Introduction
The human aurora kinase (AURK) family consists of
three genes, including AURKA, AURKB and AURKC. Their gene products
are located in different parts of the nucleus and have been
suggested to function independently during the mitotic phase
(M-phase) of the cell cycle (1–3). AURKB
appears in the nucleus at the initial synthesis phase, and is
involved in the regulation of cytokinesis by binding to several
proteins containing the inhibitor Survivin (4,5). It has
been reported that AURKs are overexpressed in tumor cells and,
therefore, they are thought to be potential molecular targets for
the treatment of malignant tumors (6–9). A number
of inhibitors of AURK (ZM447439, VX-680, AT9283, AZD1152, MLN8054
and MLN8237) have been developed (6).
These agents inhibit AURKA and AURKB to varying degrees, and some
are currently in phase I clinical trials (10). The previously described inhibitor
AZD1152 is a prodrug that changes to the active form AZD1152-hQPA
in the cytoplasm, which has a dominant effect on AURKB (11).
Chemotherapy using anticancer drugs, such as
platinum-based therapies or taxanes, and radiotherapy are the most
commonly employed strategies for the treatment of gynecological
malignant tumors (12,13). AURK inhibitors are thought to be an
effective molecular-targeting drug for gynecological malignant
tumors (6), and clinical trials for
their use against leukemia and other cancers are underway (10). In the future, there is a possibility
that ARUK inhibitors may be used in combination with anticancer
agents. However, it is unknown which anticancer agents would
function most effectively in combination with AURK inhibitors. Sun
et al (14) reported that the
AURKB inhibitor, VX-680, downregulated nuclear factor (NF)-κB
expression and increased the sensitivity of tumor cells to
anticancer agents. Therefore, evaluation of the cellular expression
or activity of NF-κB may emerge as an important basis for the use
of AURKB inhibitors.
Previously, we reported that cisplatin-resistant
HCP4 cells, which are derived from the HeLa human cervical cancer
cell line, overexpressed AURKB. Furthermore, when treated with
AZD1152-hQPA, an AURKB inhibitor, the colony formation activity of
cisplatin-resistant cells was shown to be significantly decreased,
as compared with HeLa cells (15).
Based on these results, it was hypothesized that a combination of
cisplatin and molecular-targeting drugs may have a synergistic
cytotoxic effect on malignant tumor cells. However, the present
study demonstrated that an AURKB-specific small interfering RNA
(siRNA) and AZD1152-hQPA antagonized the cytotoxic effect of
cisplatin, whereas it had a synergistic effect on
all-trans-retinoic acid (ATRA) and synthetic retinoids. These two
different effects were thought to be due to differences in the
expression levels of AURKB induced by treatment with specific
anticancer agents. The present study aimed to investigate the
expression levels of AURKB in the HeLa and HCP4 human cervical
cancer cells lines, and propose a strategy for combination therapy
involving AURKB inhibitors and anticancer agents.
Materials and methods
Cell culture
HeLa cells and their derived cisplatin-resistant
HCP4 cells were established and kindly gifted by Professor
Shin-Ichi Akiyama (Department of Molecular Oncology, Graduate
School Medical and Dental Science, Kagoshima University, Kagoshima,
Japan) (15). Both cell lines were
cultured in RPMI 1640 Medium, GlutaMAX™ supplement (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
fetal bovine serum (HyClone; GE Healthcare Life Sciences, Logan,
UT, USA) in 5% CO2 at 37°C.
Antibodies and anticancer agents
Rabbit anti-AURKB (cat. no. 1788-1) and mouse
anti-β-actin (cat. no. sc-47778) monoclonal antibodies were
purchased from Epitomics (Burlingame, CA, USA) and Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA), respectively. Cisplatin,
etoposide and ATRA were purchased from Sigma-Aldrich; Merck
Millipore (Darmstadt, Germany). Doxorubicin was obtained from Kyowa
Hakko Kogyo, Co., Ltd. (Tokyo, Japan). AZD1152-hQPA was purchased
from Chemietek (Indianapolis, IN, USA); it was dissolved in
dimethyl sulfoxide to a concentration of 10 mM and stored at −20°C.
Synthetic retinoids, Am80 (Tamibarotene) and TAC-101 were kindly
gifted by Dr Shudo Koichi of the Research Foundation ITSUU
Laboratory (Tokyo, Japan).
Knockdown analysis using siRNAs
Knockdown of AURKB in HeLA cells was performed using
AURKB-specific siRNA, as described previously (15). The following 25-bp double-stranded RNA
oligonucleotides were commercially generated (Invitrogen; Thermo
Fisher Scientific, Inc.): AURKB-specific siRNA,:
5′-UUUAGGUCCACCUUGACGAUGCGGC-3′ and
5′-GCCGCAUCGUCAAGGUGGACCUAAA-3′. A total of 200 pmol siRNA was
mixed with 5 µl Lipofectamine 2000 (Qiagen GmbH, Hilden, Germany)
according to the manufacturer's protocol. After 20 min,
5×105 cells were gently mixed and incubated for
additional 20 min. Transfected cells were used for western blotting
and colony formation assay. Negative control siRNA was purchased
from Invitrogen (Thermo Fisher Scientific, Inc.).
