
30:1275–1284. 2013.PubMed/NCBI
|
|
43
|
Liu J, Zhang C, Lin M, Zhu W, Liang Y,
Hong X, Zhao Y, Young KH, Hu W and Feng Z: Glutaminase 2 negatively
regulates the PI3K/AKT signaling and shows tumor suppression
activity in human hepatocellular carcinoma. Oncotarget.
5:2635–2647. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pellegrino R, Calvisi DF, Neumann O,
Kolluru V, Wesely J, Chen X, Wang C, Wuestefeld T, Ladu S, Elgohary
N, et al: EEF1A2 inactivates p53 by way of PI3K/AKT/mTOR-dependent
stabilization of MDM4 in hepatocellular carcinoma. Hepatology.
59:1886–1899. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou Q, Wong CH, Lau CP, Hui CW, Lui VW,
Chan SL and Yeo W: enhanced antitumor activity with combining
effect of mTOR inhibition and microtubule stabilization in
hepatocellular carcinoma. Int J Hepatol. 2013:1038302013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Khare S, Zhang Q and Ibdah JA: Epigenetics
of hepatocellular carcinoma: Role of microRNA. World J
Gastroenterol. 19:5439–5445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Law PT and Wong N: Emerging roles of
microRNA in the intracellular signaling networks of hepatocellular
carcinoma. J Gastroenterol Hepatol. 26:437–449. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhu Z, Zhang X, Wang G and Zheng H: Role
of MicroRNAs in Hepatocellular Carcinoma. Hepat Mon. 14:e186722014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu L, Beckebaum S, Iacob S, Wu G, Kaiser
GM, Radtke A, Liu C, Kabar I, Schmidt HH, Zhang X, et al:
MicroRNA-101 inhibits human hepatocellular carcinoma progression
through EZH2 downregulation and increased cytostatic drug
sensitivity. J Hepatol. 60:590–598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tsai WC, Hsu PW, Lai TC, Chau GY, Lin CW,
Chen CM, Lin CD, Liao YL, Wang JL, Chau YP, et al: MicroRNA-122, a
tumor suppressor microRNA that regulates intrahepatic metastasis of
hepatocellular carcinoma. Hepatology. 49:1571–1582. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu T, Zhu Y, Xiong Y, Ge YY, Yun JP and
Zhuang SM: MicroRNA-195 suppresses tumorigenicity and regulates
G1/S transition of human hepatocellular carcinoma cells.
Hepatology. 50:113–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gramantieri L, Fornari F, Ferracin M,
Veronese A, Sabbioni S, Calin GA, Grazi GL, Croce CM, Bolondi L and
Negrini M: MicroRNA-221 targets Bmf in hepatocellular carcinoma and
correlates with tumor multifocality. Clin Cancer Res. 15:5073–5081.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang Y, Takahashi S, Tasaka A, Yoshima T,
Ochi H and Chayama K: Involvement of microRNA-224 in cell
proliferation, migration, invasion and anti-apoptosis in
hepatocellular carcinoma. J Gastroenterol Hepatol. 28:565–575.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tischoff I and Tannapfe A: DNA methylation
in hepatocellular carcinoma. World J Gastroenterol. 14:1741–1748.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liew CT, Li HM, Lo KW, Leow CK, Chan JY,
Hin LY, Lau WY, Lai PB, Lim BK, Huang J, et al: High frequency of
p16INK4A gene alterations in hepatocellular carcinoma. Oncogene.
18:789–795. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Qin Y, Liu JY, Li B, Sun ZL and Sun ZF:
Association of low p16INK4a and p15INK4b mRNAs expression with
their CpG islands methylation with human hepatocellular
carcinogenesis. World J Gastroenterol. 10:1276–1280.
2004.PubMed/NCBI
|
|
58
|
Mori T, Nomoto S, Koshikawa K, Fujii T,
Sakai M, Nishikawa Y, Inoue S, Takeda S, Kaneko T and Nakao A:
Decreased expression and frequent allelic inactivation of the RUNX3
gene at 1p36 in human hepatocellular carcinoma. Liver Int.
