Introduction
Nedaplatin, a platinum derivative synthesized to
overcome problems of cisplatin resistance, was developed by
Shionogi Pharmaceutical Company in 1983 with the aim to provide a
treatment with effectiveness similar to cisplatin, but with less
nephrotoxicity and gastrointestinal toxicities (1). Nedaplatin produces promising response
rates in clinical trials as a monotherapy for the treatment of
squamous cell carcinoma of the uterus, cervix, head and neck,
ovary, lung and esophagus (2–4). Nedaplatin exerts anti-tumor effects
following uptake into tumor cells by binding to DNA bases and
inhibiting DNA replication, similar to cisplatin and carboplatin
(5). However, the ability of cancer
cells to become resistant to nedaplatin remains a notable obstacle
to successful chemotherapy; resistance of numerous species of
cancer cells to nedaplatin can be reversed by combining with
chemotherapeutic agents, including 5-fluorouracil, docetaxel and
vindesine (6–8). The identification of new chemotherapy
regimens incorporating nedaplatin with other chemotherapeutic
agents can enhance the knowledge of nedaplatin resistance and
support the development of nedaplatin-based approaches to cancer
therapy.
The B-cell lymphoma 2 (Bcl-2) family members serve
as primary regulators of apoptosis and include both pro- and
anti-apoptotic molecules (9).
Overexpression of anti-apoptotic Bcl-2 family proteins has been
associated with chemotherapy resistance in multiple human cancers
(10). Myeloid cell leukemia 1
(Mcl-1), an anti-apoptotic protein in the Bcl-2 family, is
frequently observed in numerous tumor types and contributes to
chemotherapeutic resistance (11).
ABT-737 is a small molecule inhibitor of Bcl-2
family proteins. It can bind Bcl-2 and B-cell lymphoma-extra large
(Bcl-xL) with high affinity. ABT-737 has shown single agent and
combination therapy efficacy against multiple myeloma, acute
myeloid leukemia, lymphoma and solid tumor cell lines (12). The resistance to ABT-737 correlates
with the overexpression of Mcl-1 protein in several cancer cell
lines (13,14). The present study exhibited for the
first time that combing nedaplatin and ABT-737 has substantial
synergistic anti-cancer efficacy. These enhanced anti-tumor
activities were accompanied by the promotion of apoptosis. The
promotion of Mcl-1 degradation was involved in the synergistic
anti-cancer effect by combining nedaplatin with ABT-737. These
results indicated that combining nedaplatin with ABT-737 may be an
effective therapeutic strategy to achieve synergistic anti-cancer
activities.
Materials and methods
Materials
In total, 3.01 mg nedaplatin from Selleck Chemicals
(Houston, TX, USA) was dissolved in 1 ml dimethyl sulfoxide.
ABT-737 was synthesized according to the literature (12), and its purity was determined to be
>99% by high performance liquid chromatography. MG132 was
purchased from Selleck Chemicals. All the following antibodies used
for western blotting are rabbit anti-human: Anti-Mcl-1 polyclonal
antibody (dilution, 1:500; catalog no., SC-819), anti-PARP
polyclonal antibody (dilution, 1:500; catalog no., SC-7150) and
anti-procaspase-3 polyclonal antibody (dilution, 1:500; catalog
no., SC-7148) (Santa Cruz Biotechnology, Dallas, TX, USA); and
anti-cleaved-caspase-3 monoclonal antibody (dilution, 1:1,000;
catalog no., 9668; Cell Signaling Technology, Danvers, MA, USA).
The mouse anti-human antibodies for western blotting are as
follows: Anti-x-linked inhibition of apoptosis protein (XIAP)
monoclonal antibody (dilution, 1:500; catalog no., SC-55550; Santa
Cruz Biotechnology); and anti-β-actin monoclonal antibody
(dilution, 1:2,000; catalog no., BD-612656; BD Biosciences,
Franklin Lakes, NJ, USA).
Cell culture
Human lung cancer A549, NCI-H1299 and 95-D cell
lines, prostate cancer PC-3 cell line and ovarian cancer SKOV3 cell
line were obtained from Shanghai Institute of Biochemistry and Cell
Biology (Shanghai, China). All cell lines were tested and
authenticated for genotypes by DNA fingerprinting. The 95-D,
NCI-H1299 and SKOV3 cells were maintained in RPMI-1640 (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA), and the PC-3 and A549 cells were grown in Ham's
F12 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum. All the cell lines were maintained in
a humidified atmosphere of 95% air plus 5% CO 2 at
37°C.
Sulforhodamine blue (SRB) assay
The anti-proliferative activity of nedaplatin plus
ABT-737 was detected by SRB (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) assay. Briefly, cancer cells were fixed with
10% trichoroacetic acid solution. Subsequent to washing, 0.4% SRB
solution (100 µl per well) was added into each well. Following 20
min staining, wells were rinsed with 1% acetic acid to remove
unbound dye, and then left to air dry. Subsequently, 100 µl
Tris-base lye (10 mmol/l; Biosharp, Hefei, China) was added,
followed by 10-min oscillation. The absorbance was then recorded at
515 nm using a multi-scan spectrum (Thermo Scientific Multiskan Go
1510; Thermo Fisher Scientific, Inc.).
Colony formation assay
Cancer cells were plated at 500–1,000 cells per
dish, and then cells were exposed to nedaplatin (1 µM), ABT-737 (1
µM) or a combination of the two. After 14 days, dishes were stained
by crystal violet and colony numbers were counted.
Propidium iodide (PI) staining
Sub-G1 analysis following PI staining was used to
detect apoptosis. A549 cells (3×105/well) were seeded
into 6-well plates and exposed to either nedaplatin (20 µM) or
ABT-737 (5 µM), or the two agents together. 95-D cells
(3×105/well) were seeded into 6-well plates and exposed
to either nedaplatin (10 µM) or ABT-737 (10 µM), or the two agents
together. Cells were harvested and washed with phosphate-buffered
saline (PBS) three times and fixed with pre-cooled 70% ethanol at
−20°C overnight. Cells were washed and resuspended in 500 µl PBS
containing 50 µg/ml RNase at 37°C for 30 min. The cells were then
stained with 5 µg PI at room temperature for 30 min. For each
sample, 2×104 cells were collected and analyzed using a
FACS-Calibur cytometer (Becton Dickinson, San Jose, CA, USA), and
the data were analyzed using Cellquest Software (version 6.0;
Becton Dickinson, San Jose, CA, USA).