Cell viability assays and combined
effects of anticancer agents
Briefly, HeLa cells (1×103) were seeded
into 96-well plates for 24 h, and subsequently, cisplatin,
etoposide, doxorubicin, ATRA, Am80, TAC-101 and AZD1152-hQPA were
added to the cells at a maximum concentration at 20, 40, 1, 500,
100, 200 and 100 µM, respectively, and 2-fold serial dilutions were
performed. For combination treatment, fixed combination ratios and
2-fold serial dilutions were employed. For AURKB, specific
siRNA-transfected HeLa cells (2×103) were seeded into 96-well
plates at 24 h post-transfection, and the above indicated single
agents were added to the cells with 2-fold serial dilutions. After
72 h, the surviving cells were stained with the water-soluble
tetrazolium salt-8 (TetraColor ONE; Seikagaku Corporation, Tokyo,
Japan) for 2–3 h at 37°C, according to the manufacturer's protocol.
The absorbance was then measured at 450 nm. To measure the half
maximal inhibitory concentration (IC50) in each
experiment, CalcuSyn software version 2.0 (Biosoft, Cambridge, UK)
was used. To evaluate the synergism or antagonism of the
combination of an anticancer agent and AZD1152-hQPA, HeLa cells
were treated with an anticancer agent alone, AZD1152-hQPA alone, or
a fixed combination ratio of the anticancer agent and AZD1152-hQPA,
as decided by the IC50 values. The experiments were performed in
duplicate, with 2-fold serial dilutions. To assess whether there
was a synergistic effect on cytotoxicity, the combination index
(CI) was calculated using CalcuSyn software version 2.0. This
method enables the quantification of synergism (CI<1) and
antagonism (CI>1) at different concentrations and effect levels
(16). Based on the median effective
dose (ED)50, ED75 and ED90 of the drug
combinations, isobolograms were generated and synergy was evaluated
using CalcuSyn software version 2.0.
Western blot analysis
Preparation of whole cell lysates and western blot
analysis were performed as described previously (15). The cells were washed with PBS twice,
and then lysed in buffer containing 50 mmol/l Tris-HCl (pH 8.0), 1
mmol/l EDTA, 120 mmol/l NaCl, 0.5% (v/v) Nonidet P-40, 10% (v/v)
glycerol, 1 mmol/l phenylmethylsulfonyl fluoride and 1 mmol/l
dithiothreitol. The lysates were centrifuged at 21,000 × g
for 10 min at 4°C, and the supernatants (50 µg) were separated by
10% SDS-PAGE and then transferred onto polyvinylidene difluoride
membranes. The blotted membranes were treated with 3% (w/v) skimmed
milk in 10 mmol/l Tris, 150 mmol/l NaCl and 0.2% (v/v) Tween 20,
and then incubated for 1 h at room temperature with the
corresponding primary antibodies (1:1,000 dilution of rabbit
anti-AURKB and 1:10,000 dilution of mouse anti-β-actin). Next, the
membranes were then incubated for 40 min at room temperature with a
peroxidase-conjugated secondary antibody [anti-rabbit
immunoglobulin (Ig) G, peroxidase-linked species-specific whole
antibody from donkey (NA934; GE Healthcare Life Sciences, Chalfont,
UK) and anti-mouse IgG, peroxidase-linked species-specific whole
antibody from sheep (NA931; GE Healthcare Life Sciences)] at
1:7,500 dilution. The bound antibody was visualized using an
enhanced chemiluminescence kit (GE Healthcare Bio-Sciences,
Pittsburgh, PA, USA), and the signal intensity was quantitated
using Multi Gauge software version 3.0 (Fujifilm, Tokyo,
Japan).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was purified from the transfected- and
non-transfected HeLa cells using the RNeasy Mini kit (Qiagen, Inc.,
Valencia, CA, USA), and RT-qPCR was performed as described
previously (17). Briefly, RT from
total messenger RNA (mRNA) with random primers (Applied Biosystems;
Thermo Fisher Scientific, Inc.) was performed with the
High-Capacity cDNA Reverse Transcription kit (Thermo Fisher
Scientific, Inc.), and then PCR with TaqMan® Universal
Master Mix II with UNG (Thermo Fisher Scientific, Inc.) was
conducted on the StepOnePlus™ Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using the following
cycling parameters: 2 min at 95°C, followed by 40 cycles of 15 sec
at 95°C and 1 min at 60°C, according to the manufacturer's
protocol. The following primer sets for PCR were used:
Hs01060665_g1 for β-actin, Hs00945858_g1 for AURKB and
Hs01582072_m1 for AURKA (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The comparative Cq method was used to quantify
the gene expression (18). Values
were normalized to those for human β-actin. All samples were
analyzed in duplicate in each experiment.