25:380–388. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Park WS, Cho YG, Kim CJ, Song JH, Lee YS,
Kim SY, Nam SW, Lee SH, Yoo NJ and Lee JY: Hypermethylation of the
RUNX3 gene in hepatocellular carcinoma. Exp Mol Med. 37:276–281.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yoshikawa H, Matsubara K, Qian GS, Jackson
P, Groopman JD, Manning JE, Harris CC and Herman JG: SOCS-1, a
negative regulator of the JAK/STAT pathway, is silenced by
methylation in human hepatocellular carcinoma and shows
growth-suppression activity. Nat Genet. 28:29–35. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Okochi O, Hibi K, Sakai M, Inoue S, Takeda
S, Kaneko T and Nakao A: Methylation-mediated silencing of SOCS-1
gene in hepatocellular carcinoma derived from cirrhosis. Clin
Cancer Res. 9:5295–5298. 2003.PubMed/NCBI
|
|
62
|
Shih YL, Shyu RY, Hsieh CB, Lai HC, Liu
KY, Chu TY and Lin YW: Promoter methylation of the secreted
frizzled-related protein 1 gene SFRP1 is frequent in hepatocellular
carcinoma. Cancer. 107:579–590. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Huang J, Zhang YL, Teng XM, Lin Y, Zheng
DL, Yang PY and Han ZG: Down-regulation of SFRP1 as a putative
tumor suppressor gene can contribute to human hepatocellular
carcinoma. BMC cancer. 7:1262007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang YJ, Chen Y, Ahsan H, Lunn RM, Lee
PH, Chen CJ and Santella RM: Inactivation of the DNA repair gene
O6-methylguanine-DNA methyltransferase by promoter hypermethylation
and its relationship to aflatoxin B1-DNA adducts and p53 mutation
in hepatocellular carcinoma. Int J Cancer. 103:440–444. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Matsukura S, Soejima H, Nakagawachi T,
Yakushiji H, Ogawa A, Fukuhara M, Miyazaki K, Nakabeppu Y,
Sekiguchi M and Mukai T: CpG methylation of MGMT and hMLH1 promoter
in hepatocellular carcinoma associated with hepatitis viral
infection. Br J Cancer. 88:521–529. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li Z, Zhang H, Yang J, Hao T and Li S:
Promoter hypermethylation of DNA damage response genes in
hepatocellular carcinoma. Cell Biol Int. 36:427–432. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Schagdarsurengin U, Wilkens L, Steinemann
D, Flemming P, Kreipe HH, Pfeifer GP, Schlegelberger B and Dammann
R: Frequent epigenetic inactivation of the RASSF1A gene in
hepatocellular carcinoma. Oncogene. 22:1866–1871. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hu L, Chen G, Yu H and Qiu X:
Clinicopathological significance of RASSF1A reduced expression and
hypermethylation in hepatocellular carcinoma. Hepatol Int.
4:423–432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xu B, Di J, Wang Z, Han X, Li Z, Luo X and
Zheng Q: Quantitative analysis of RASSF1A promoter methylation in
hepatocellular carcinoma and its prognostic implications. Biochem
Biophys Res Commun. 438:324–328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhong S, Tang MW, Yeo W, Liu C, Lo YM and
Johnson PJ: Silencing of GSTP1 gene by CpG island DNA
hypermethylation in HBV-associated hepatocellular carcinomas. Clin
Cancer Res. 8:1087–1092. 2002.PubMed/NCBI
|
|
71
|
Midorikawa Y, Makuuchi M, Tang W and
Aburatani H: Microarray-based analysis for hepatocellular
carcinoma: From gene expression profiling to new challenges. World
J Gastroenterol. 13:1487–1492. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Okabe H, Satoh S, Kato T, Kitahara O,
Yanagawa R, Yamaoka Y, Tsunoda T, Furukawa Y and Nakamura Y:
Genome-wide analysis of gene expression in human hepatocellular
carcinomas using cDNA microarray: Identification of genes involved
in viral carcinogenesis and tumor progression. Cancer Res.