Determination of mitochondrial
membrane depolarization
Cells (3×105/well) were treated with
nedaplatin and/or ABT-737 for 48 h, collected, and resuspended in
fresh medium containing 10 µg/ml
5,5′,6,6′tetrachloro-1,1′,3,3′-tetraethylbenzimidazol-carbocyanine
iodide (JC-1; Sigma-Aldrich; Merck Millipore). After incubation at
37°C for 30 min, cells were analyzed by FACS-Calibur cytometer.
Western blot analysis
Proteins were extracted with lysis buffer containing
150 mM NaCl, 50 mM Tris-HCl, 0.1% sodium dodecyl sulfate, 1 mM
ethylenediaminetetraacetic acid, 0.5% deoxycholic acid, 1% NP-40,
2.0 µg/ml aprotinin, 1 mM phenylmethylsulfonylfluoride and 0.02%
sodium azide (Beyotime Institute of Biotechnology, Haimen, China).
The lysates were centrifuged at 10,000 × g for 30 min at
4°C, then the concentrations protein were determined. Proteins were
fractionated on 8–15% Tris-glycine gels, and then they were
transferred to polyvinylidene fluoride membrane (Millipore,
Bedford, MA, USA) and probed with the aforementioned primary
antibodies (dilution range 1:500–1:1,000). The proteins were
visualized with peroxidase-coupled secondary antibodies horseradish
peroxidase (HRP)-conjugated goat anti-mouse IgG, catalog no.,
GAM007; HRP-conjugated goat anti-rabbit IgG, catalog no., GAR007;
MultiSciences, Hangzhou, China) at a dilution of 1:5,000. Finally,
proteins were visualized using the enhanced chemiluminescence
detection system (PerkinElmer, Waltham, MA, USA).
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA was isolated from A549 and 95-D cells using the
TRIzol system (Thermo Fisher Scientific, Inc.), and the
concentration of RNA was determined using NanoDrop 2000 (Thermo
Fisher Scientific, Inc). Single-strand cDNA was prepared from the
purified RNA using oligo (dT) priming (Thermoscript RT kit;
Invitrogen; Thermo Fisher Scientific, Inc.), followed by SYBR-Green
qPCR (Qiagen, Hilden, Germany). The sequences of PCR primers were
as follows: Mcl-1 forward, 5′-GGGCAGGATTGTGACTCTCATT-3′ and
reverse, 5′-GATGCAGCTTTCTTGGTTTATGG-3′; glyceraldehyde 3-phosphate
dehydrogenase forward, 5′-GAGTCAACGGATTTGGTCGT-3′ and reverse,
5′-TTGATTTTGGAGGGATCTCG-3′ (Sangon Biotech, Shanghai, China).
Plasmid transfection
The pTOPO-Mcl-1 plasmid from Addgene (15) (Plasmid 21605; Cambridge, MA, USA) or
the empty vector (pTOPO) was transfected into A549 cells by
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol.