Statistical analysis
Student's t-tests were performed for statistical
analysis of the variables between the two groups with GraphPad
StatMate statistical software version IV (GraphPad Software, Inc.,
La Jolla, CA, USA). Data are presented as the mean ± standard
deviation.
Results
AZD1152-hQPA antagonizes the cytotoxic
effect of cisplatin on HeLa cells
In our previous study, we reported that the AURKB
protein was overexpressed in cisplatin-resistant PCDP5 and HCP4
cells, as compared with the parent PC3 and HeLa cells,
respectively, and was induced by cisplatin treatment in a
concentration- and dose-dependent manner (15). Furthermore, the AURKB inhibitor,
AZD1152-hQPA, sensitized cisplatin-resistant cells, but not parent
cells, to cisplatin. To confirm whether cisplatin and AZD1152-hQPA
have a synergistic cytotoxic effect on cancer cells, the present
study calculated the CI using CalcuSyn software. First, the
IC50 values of cisplatin and AZD1152-hQPA with HeLa
cells were calculated (Table I), and
were 1.87 and 14.8 µM, respectively. Based on these results, we
employed a fixed combination ratio of cisplatin-to-AZD1152-hQPA of
1:5 (Table II), and treated HeLa
cells with these agents using a single or a fixed combination
concentration to calculate the CI. As is shown in Table III, the CIs at ED50,
ED75 and ED90 were 1.35, 1.46 and 1.62,
respectively, which suggested that, as the concentration of the
drugs was elevated, the CI increased. These results indicated that
cisplatin and AZD1152-hQPA did not have a synergistic cytotoxic
effect, but an antagonistic cytotoxic effect, on HeLa cells.
 | Table I.Evaluation of IC50. |
Table I.
Evaluation of IC50.
| Drug | HeLa
IC50 | HCP4
IC50 | IC50
ratioa |
|---|
| Cisplatin (µM) | 1.87±0.07 | 65.53±0.83 | 35.0 |
| Etoposide (µM) | 9.72±0.14 | 15.84±0.21 | 1.6 |
| Doxorubicin
(nM) | 87.75±0.09 | 146.74±7.04 | 1.7 |
| ATRA (µM) | 89.43±0.22 | 84.34±1.31 | 0.9 |
| Am80 (µM) | 14.66±0.18 | 14.82±0.48 | 1.0 |
| TAC-101 (µM) | 34.12±1.20 | 38.75±0.36 | 1.1 |
| AZD1152-hQPA
(µM) | 14.77±0.42 | 31.22±0.15 | 2.1 |
 | Table II.Fixed combination ratio. |
Table II.
Fixed combination ratio.
|
| Fixed combination
ratio |
|---|
|
|
|
|---|
| Drug
combination | HeLa cells | HCP4 cells |
|---|
|
Cisplatin/AZD1152-hQPA | 1:5 | 2:1 |
|
Etoposide/AZD1152-hQPA | 2:5 | 1:2 |
|
Doxorubicin/AZD1152-hQPA | 1:200 | 1:100 |
|
ATRA/AZD1152-hQPA | 5:1 | 3:1 |
|
Am80/AZD1152-hQPA | 1:1 | 1:2 |
|
TAC-101/AZD1152-hQPA | 2:1 | 1:1 |
 | Table III.CI values for various concentrations
of anticancer agents. |
Table III.
CI values for various concentrations
of anticancer agents.
|
| HeLa CI | HCP4 CI |
|---|
|
|
|
|
|---|
| Drug |
ED50 |
ED75 |
ED90 |
ED50 |
ED75 |
ED90 |
|---|
| Cisplatin | 1.35 | 1.46 | 1.62 | 0.95 | 94.11 | 56,116 |
| Etoposide | 0.93 | 1.13 | 2.44 | 1.57 | 0.44 | 0.22 |
| Doxorubicin | 1.18 | 1.56 | 2.48 | 1.71 | 0.66 | 0.34 |
| ATRA | 0.36 | 0.46 | 0.77 | 0.88 | 0.50 | 5.44 |
| Am80 | 0.54 | 0.41 | 0.41 | 0.55 | 0.28 | 2.74 |
| TAC-101 | 0.52 | 0.39 | 0.46 | 0.83 | 0.61 | 23.09 |
Subsequently, the effect of the combined
administration of cisplatin and AZD1152-hQPA on cisplatin-resistant
HCP4 cells derived from HeLa cells was investigated. From the
result of IC50 values for HCP4 (Table I), HCP4 cells were 35-times more
resistant to cisplatin than HeLa cells. Therefore, a fixed
combination ratio of cisplatin-to-AZD1152-hQPA of 2:1 was employed
(Table II). As is shown in Table III, the CI of cisplatin and
AZD1152-hQPA was very high, especially when used at ED75
and ED90, indicating that they had a strong antagonistic
cytotoxic effect on HCP4 cells. The antagonistic cytotoxic effect
on HCP4 cells was much higher than that of HeLa cells.