61:2129–2137. 2001.PubMed/NCBI
|
|
73
|
Shirota Y, Kaneko S, Honda M, Kawai HF and
Kobayashi K: Identification of differentially expressed genes in
hepatocellular carcinoma with cDNA microarrays. Hepatology.
33:832–840. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Nomoto S, Kanda M, Okamura Y, Nishikawa Y,
Qiyong L, Fujii T, Sugimoto H, Takeda S and Nakao A: Epidermal
growth factor-containing fibulin-like extracellular matrix protein
1, EFEMP1, a novel tumor-suppressor gene detected in hepatocellular
carcinoma using double combination array analysis. Ann Surg Oncol.
17:923–932. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhang LH and Ji JF: Molecular profiling of
hepatocellular carcinomas by cDNA microarray. World J
Gastroenterol. 11:463–468. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kanda M, Nomoto S, Okamura Y, Nishikawa Y,
Sugimoto H, Kanazumi N, Takeda S and Nakao A: Detection of
metallothionein 1G as a methylated tumor suppressor gene in human
hepatocellular carcinoma using a novel method of double combination
array analysis. Int J Oncol. 35:477–483. 2009.PubMed/NCBI
|
|
77
|
Okamura Y, Nomoto S, Kanda M, Li Q,
Nishikawa Y, Sugimoto H, Kanazumi N, Takeda S and Nakao A: Leukemia
inhibitory factor receptor (LIFR) is detected as a novel suppressor
gene of hepatocellular carcinoma using double-combination array.
Cancer Lett. 289:170–177. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kanda M, Nomoto S, Okamura Y, Hayashi M,
Hishida M, Fujii T, Nishikawa Y, Sugimoto H, Takeda S and Nakao A:
Promoter hypermethylation of fibulin 1 gene is associated with
tumor progression in hepatocellular carcinoma. Mol Carcinog.
50:571–579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Okamura Y, Nomoto S, Kanda M, Hayashi M,
Nishikawa Y, Fujii T, Sugimoto H, Takeda S and Nakao A: Reduced
expression of reelin (RELN) gene is associated with high recurrence
rate of hepatocellular carcinoma. Ann Surg Oncol. 18:572–579. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hayashi M, Nomoto S, Kanda M, Okamura Y,
Nishikawa Y, Yamada S, Fujii T, Sugimoto H, Takeda S and Kodera Y:
Identification of the A kinase anchor protein 12 (AKAP12) gene as a
candidate tumor suppressor of hepatocellular carcinoma. J Surg
Oncol. 105:381–386. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wong QW, Lung RW, Law PT, Lai PB, Chan KY,
To KF and Wong N: MicroRNA-223 is commonly repressed in
hepatocellular carcinoma and potentiates expression of Stathmin1.
Gastroenterology. 135:257–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Shen J, Wang S, Zhang YJ, Kappil M, Wu HC,
Kibriya MG, Wang Q, Jasmine F, Ahsan H, Lee PH, et al: Genome-wide
DNA methylation profiles in hepatocellular carcinoma. Hepatology.