Statistical analysis
Two-tailed Student's t–test was used to
detect the significance of differences between the experimental
conditions. P<0.05 was considered to indicate a statistically
significant difference. Combination index (CI) was used to quantify
drug synergism, based on the multiple drug-effect equation of
Chou-Talalay (16). For in
vitro experiments, CI values were calculated for each
concentration of nedaplatin, ABT-737 and the combination of the two
in SRB assays using CalcuSyn (version 2.0; Biosoft, Cambridge, UK),
and the mean CI values were presented.
Results
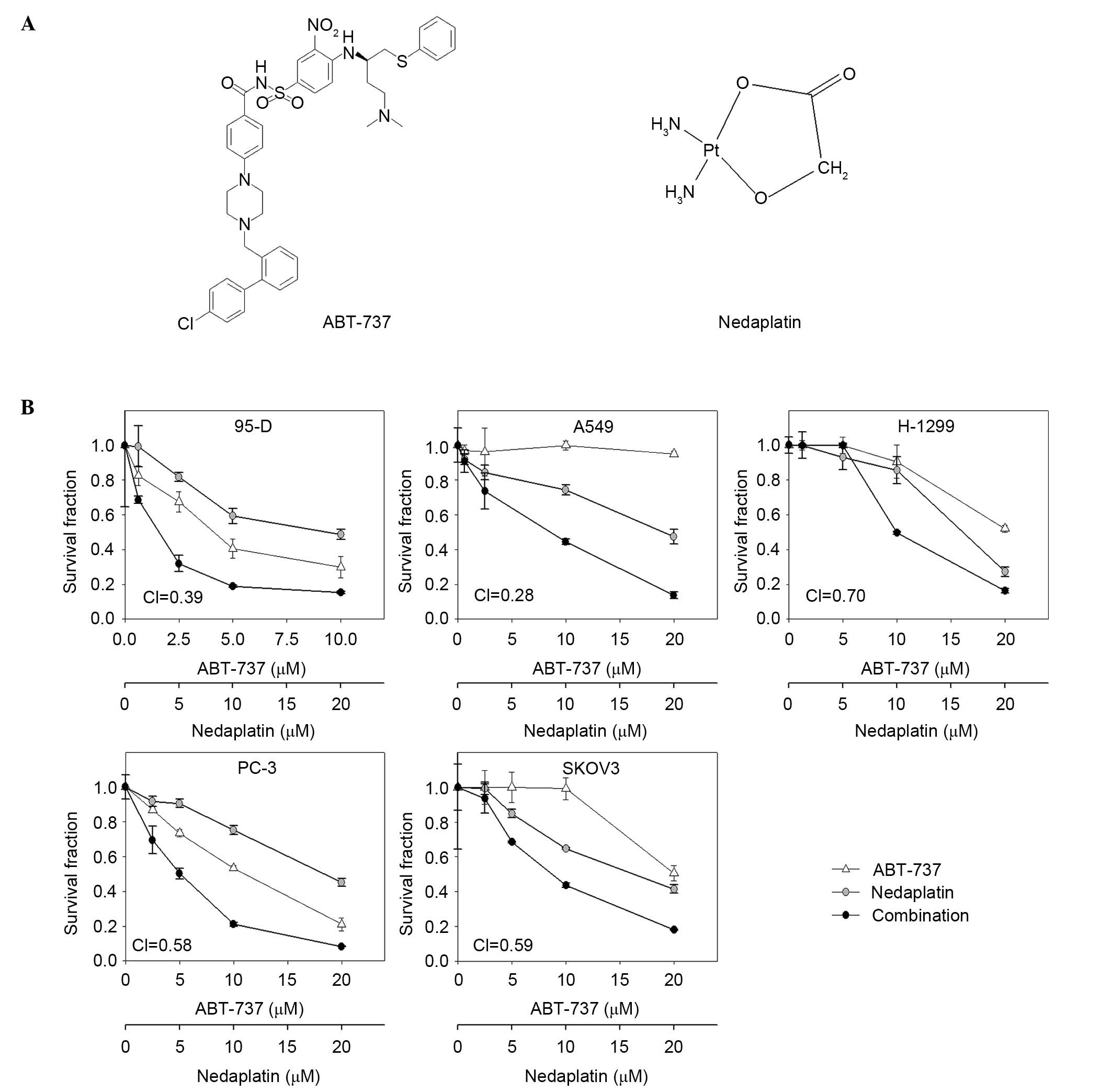
Nedaplatin plus ABT-737 inhibited the
proliferation of human cancer cell lines
The sensitivities of 5 human cancer cell lines to
nedaplatin, ABT-737, or nedaplatin combined with ABT-737 were
detected by SRB assay, and the survival curves are shown in
Fig. 1. The fixed-ratio
concentrations of nedaplatin and ABT-737 were used and CI values
were calculated using CalcuSyn Software to assess combination
activity (95-D, nedaplatin vs. ABT-737, 2:1; other cell lines,
nedaplatin vs. ABT-737, 1:1). Nedaplatin plus ABT-737 showed
synergistic effects in 5 human cancer cell lines, with the CI
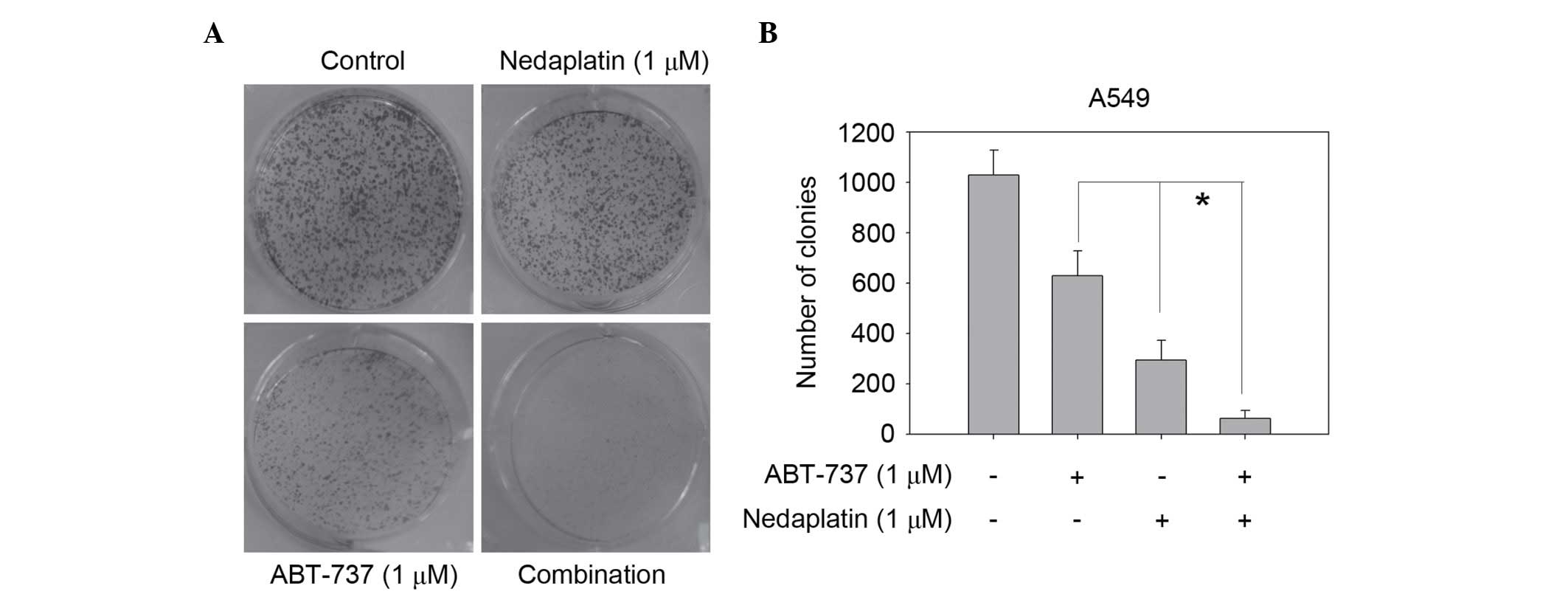
values <0.7. Nedaplatin at 1 µM and ABT-737 at 1 µM alone had
limited effects on suppressing A549 cell colony formation; however,
the combination almost eliminated colony formation in the colony
formation assay (Fig. 2). Thus,
combination was much more effective than either single agent in
inhibiting the proliferation of cancer cells.
Nedaplatin plus ABT-737 induced
enhanced apoptosis in A549 and 95-D cells
To investigate whether the cytotoxic effects of
treatments were linked to the enhancement of apoptosis, PI staining
was used to detect apoptosis in A549 and 95-D cells, which showed
strong synergistic anti-cancer effects in cytotoxicity assay. As
shown in Fig. 3A (top panel), the
percentage of apoptotic A549 cells was markedly increased in the
combination treatment group (66.4%) compared to ABT-737 (10.18%) or
nedaplatin (19.81%) alone (ABT-737 vs. combination, P=0.004;
nedaplatin vs. combination, P=0.005). Similarly, the apoptotic 95-D
cells were significantly higher in the combination treatment group
compared with the single treatment group (ABT-737 vs. combination,
P=0.008; nedaplatin vs. combination, P=0.009; Fig. 3B and C).