Effect of anticancer agents on AURKB
expression
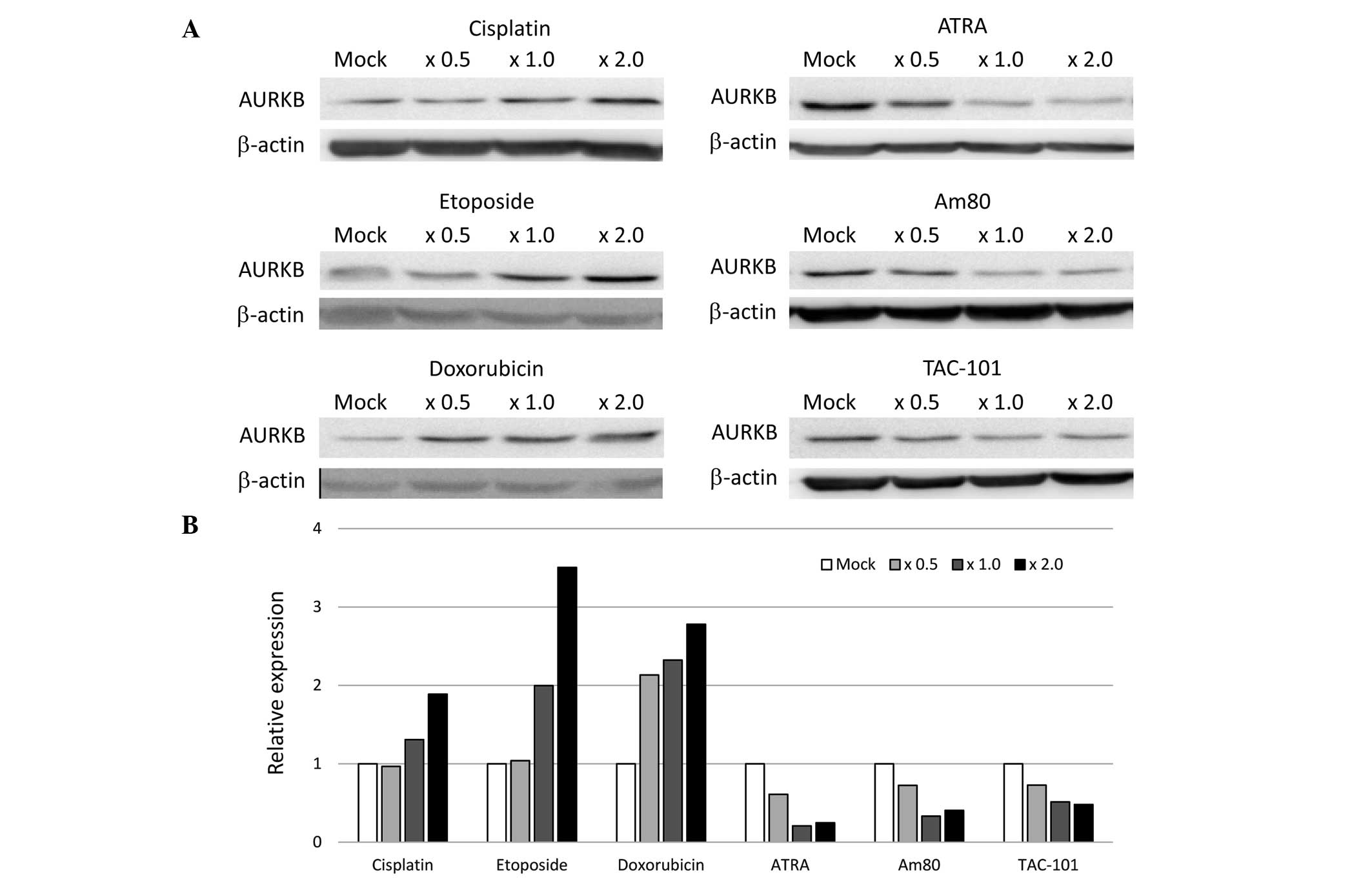
Consistent with our previous report (15), cisplatin treatment of HeLa cells
induced the protein expression of AURKB in a
concentration-dependent manner (Fig.