55:1799–1808. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Okamura Y, Nomoto S, Hayashi M, Hishida M,
Nishikawa Y, Yamada S, Fujii T, Sugimoto H, Takeda S, Kodera Y and
Nakao A: Identification of the bleomycin hydrolase gene as a
methylated tumor suppressor gene in hepatocellular carcinoma using
a novel triple-combination array method. Cancer Lett. 312:150–157.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hishida M, Nomoto S, Inokawa Y, Hayashi M,
Kanda M, Okamura Y, Nishikawa Y, Tanaka C, Kobayashi D, Yamada S,
et al: Estrogen receptor 1 gene as a tumor suppressor gene in
hepatocellular carcinoma detected by triple-combination array
analysis. Int J Oncol. 43:88–94. 2013.PubMed/NCBI
|
|
85
|
Inokawa Y, Nomoto S, Hishida M, Hayashi M,
Kanda M, Nishikawa Y, Takeda S, Sugimoto H, Fujii T, Yamada S and
Kodera Y: Detection of doublecortin domain-containing 2 (DCDC2), a
new candidate tumor suppressor gene of hepatocellular carcinoma, by
triple combination array analysis. J Exp Clin Cancer Res.
32:652013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Inokawa Y, Nomoto S, Hishida M, Hayashi M,
Kanda M, Nishikawa Y, Takeda S, Fujiwara M, Koike M, Sugimoto H, et
al: Dynamin 3: A new candidate tumor suppressor gene in
hepatocellular carcinoma detected by triple combination array
analysis. Onco Targets Ther. 6:1417–1424. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hayashi M, Nomoto S, Hishida M, Inokawa Y,
Kanda M, Okamura Y, Nishikawa Y, Tanaka C, Kobayashi D, Yamada S,
et al: Identification of the collagen type 1 α 1 gene (COL1A1) as a
candidate survival-related factor associated with hepatocellular
carcinoma. BMC Cancer. 14:1082014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Takano N, Hishida M, Inokawa Y, Hayashi M,
Kanda M, Nishikawa Y, Iwata N, Kobayashi D, Tanaka C, Yamada S, et
al: CCNJ detected by triple combination array analysis as a
tumor-related gene of hepatocellular carcinoma. Int J Oncol.
46:1963–70. 2015.PubMed/NCBI
|
|
89
|
Hishida M, Inokawa Y, Takano N, Nishikawa
Y, Iwata N, Kanda M, Tanaka C, Kobayashi D, Yamada S, Nakayama G,
et al: Protein tyrosine kinase 7: A hepatocellular
carcinoma-related gene detected by triple-combination array. J Surg
Res. 195:444–453. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Revill K, Wang T, Lachenmayer A, Kojima K,
Harrington A, Li J, Hoshida Y, Llovet JM and Powers S: Genome-wide
methylation analysis and epigenetic unmasking identify tumor
suppressor genes in hepatocellular carcinoma. Gastroenterology.
145:1424–1435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Nishida N, Nishimura T, Nakai T, Chishina
H, Arizumi T, Takita M, Kitai S, Yada N, Hagiwara S, Inoue T, et
al: Genome-wide profiling of DNA methylation and tumor progression
in human hepatocellular carcinoma. Dig Dis. 32:658–663. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Sherman M: Hepatocellular carcinoma:
Epidemiology, surveillance, and diagnosis. Semin Liver Dis.
30:3–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Fattovich G, Stroffolini T, Zagni I and
Donato F: Hepatocellular carcinoma in cirrhosis: Incidence and risk
factors. Gastroenterology. 127:5:Suppl 1. S35–S50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ladep NG, Lesi OA, Mark P, Lemoine M,
Onyekwere C, Afihene M, Crossey MM and Taylor-Robinson SD: Problem
of hepatocellular carcinoma in West Africa. World J Hepatol.
6:783–792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Perz JF, Armstrong GL, Farrington LA,
Hutin YJ and Bell BP: The contributions of hepatitis B virus and
hepatitis C virus infections to cirrhosis and primary liver cancer
worldwide. J Hepatol. 45:529–538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kao JH and Chen DS: Global control of
hepatitis B virus infection. Lancet Infect Dis. 2:395–403. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lavanchy D: The global burden of hepatitis
C. Liver Int. 29(Suppl 1): S74–S81. 2009. View Article : Google Scholar
|
|
98
|
El-Serag HB: Hepatocellular carcinoma and
hepatitis C in the United States. Hepatology. 36(5): Suppl 1.