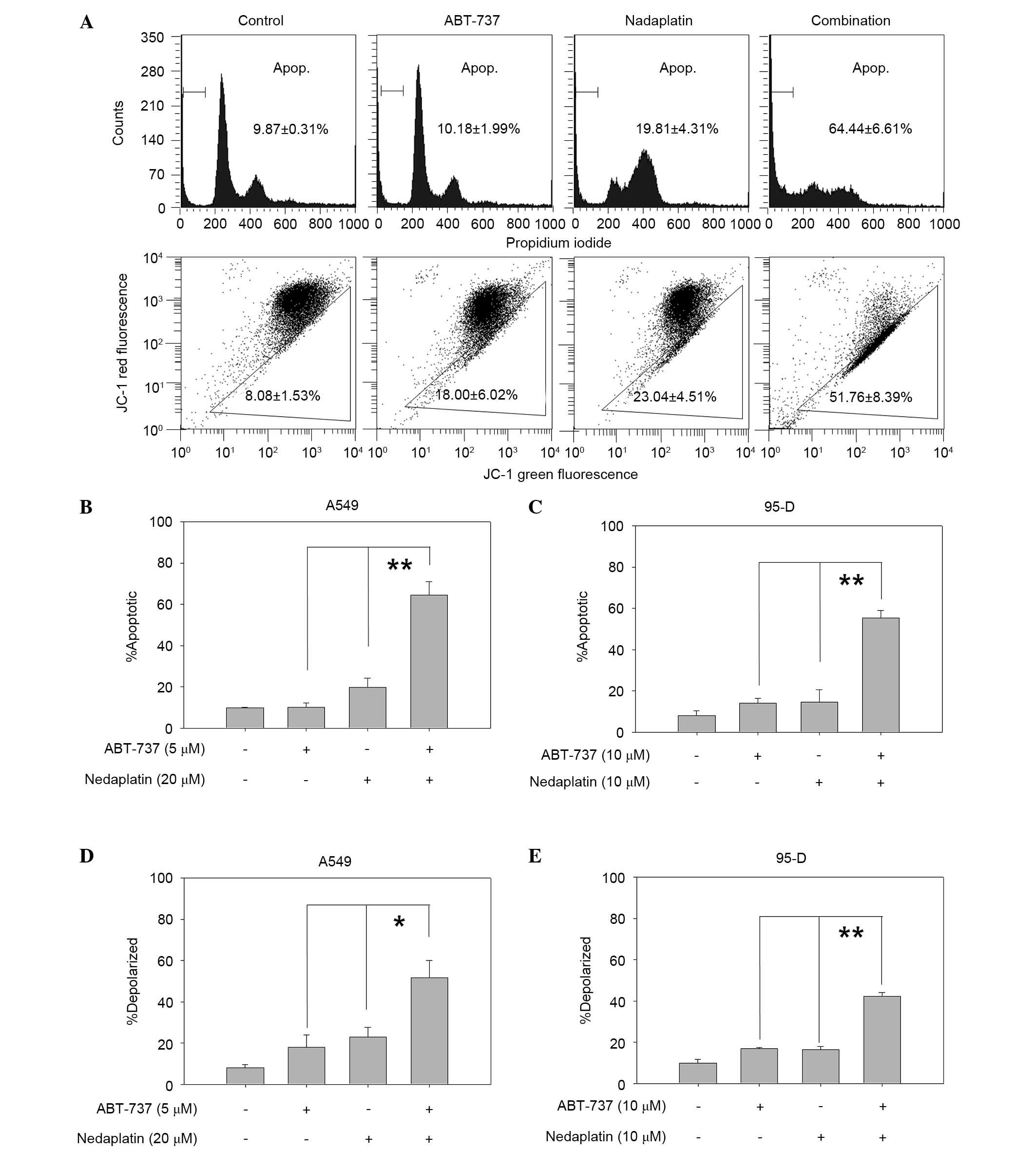 | Figure 3.Nedaplatin plus ABT-737 caused
enhanced apoptosis. (A) A549 cells were treated with ABT-737 (5
µM), nedaplatin (20 µM), or a combination of the two for 48 h, and
then cells were incubated with PI (top) or JC-1 (bottom) and
analyzed by flow cytometry. (B) A549 and (C) 95-D cells in 6-well
plates were exposed to the compounds for 48 h and then cells were
analyzed by flow cytometry after PI staining. (D) A549 and (E) 95-D
cells were exposed to compounds for 48 h and then cells were
analyzed by flow cytometry after JC-1 staining. The experiments
were repeated three times and error bars represented the standard
deviation. *P<0.05, **P<0.01. JC-1,
5,5′,6,6′tetrachloro-1,1′,3,3′-tetraethylbenzimidazol-carbocyanine
iodide; PI, propidium iodide. |
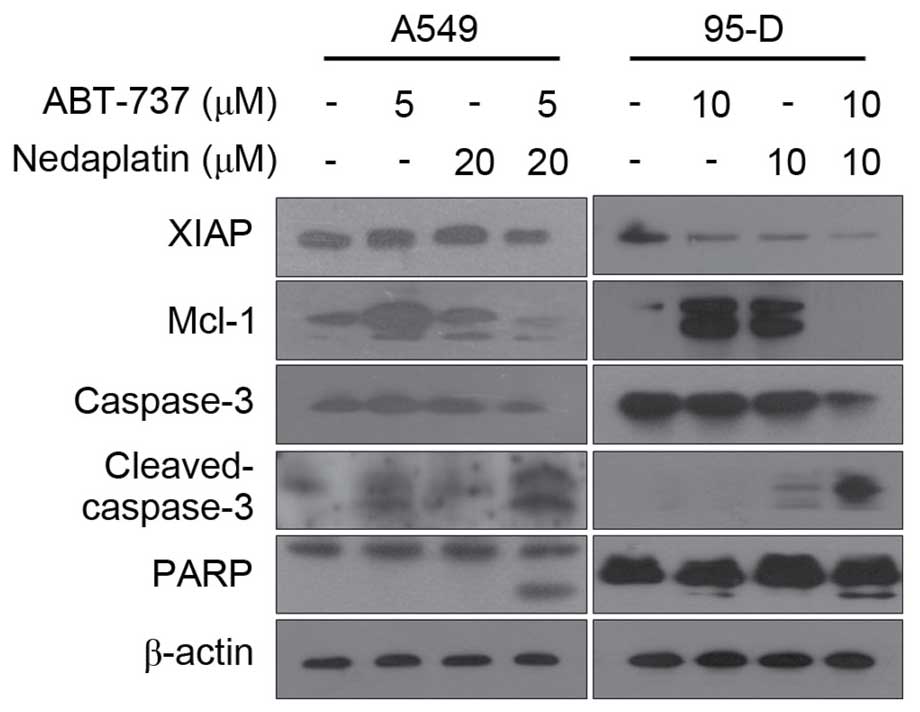
To further assess whether increased ABT-737-mediated
apoptosis by nedaplatin-induced caspase activation, immunoblot
analysis was performed. As shown in Fig.