1). To confirm whether AURKB protein expression was
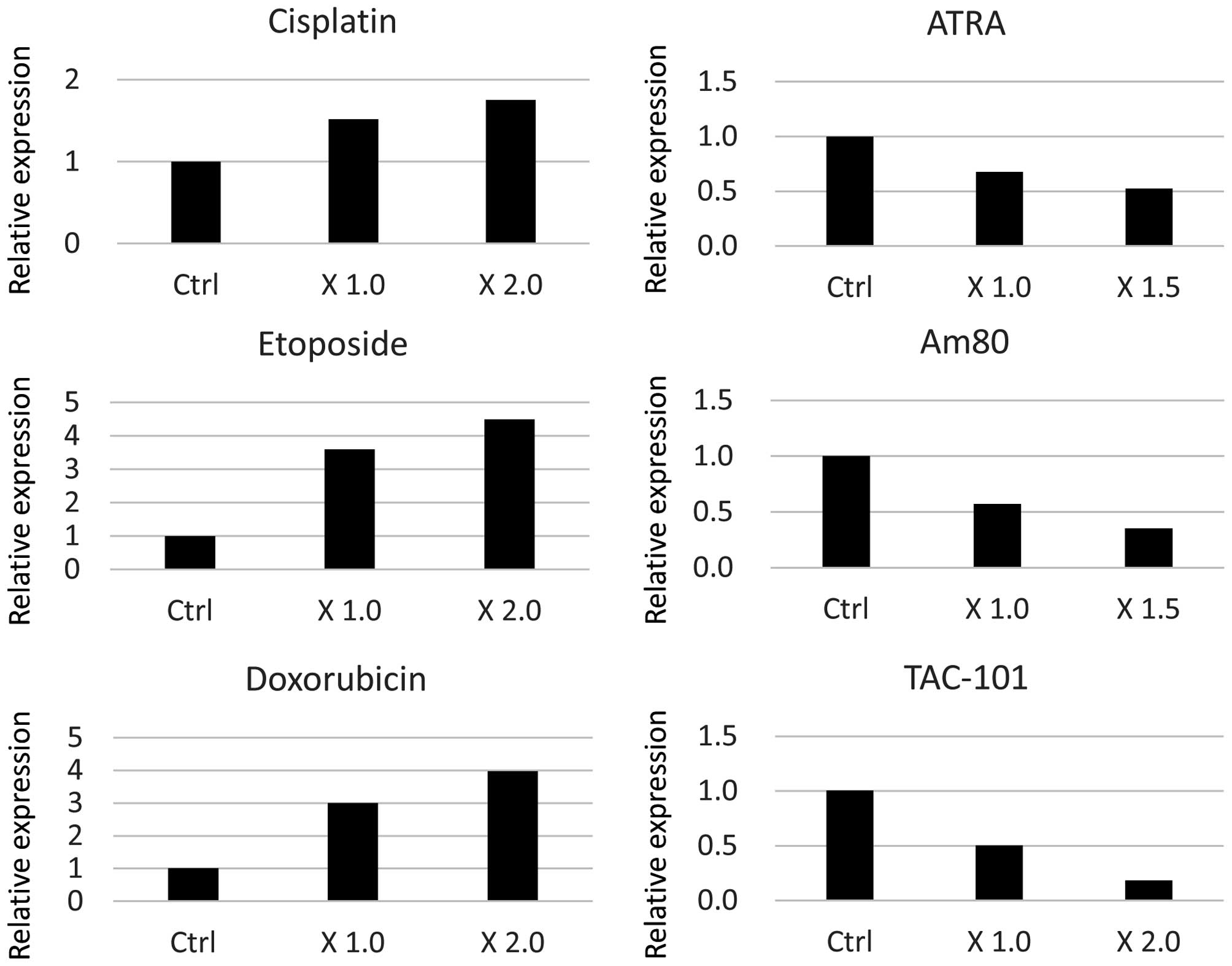
transcriptionally induced by cisplatin, RT-qPCR was performed, and
demonstrated that AURKB mRNA expression was upregulated by
cisplatin (Fig. 2). It was
hypothesized that the level of AURKB protein induced by anticancer
agents might influence the cytotoxic effect of AZD1152-hQPA.
Therefore, the effect of several anticancer agents on AURKB
expression was examined. Notably, etoposide and doxorubicin
increased both the protein and mRNA expression levels of AURKB
(Figs. 1 and 2). Conversely, ATRA, synthetic retinoids,
Am80 and TAC-101 decreased the protein and mRNA expression levels
of AURKB (Figs. 1 and 2). In addition, the mRNA expression levels
of AURKA in HeLa cells treated with various anticancer agents were
evaluated. Cisplatin, etoposide and doxorubicin increased the mRNA
expression levels of AURKA, whereas ATRA, Am80 and TAC-101
decreased the mRNA expression levels of AURKA and AURKB (data not
shown).