S74–S83. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Donato F, Tagger A, Gelatti U, Parrinello
G, Boffetta P, Albertini A, Decarli A, Trevisi P, Ribero ML,
Martelli C, et al: Alcohol and hepatocellular carcinoma: The effect
of lifetime intake and hepatitis virus infections in men and women.
Am J Epidemiol. 155:323–331. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Baffy G, Brunt EM and Caldwell SH:
Hepatocellular carcinoma in non-alcoholic fatty liver disease: An
emerging menace. J Hepatol. 56:1384–1391. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
White DL, Kanwal F and El-Serag HB:
Association between nonalcoholic fatty liver disease and risk for
hepatocellular cancer, based on systematic review. Clin
Gastroenterol Hepatol. 10:1342–1359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Poon RT, Fan ST, Lo CM, Liu CL and Wong J:
Long-term survival and pattern of recurrence after resection of
small hepatocellular carcinoma in patients with preserved liver
function: Implications for a strategy of salvage transplantation.
Ann Surg. 235:373–382. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Senthilnathan S, Memon K, Lewandowski RJ,
et al: Extrahepatic metastases occur in a minority of
hepatocellular carcinoma patients treated with locoregional
therapies: Analyzing patterns of progression in 285 patients.
Hepatology. 55:1432–1442. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhou XD, Tang ZY, Yang BH, Lin ZY, Ma ZC,
Ye SL, Wu ZQ, Fan J, Qin LX and Zheng BH: Experience of 1000
patients who underwent hepatectomy for small hepatocellular
carcinoma. Cancer. 91:1479–1486. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Shimada M, Hamatsu T, Yamashita Y,
Rikimaru T, Taguchi K, Utsunomiya T, Shirabe K and Sugimachi K:
Characteristics of multicentric hepatocellular carcinomas:
Comparison with intrahepatic metastasis. World J Surg. 25:991–995.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wang B, Xia CY, Lau WY, Lu XY, Dong H, Yu
WL, Jin GZ, Cong WM and Wu MC: Determination of clonal origin of
recurrent hepatocellular carcinoma for personalized therapy and
outcomes evaluation: A new strategy for hepatic surgery. J Am Coll
Surg. 217:1054–1062. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Nomoto S, Yamashita K, Koshikawa K, Nakao
A and Sidransky D: Mitochondrial D-loop mutations as clonal markers
in multicentric hepatocellular carcinoma and plasma. Clin Cancer
Res. 8:481–487. 2002.PubMed/NCBI
|
|
108
|
Nomoto S, Kinoshita T, Kato K, Otani S,
Kasuya H, Takeda S, Kanazumi N, Sugimoto H and Nakao A:
Hypermethylation of multiple genes as clonal markers in
multicentric hepatocellular carcinoma. Br J Cancer. 97:1260–1265.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Nomoto S, Hishida M, Inokawa Y, Sugimoto H
and Kodera Y: Management of hepatocellular carcinoma should
consider both tumor factors and background liver factors.
Hepatobiliary Surg Nutr. 3:82–85. 2014.PubMed/NCBI
|
|
110
|
Utsunomiya T, Shimada M, Morine Y, Tajima
A and Imoto I: Specific molecular signatures of non-tumor liver
tissue may predict a risk of hepatocarcinogenesis. Cancer Sci.
105:749–754. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Utsunomiya T, Shimada M, Imura S, Morine
Y, Ikemoto T and Mori M: Molecular signatures of noncancerous liver
tissue can predict the risk for late recurrence of hepatocellular
carcinoma. J Gastroenterol. 45:146–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Okamoto M, Utsunomiya T, Wakiyama S,
Hashimoto M, Fukuzawa K, Ezaki T, Hanai T, Inoue H and Mori M:
Specific gene-expression profiles of noncancerous liver tissue
predict the risk for multicentric occurrence of hepatocellular
carcinoma in hepatitis C virus-positive patients. Ann Surg Oncol.