4, combined treatment markedly activated caspase-3 and PARP in
both A549 and 95-D cells. In addition, combination treatment also
resulted in potentiation of XIAP downregulation in A549 and 95-D
cells. Overall, nedaplatin and ABT-737 showed a synergistic effect
by inducing apoptosis in cancer cells.
Apoptosis induced by nedaplatin plus
ABT-737 was through mitochondrial pathway
Rapid loss of mitochondrial membrane potential and
the release of cytochrome c are considered to be the
hallmarks of mitochondrial dysfunction, which can induce the
activation of caspases and lead to apoptosis (10). Thus, the present study investigated
whether or not apoptosis induced by nedaplatin plus ABT-737 was
triggered by mitochondrial dysfunction. As shown in Fig. 3A (bottom panel), nedaplatin plus
ABT-737 resulted in an increased percentage of mitochondrial
membrane depolarized A549 cells than compared with a single agent
used alone (51.76% in combination treated cells, 23.04% in
nedaplatin-treated cells, 18.00% in ABT-737-treated cells and 8.08%
in control group; ABT-737 vs. combination, P=0.03; nedaplatin vs.
combination, P=0.04). Combination treatment with nedaplatin and
ABT-737 resulted in increased mitochondrial membrane potential in
A549 and 95-D cell lines (Fig. 3D and
E).
Combination of nedaplatin and ABT-737
promoted the degradation of Mcl-1
It has been reported that high levels of Mcl-1 may
confer resistance to ABT-737 in several solid tumors (17). Thus, the present study examined the
involvement of Mcl-1 in nedaplatin and ABT-737 combination
treatment. Notably, it was found that treatment with nedaplatin or
ABT-737 alone increased the expression of Mcl-1 in A549 and 95-D
cells, whereas Mcl-1 expression was markedly decreased in the
nedaplatin plus ABT-737 combination group, suggesting that Mcl-1
may be involved in the synergistic effect (Fig. 4). To determine whether the synergistic
reduction of Mcl-1 protein by nedaplatin and ABT-737 combination
treatment was the result of transcriptional inhibition, the level
of Mcl-1 mRNA expression was assessed by RT-PCR in A549 and 95-D
cells treated with ABT-737, nedaplatin, or a combination of the two
for 12 h. As shown in Fig. 5A, no
apparent synergist inhibitory effects on Mcl-1 mRNA expression
levels were observed in the nedaplatin plus ABT-737 group in A549
and 95-D cells, whereas the Mcl-1 mRNA expression level was
upregulated in nedaplatin plus ABT-737 combination-treated cells.
Therefore, these data revealed that the synergistic reduction of
Mcl-1 protein by nedaplatin and ABT-737 combination treatment was
not the result of transcriptional inhibition. Thus, it was
hypothesized that the putative ubiquitination of Mcl-1 in response
to nedaplatin and ABT-737 combination treatment may play a key role
in the synergistic effect. To further investigate this hypothesis,
A549 cells were treated with CHX (200 mg/ml) to block new protein
synthesis and observed Mcl-1 degradation in the presence of 5 µM
ABT-737 and/or 20 µM nedaplatin. The half-life of Mcl-1 was
compared in A549 cells treated with CHX in the presence of
nedaplatin, ABT-737, or a combination of the two. Fig. 5B showed that the level of Mcl-1
protein decreased more rapidly in the nedaplatin plus ABT-737 group
compared with either of the single-agent groups. Addition of the
proteasome inhibitor MG132 attenuated combination-mediated Mcl-1
degradation (Fig. 5C). These data
suggested that a promotion of Mcl-1 degradation was involved in the
synergistic effect of nedaplatin and ABT-737.