AURKB expression status is affected by
anticancer agents, which influences the cytotoxic effect of
AZD1152-hQPA
To investigate whether the AURKB expression status
affects the cytotoxic effect of AZD1152-hQPA, CI values were
calculated for the combined administration of AZD1152-hQPA with
various anticancer agents. In HeLa cells, etoposide and
doxorubicin, as well as cisplatin, increased the expression of
AURKB and the CI value was >1 in all cases, with the exception
of etoposide at ED50 (Table
III). The highest CI for each agent was observed at
ED90 for all cases. In contrast, the CI values for ATRA,
Am80 and TAC-101 m, which decreased AURKB expression, was <1 for
all cases. The CI value for ATRA gradually became higher as the
concentration was increased; however, Am80 and TAC-101 retained low
CI values, despite increased concentrations. In cisplatin-resistant
HCP4 cells, the CI values of ATRA, Am80 and TAC-101 were <1 at
ED50 and ED75, but were >1 at
ED90 (Table III).
Furthermore, the CI of etoposide and doxorubicin at ED75
and ED90 was <1 when tested using HeLa cells. As is
shown in Table I, cisplatin-resistant
HCP4 cells had a multidrug-resistant phenotype, but this mechanism
had no effect on the cytotoxicity of ATRA and synthetic
retinoids.
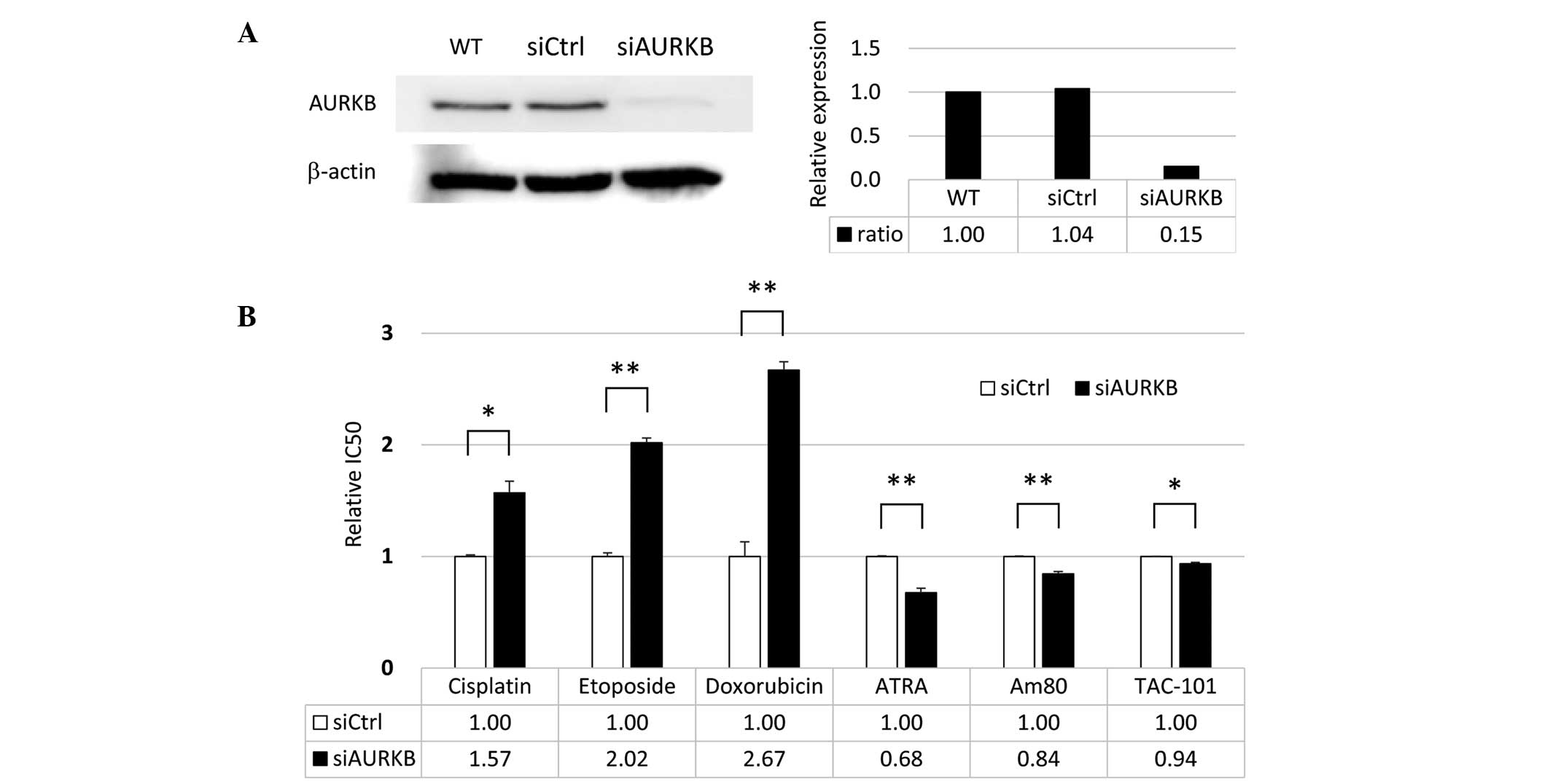
Knockdown of AURKB and the cytotoxic
effects of anticancer agents
AZD1152-hQPA decreases the activity of AURKB
(11). To investigate whether
knockdown of AURKB affected the cytotoxic effect of ATRA, Am80 and
TAC-101, HeLa cells were transfected with AURKB-specific siRNA and
treated with these agents. As is shown in Fig. 3A, the expression of AURKB was
downregulated in AURKB-specific siRNA-knockdown HeLa cells, as
compared with cells transfected with control siRNA. Knockdown of
AURKB increased the IC50 values of cisplatin, etoposide
and doxorubicin, and decreased the IC50 values of ATRA,
Am80 and TAC-101, as compared with the control (Fig. 3B).