13:947–954. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Utsunomiya T, Ishikawa D, Asanoma M,
Yamada S, Iwahashi S, Kanamoto M, Arakawa Y, Ikemoto T, Morine Y,
Imura S, et al: Specific miRNA expression profiles of non-tumor
liver tissue predict a risk for recurrence of hepatocellular
carcinoma. Hepatol Res. 44:631–638. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Hoshida Y, Villanueva A, Kobayashi M, Peix
J, Chiang DY, Camargo A, Gupta S, Moore J, Wrobel MJ, Lerner J, et
al: Gene expression in fixed tissues and outcome in hepatocellular
carcinoma. N Engl J Med. 359:1995–2004. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Nomoto S, Hishida M, Inokawa Y, Takano N,
Kanda M, Nishikawa Y, Fujii T, Koike M, Sugimoto H and Kodera Y:
Expression analysis of THOP1 in background liver, a prognostic
predictive factor in hepatocellular carcinoma, extracted by
multiarray analysis. Ann Surg Oncol. 21(Suppl 3): S443–S450. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Sonohara F, Nomoto S, Inokawa Y, Hishida
M, Takano N, Kanda M, Nishikawa Y, Fujii T, Koike M, Sugimoto H and
Kodera Y: High expression of Janus kinase 2 in background normal
liver tissue of resected hepatocellular carcinoma is associated
with worse prognosis. Oncol Rep. 33:767–773. 2015.PubMed/NCBI
|
|
117
|
Ammerpohl O, Pratschke J, Schafmayer C,
Haake A, Faber W, von Kampen O, Brosch M, Sipos B, von Schönfels W,
Balschun K, et al: Distinct DNA methylation patterns in cirrhotic
liver and hepatocellular carcinoma. Int J Cancer. 130:1319–1328.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Um TH, Kim H, Oh BK, Kim MS, Kim KS, Jung
G and Park YN: Aberrant CpG island hypermethylation in dysplastic
nodules and early HCC of hepatitis B virus-related human multistep
hepatocarcinogenesis. J Hepatol. 54:939–947. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Arai E, Ushijima S, Gotoh M, Ojima H,
Kosuge T, Hosoda F, Shibata T, Kondo T, Yokoi S, Imoto I, et al:
Genome-wide DNA methylation profiles in liver tissue at the
precancerous stage and in hepatocellular carcinoma. Int J Cancer.
125:2854–2862. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yoshida T, Kato J, Maekita T, Yamashita S,
Enomoto S, Ando T, Niwa T, Deguchi H, Ueda K, Inoue I, et al:
Altered mucosal DNA methylation in parallel with highly active
Helicobacter pylori-related gastritis. Gastric Cancer. 16:488–497.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Lee KH, Hwang D, Kang KY, Lee S, Kim DY,
Joo YE and Lee JH: Frequent promoter methylation of CDH1 in
non-neoplastic mucosa of sporadic diffuse gastric cancer.
Anticancer Res. 33:3765–3774. 2013.PubMed/NCBI
|
|
122
|
Kadara H, Fujimoto J, Yoo SY, Maki Y,
Gower AC, Kabbout M, Garcia MM, Chow CW, Chu Z, Mendoza G, et al:
Transcriptomic architecture of the adjacent airway field
cancerization in non-small cell lung cancer. J Natl Cancer Inst.
106:dju0042014. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zheng Z, Liang W, Wang D, Schroder PM, Ju
W, Wu L, Zheng Z, Shang Y, Guo Z and He X: Adjuvant chemotherapy
for patients with primary hepatocellular carcinoma: A
meta-analysis. Int J Cancer. 136:E751–E759. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kubo S, Takemura S, Sakata C, Urata Y and
Uenishi T: Adjuvant therapy after curative resection for
hepatocellular carcinoma associated with hepatitis virus. Liver
Cancer. 2:40–46. 2013. View Article : Google Scholar : PubMed/NCBI
|