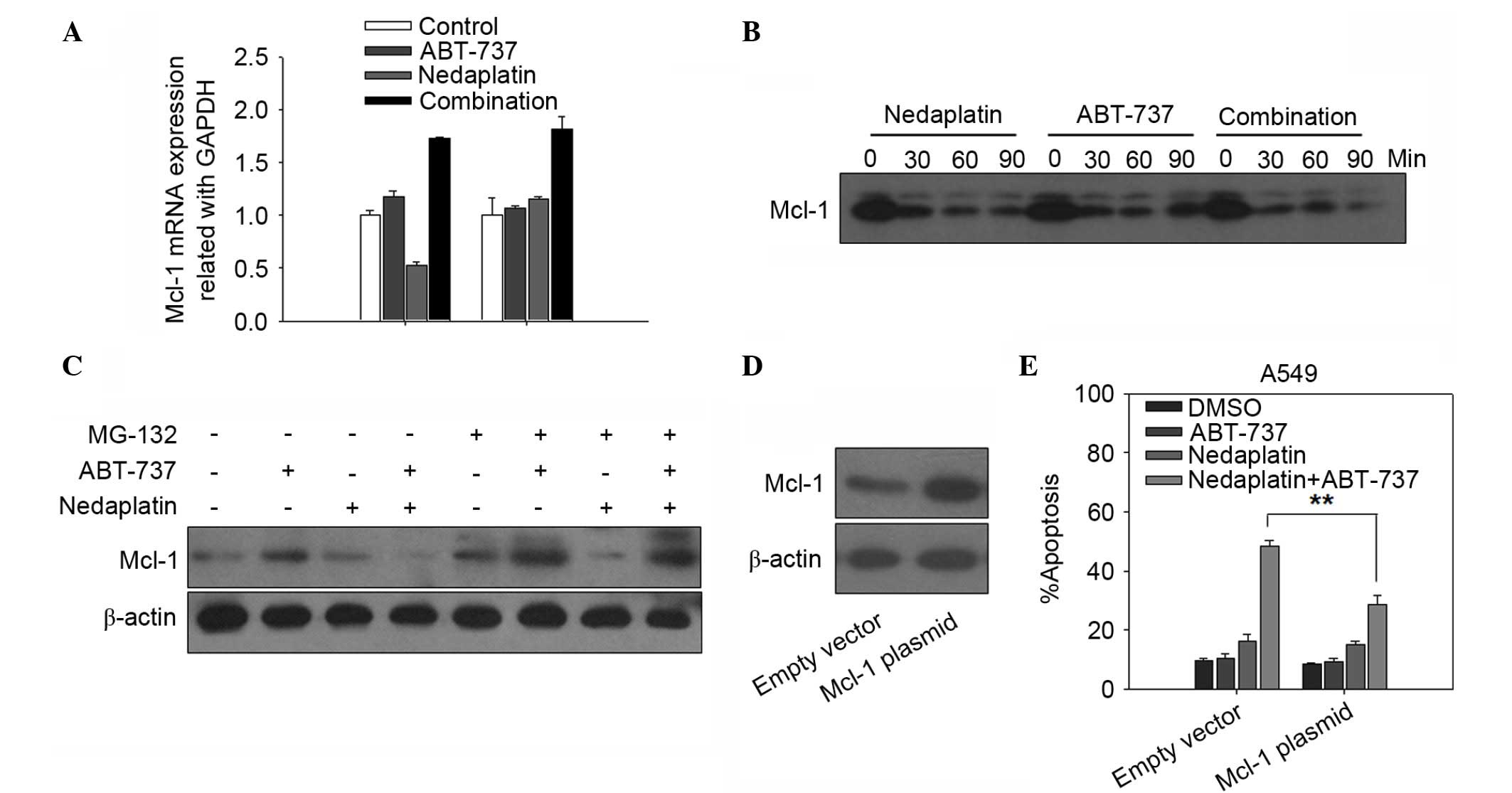 | Figure 5.The involvement of Mcl-1 in the
enhanced apoptosis synergistically induced by nedaplatin and
ABT-737. (A) Mcl-1 mRNA levels were evaluated by RT-PCR in A549 and
95-D cells treated with ABT-737, nedaplatin, or a combination of
the two for 12 h. (B) Cells were treated with CHX (200 mg/ml) to
block new protein synthesis, and the degradation of Mcl-1 in the
presence of 5 µM ABT-737 and/or 20 µM nedaplatin at 30, 60 and 90
min was detected by western blotting. (C) A549 cells were
pretreated with or without 1 µM MG132 for 30 min, then the cells
were treated with ABT-737 (5 µM), nedaplatin (20 µM), or a
combination of the two for 24 h. Cell lysates were prepared for
western blot analysis. (D) A549 cells were transfected with Mcl-1
plasmid and empty vector according to manufacturer's protocol. A
total of 48 h after transfection, cell lysates were prepared for
western blot analysis. (E) The ratio of apoptosis in A549 cells
that had been transfected with Mcl-1 plasmid or empty vector and
then treated with 20 µM nedaplatin, either alone or in combination
with 5 µM ABT-737 for 48 h were examined. Quantification of the
apoptotic cells by PI staining was repeated three times, and the
standard deviation was represented as error bars. **P<0.01.
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Mcl-1, myeloid
cell leukemia 1; DMSO, dimethyl sulfoxide; PI, propidium
iodide. |
Overexpression of Mcl-1 rescued cells
from synergistic killing by the combination of nedaplatin and
ABT-737
To further evaluate whether the downregulation of
Mcl-1 was required for nedaplatin plus ABT-737-induced apoptosis,
the expression of Mcl-1 protein was successfully increased via
transfecting pTOPO-Mcl-1 plasmid to A549 cells (Fig. 5D). Mcl-1 overexpression significantly
decreased apoptosis in A549 cells treated with nedaplatin plus
ABT-737 (Fig. 5E). The present
results indicated that downregulation of Mcl-1 may contribute to
the synergistic killing of cells by the combination of nedaplatin
and ABT-737.
Discussion
Platinum compounds are widely used in the treatment
of a number of solid malignancies. Platinum compounds exhibit
individual characteristics, although they share similar chemical
structures and cytotoxic mechanisms (18). As single agents, high response rates
have been observed in first-line chemotherapy, but the majority of
patients will relapse and subsequently prove resistant to platinum
compounds (19). In addition, severe
nephrotoxicity and gastrointestinal toxicity also limit their
clinical application (20). Thus, it
is urgent to develop a new anti-tumor combination therapy with a
lower concentration of platinum compounds and a high anti-cancer
efficacy. Nedaplatin is a second-generation platinum complex that
is ~10 times as soluble in water as cisplatin (1). Additionally, nedaplatin is considered to
have more pronounced anti-cancer activity, but less nephrotoxicity
and gastrointestinal toxicity (21).
Thus, high anti-cancer efficacy and limiting toxicity have made
nedaplatin an attractive compound for combination therapy.
The experimental strategies under investigation
aimed at overcoming platinum compounds resistance include
introduction of functional genes (p53 and p21), or of genes that
interfere with apoptotic pathways, such as Bcl-XL and Bcl-2
pathways. These are likely to contribute to tumor treatment,
particularly in combination with regimens using platinum compounds
(22). Regimens containing
cisplatin/carboplatin together with the Bcl-2 inhibitor ABT-737
show high anti-cancer efficacy by modulation of the Mcl-1/Noxa axis
(23). To the best of our knowledge,
the present results indicated for the first time that the
synergistic anti-tumor effect observed in vitro by combining
nedaplatin with ABT-737 may be observed in several human cancer
cell lines. The significant decline of the survival curves in the
nedaplatin plus ABT-737 group strongly demonstrated that
combination of the agents showed synergy in 5 human solid tumor
cell lines, consisting of the PC-3, SKOV3, A549, NCI-H1299 and 95-D
cell lines.