Discussion
AURKs are important molecules involved in mitosis,
and AURKA and AURKB have been shown to be overexpressed in several
types of cancer (1). Therefore, AURKs
are potential novel molecular targets for the prevention of cancer
proliferation, and clinical trials have been performed (10). It has been reported that AURKB
inhibitors are able to enhance the cytotoxic effects of anticancer
agents (19–21). Fu et al (19) reported that the AURK inhibitor VE 465
was able to enhance the antitumor activity of carboplatin in human
ovarian cancer cells. Fiskus et al (20) used a combination treatment involving
the pan-AURK inhibitor MK-0457 and vorinostat in human breast
cancer cells, and demonstrated a synergistic effect in both in
vitro and in vivo assays. Therefore, the present study
aimed to investigate the cytotoxic effect of the AURKB-specific
inhibitor, AZD1152-hQPA, and cisplatin on the HeLa cervical
adenocarcinoma cell line, and demonstrated that the effects were
antagonistic. Previously, we reported that the expression of AURKB
was increased following treatment of cancer cells with cisplatin
(15). It was hypothesized that the
induction of AURKB by cisplatin treatment may alter the sensitivity
of cancer cells to AZD1152-hQPA, and it was decided that we would
search for anticancer agents that increased or decreased AURKB
expression. In the present study, it was demonstrated that
doxorubicin, etoposide and cisplatin increased the expression of
AURKB, while ATRA, Am80 and TAC-101 decreased its expression.
Subsequently, the combined effects of these anticancer agents with
AZD1152-hQPA were investigated, their CIs were calculated, and it
was demonstrated that the combinations that increased AURKB
expression showed antagonistic cytotoxic effects on HeLa cells. In
contrast, the combination of AZD1152-hQPA with anticancer agents
that decreased the expression of AURKB showed synergistic cytotoxic
effects on HeLa cells. These results indicated that AURKB
expression may influence the cytotoxic effect of AZD1152-hQPA. To
the best of our knowledge, there has been no previous report
showing a synergistic cytotoxic effect for AZD1152-hQPA used in
combination with anticancer agents. Zhang and Zhang (21) reported that ZM447439, an AURKB
inhibitor, suppressed the growth of SiHa cervical cancer cells and
enhanced their chemosensitivity to cisplatin, which was
inconsistent with the results of the present study. Both
AZD1152-hQPA and ZM447439 also inhibited AURKA, but the effect of
AZD1152-hQPA on AURKB inhibition was greater than ZM447439
(6). Furthermore, AURKA expression
was increased by cisplatin in the present study. This discrepancy
might be due to differing specificities for AURKs or the type of
cells used.
In the present study, cisplatin, doxorubicin and
etoposide increased the cellular expression of AURKB, while ATRA,
Am80 and TAC-101 decreased it. Notably, these anticancer agents
regulated AURKB expression at the transcriptional level, as
demonstrated by RT-qPCR. From sequence alignment analysis, the Alu
sequence upstream of the transcription start site of the AURKB gene
was identified, and it was observed that the promoter region
contained ~230 bp (data not shown). the promoter region of the
AURKB gene is ~230 bp (data not shown). Kimura et al
(22) reported that the E2 family
(E2F) of transcription factors promote the transcription of the
AURKB gene via a cell-cycle-dependent element (CDE) in the promoter
region. Ianari et al (23)
reported that treatment with cisplatin or doxorubicin increased
E2F-1 expression, and E2F-1 Ser403 phosphorylation was induced by
doxorubicin (24). Conversely, E2F-1
expression, induced by treatment with estrogen, was inhibited by
treatment with a trans-retinoic acid (25). Therefore, the CDE/E2F-1 pathway may be
associated with AURKB gene expression and regulated by anticancer
agents. Notably, the effects of anticancer agents on the cell cycle
in previous studies were different. Cisplatin, doxorubicin and
etoposide induced G2/M-phase cell cycle arrest (26–28), while
ATRA, Am80 and TAC-101 induced G1 cell cycle arrest (29–31).