The present results also suggested that synergism
achieved by combining nedaplatin with ABT-737 was accompanied by
enhanced apoptosis. Mitochondria play a key role in apoptosis,
mitochondrial outer membrane permeabilization leads to cytochrome
c release and Bcl-2-associated X protein translocation
(24). The current results suggested
that loss of mitochondrial membrane potential was significantly
greater in the nedaplatin plus ABT-737 group compared with the
single agent group, indicating that nedaplatin plus ABT-737 may
activate the mitochondrial-mediated apoptosis pathway in 95-D and
A549 cells. Marked overexpression of cleaved-caspase-3 and PARP was
observed in 95-D/A549 cells following nedaplatin and ABT-737
combination treatment. XIAP, plays a critical role in the
regulation of apoptosis through inhibiting caspases (25). The present data suggested that the
synergistic effect on apoptosis induced by nedaplatin plus ABT-737
was accompanied by a large reduction of XIAP.
The Bcl-2 family members control the vital step in
the intrinsic apoptotic pathway (13). ABT-737 and its orally active analog
ABT-263 are the most potent Bcl-2/Bcl-XL inhibitors, but they have
a much lower affinity for Mcl-1. Thus, they are not effective in
certain cancer types with high levels of Mcl-1 as single agents
(26). The up-regulation of Mcl-1,
which is inducible upon treatment with ABT-737 in resistant cancer
cells, has been confirmed to be involved in the resistance to
ABT-737 (27). Platinum derivatives,
such as cisplatin and carboplatin, have been described to reduce
expression of Mcl-1 (23,28). Whether the potentiating effect of
nedaplatin on ABT-737 response was also due to an inhibition effect
on Mcl-1 expression was assessed. The current results showed that
ABT-737-treatment increased the expression of Mcl-1 in
ABT-737-resistant A549 and 95-D cells. However, nedaplatin plus
ABT-737 exerted a synergistic anti-cancer effect by decreasing the
expression of Mcl-1 protein. Thus, it was hypothesized that low
expression of Mcl-1 contributed to the synergistic effect in cancer
cell lines. As in all proteins, the equilibrium between production
and degradation determines the protein level of Mcl-1, and the
stability of Mcl-1 could be critically important in numerous
physiologic and pathologic situations (17). Firstly, it was postulated that the
synergistic reduction of Mcl-1 protein by nedaplatin plus ABT-737
was the result of transcriptional inhibition. However, no apparent
inhibitory effects on Mcl-1 mRNA levels were observed in the
combination treated group, suggesting that the synergistic decrease
of Mcl-1 protein by nedaplatin plus ABT-737 was not the result of
transcriptional inhibition. Subsequently, the present data
indicated that Mcl-1 protein level decreased more rapidly in the
combination treatment group than that in single-agent groups.
Furthermore, addition of the proteasome inhibitor MG132 attenuated
combination-mediated Mcl-1 degradation. These data suggested that a
promotion of Mcl-1 degradation was involved in the synergistic
effect of nedaplatin and ABT-737. Additionally, Mcl-1
overexpression significantly decreased apoptosis in A549 cells
treated with nedaplatin plus ABT-737. These results indicated that
Mcl-1 may be involved in the synergistic anti-cancer effect of
nedaplatin and ABT-737 combination.
In conclusion, the present study reports evidence
showing apoptosis induction is strongly reinforced when ABT-737 is
combined with nedaplatin. In addition, nedaplatin plus ABT-737
exerts a synergistic effect on cancer cells through the ability of
the agents to downregulate Mcl-1, and combination of the agents
accelerates the proteasome-mediated degradation of Mcl-1. The
present study has revealed nedaplatin as a pertinent sensitizer to
ABT-737 through inhibition of Mcl-1, which opens up new avenues for
this promising BH3-mimetic molecule in the clinic. Furthermore, the
strategy of combining ABT-737 with nedaplatin appears to be an
attractive option for reversing resistance to nedaplatin.
Acknowledgements
The authors gratefully acknowledge financial support
from National Natural Science Foundation of China (grant nos.
81272473 and 81302806), Public-Service Technology Research Plan of
Zhejiang Province (grant no. 2016C33210), High-level Talents Coming
Back From Abroad Innovation and Entrepreneurship Program in
Hangzhou (grant no. 2051), Zhejiang Provincial Foundation of
Natural Science (grant nos. LQ16H310004 and LQ15H310001), Science
Research Foundation of Zhejiang Health Bureau & the Ministry of
Health (grant no. WKJ2012-2-022), Zhejiang Provincial Program for
the Cultivation of High-level Innovative Health talents (grant no.
2010-190–4), Student Research Fund of Zhejiang University City
College (grant no. X2016556121), Scientific and Technological
Developing Scheme of Hangzhou City (grant no. 20150733Q14) and
Hangzhou 131 Talents Project.
Glossary
Abbreviations
Abbreviations:
|
Mcl-1
|
myeloid cell leukemia 1
|
|
SRB
|
sulforhodamine blue
|
|
JC1
|
5,5′,6,6′-tetrachloro-1,1′3,3′-tetraethyl-benzimidazol-carbocyanine
iodide
|
|
PI
|
propidium iodide
|
|
CI
|
combination index
|
References
|
1
|
Shimada M, Itamochi H and Kigawa J:
Nedaplatin: A cisplatin derivative in cancer chemotherapy. Cancer
Manag Res. 5:67–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Inuyama Y, Miyake H, Horiuchi M, Hayasaki
K, Komiyama S and Ota K: An early phase II clinical study of
cis-diammine glycolato platinum, 254-S, for head and neck cancers.
Gan To Kagaku Ryoho. 19:863–869. 1992.(In Japanese). PubMed/NCBI
|
|
3
|
Furuse K, Fukuoka M, Kurita Y, Ariyoshi Y,
Niitani H, Yoneda S, Fujii M, Hasegawa K, Nishiwaki Y, Tamura M, et
al: A phase II clinical study of cis-diammine glycolato platinum,
254-S, for primary lung cancer. Gan To Kagaku Ryoho. 19:879–884.
1992.(In Japanese). PubMed/NCBI
|
|
4
|
He YF, Ji CS, Hu B, Fan PS, Hu CL, Jiang
FS, Chen J, Zhu L, Yao YW and Wang W: A phase II study of
paclitaxel and nedaplatin as front-line chemotherapy in Chinese
patients with metastatic esophageal squamous cell carcinoma. World
J Gastroenterol. 19:5910–5916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jung Y and Lippard SJ: Direct cellular
responses to platinum-induced DNA damage. Chem Rev. 107:1387–1407.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamashita H, Nakagawa K, Tago M, Igaki H,
Nakamura N, Shiraishi K, Sasano N and Ohtomo K: Radiation therapy
combined with cis-diammine-glycolatoplatinum (nedaplatin) and
5-fluorouracil for Japanese stage II–IV esophageal cancer compared
with cisplatin plus 5-fluorouracil regimen: A retrospective study.
Dis Esophagus. 19:15–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshioka T, Sakayori M, Kato S, Chiba N,
Miyazaki S, Nemoto K, Shibata H, Shimodaira H, Ohtsuka K, Kakudo Y,
et al: Dose escalation study of docetaxel and nedaplatin in
patients with relapsed or refractory squamous cell carcinoma of the
esophagus pretreated using cisplatin, 5-fluorouracil, and
radiation. Int J Clin Oncol. 11:454–460. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takigawa N, Segawa Y, Ueoka H, Kiura K,
Tabata M, Shibayama T, Takata I, Miyamoto H, Eguchi K and Harada M:
Combination of nedaplatin and vindesine for treatment of relapsed
or refractory non-small-cell lung cancer. Cancer Chemother
Pharmacol. 46:272–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou P, Qian L, Kozopas KM and Craig RW:
Mcl-1, a Bcl-2 family member, delays the death of hematopoietic
cells under a variety of apoptosis-inducing conditions. Blood.
89:630–643. 1997.PubMed/NCBI
|
|
10
|
Kang MH and Reynolds CP: Bcl-2 inhibitors:
Targeting mitochondrial apoptotic pathways in cancer therapy. Clin
Cancer Res. 15:1126–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Belmar J and Fesik SW: Small molecule
Mcl-1 inhibitors for the treatment of cancer. Pharmacol Ther.
145:76–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang C, Shi J, Mao SY, Xu YS, Zhang D,
Feng LY, Zhang B, Yan YY, Wang SC, Pan JP, et al: Role of p38 MAPK
in enhanced human cancer cells killing by the combination of
aspirin and ABT-737. J Cell Mol Med. 19:408–417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yecies D, Carlson NE, Deng J and Letai A:
Acquired resistance to ABT-737 in lymphoma cells that up-regulate
MCL-1 and BFL-1. Blood. 115:3304–3313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Konopleva M, Contractor R, Tsao T, Samudio
I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, et al:
Mechanisms of apoptosis sensitivity and resistance to the BH3
mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 10:375–388.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maurer U, Charvet C, Wagman AS, Dejardin E
and Green DR: Glycogen synthase kinase-3 regulates mitochondrial
outer membrane permeabilization and apoptosis by destabilization of
MCL-1. Mol Cell. 21:749–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang C, Cai TY, Zhu H, Yang LQ, Jiang H,
Dong XW, Hu YZ, Lin NM, He QJ and Yang B: Synergistic antitumor
activity of gemcitabine and ABT-737 in vitro and in vivo through
disrupting the interaction of USP9X and Mcl-1. Mol Cancer Ther.
10:1264–1275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yonezawa A and Inui K: Organic cation
transporter OCT/SLC22A and H(+)/organic cation antiporter
MATE/SLC47A are key molecules for nephrotoxicity of platinum
agents. Biochem Pharmacol. 81:563–568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giaccone G: Clinical perspectives on
platinum resistance. Drugs. 59(Suppl 4): S9–S17, S37-S88. 2000.
View Article : Google Scholar
|
|
20
|
Piccart MJ, Lamb H and Vermorken JB:
Current and future potential roles of the platinum drugs in the
treatment of ovarian cancer. Ann Oncol. 12:1195–1203. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuwahara A, Yamamori M, Nishiguchi K,
Okuno T, Chayahara N, Miki I, Tamura T, Inokuma T, Takemoto Y,
Nakamura T, et al: Replacement of cisplatin with nedaplatin in a
definitive 5-fluorouracil/cisplatin-based chemoradiotherapy in
Japanese patients with esophageal squamous cell carcinoma. Int J
Med Sci. 6:305–311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boulikas T and Vougiouka M: Recent
clinical trials using cisplatin, carboplatin and their combination
chemotherapy drugs (review). Oncol Rep. 11:559–595. 2004.PubMed/NCBI
|
|
23
|
Simonin K, N'Diaye M, Lheureux S,
Loussouarn C, Dutoit S, Briand M, Giffard F, Brotin E,
Blanc-Fournier C and Poulain L: Platinum compounds sensitize
ovarian carcinoma cells to ABT-737 by modulation of the Mcl-1/Noxa
axis. Apoptosis. 18:492–508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lakhani SA, Masud A, Kuida K, Porter GA
Jr, Booth CJ, Mehal WZ, Inayat I and Flavell RA: Caspases 3 and 7:
Key mediators of mitochondrial events of apoptosis. Science.
311:847–851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martins LM: The serine protease Omi/HtrA2:
A second mammalian protein with a Reaper-like function. Cell Death
Differ. 9:699–701. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Doi K, Li R, Sung SS, Wu H, Liu Y, Manieri
W, Krishnegowda G, Awwad A, Dewey A, Liu X, et al: Discovery of
marinopyrrole A (maritoclax) as a selective Mcl-1 antagonist that
overcomes ABT-737 resistance by binding to and targeting Mcl-1 for
proteasomal degradation. J Biol Chem. 287:10224–10235. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang S and Sinicrope FA: BH3 mimetic
ABT-737 potentiates TRAIL-mediated apoptotic signaling by
unsequestering Bim and Bak in human pancreatic cancer cells. Cancer
Res. 68:2944–2951. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang C, Kaushal V, Shah SV and Kaushal GP:
Mcl-1 is downregulated in cisplatin-induced apoptosis and
proteasome inhibitors restore Mcl-1 and promote survival in renal
tubular epithelial cells. Am J Physiol Renal Physiol.
292:F1710–F1717. 2007. View Article : Google Scholar : PubMed/NCBI
|