Further analysis is required to elucidate the associations among
AURKB expression and anticancer agents.
Previously, we reported that ARUKB expression in
cisplatin-resistant HCP4 cells was upregulated, as compared with
parent HeLa cells, and demonstrated that HCP4 cells were
hypersensitive to AZD1152-hQPA using colony formation assays
(15). However, in the present study,
cytotoxicity assays demonstrated that HCP4 cells were resistant to
AZD1152-hQPA. This discrepancy may be due to differences in the
assays performed and the growth rates of each cell line.
Furthermore, upregulation of AURKB expression in HCP4 cells may
have contributed to induce resistance to AZD1152-hQPA. Unlike HeLa
cells, the combined treatment of AZD1152-hQPA and anticancer agents
had a biphasic effect in cisplatin-resistant HCP4 cells: the
ED75 of ATRA and synthetic retinoids was synergistic,
while the ED90 of these agents was antagonistic. This
biphasic effect may also be influenced by the slow growth rate of
HCP4 cells. Further analysis is required to develop an effective
method to overcome cisplatin resistance when the combination of
AURK inhibitors and anticancer agents is used.
In the present study, AZD1152-hQPA had an
antagonistic effect on cisplatin. Therefore, whether AURKB
knockdown was able to inhibit the cytotoxic effect of cisplatin was
investigated. AURKB knockdown resulted in the resistance of HeLa
cells to cisplatin, doxorubicin and etoposide, while it sensitized
the cells to ATRA, Am80 and TAC-101, as well as AZD1152-hQPA. These
results suggested that strategies involving AURKB inhibition or
knockdown may have similar effects as anticancer agents.
In conclusion, the results of the present study
suggested that a combination of molecular-targeting drugs against
AURKB and anticancer agents may influence the cytotoxic effects in
cells. The optimal combination therapy may be determined by
revealing these mechanisms.
Acknowledgements
This study was supported in part by Grants-in-Aid
for Scientific Research from the Ministry for Education, Culture,
Sports, Science and Technology of Japan (grant no. 24591987).
References
|
1
|
Kollareddy M, Dzubak P, Zheleva D and
Hajduch M: Aurora kinases: Structure, functions and their
association with cancer. Biomed Pap Med Fac Univ Palacky Olomouc
Czech Repub. 152:27–33. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hochegger H, Hégarat N and Pereira-Leal
JB: Aurora at the pole and equator: Overlapping functions of Aurora
kinases in the mitotic spindle. Open Biol. 3:1201852013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vas AC and Clarke DJ: Aurora B kinases
restrict chromosome decondensation to telophase of mitosis. Cell
Cycle. 7:293–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dutertre S, Descamps S and Prigent C: On
the role of aurora-A in centrosome function. Oncogene.
21:6175–6183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carmena M, Ruchaud S and Earnshaw WC:
Making the Auroras glow: Regulation of Aurora A and B kinase
function by interacting proteins. Curr Opin Cell Biol. 21:796–805.
2002. View Article : Google Scholar
|
|
6
|
Umene K, Banno K, Kisu I, Yanokura M,
Nogami Y, Tsuji K, Masuda K, Ueki A, Kobayashi Y, Yamagami W, et
al: Aurora kinase inhibitors: Potential molecular-targeted drugs
for gynecologic malignant tumors. Biomed Rep. 1:335–340.
2013.PubMed/NCBI
|
|
7
|
Mehra R, Serebriiskii IG, Burtness B,
Astsaturov I and Golemis EA: Aurora kinases in head and neck
cancer. Lancet Oncol. 14:e425–e435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baldini E, Sorrenti S, D'Armiento E,
Prinzi N, Guaitoli E, Favoriti P, Gnessi L, Moretti C, Bianchini M,
Alessandrini S, et al: Aurora kinases: New molecular targets in
thyroid cancer therapy. Clin Ter. 163:e457–e462. 2012.PubMed/NCBI
|
|
9
|
Kelly KR, Ecsedy J, Mahalingam D, Nawrocki
ST, Padmanabhan S, Giles FJ and Carew JS: Targeting aurora kinases
in cancer treatment. Curr Drug Targets. 12:2067–2078. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheung CH, Sarvagalla S, Lee JY, Huang YC
and Coumar MS: Aurora kinase inhibitor patents and agents in
clinical testing: An update (2011–2013). Expert Opin Ther Pat.
24:1021–1038. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mortlock AA, Foote KM, Heron NM, Jung FH,
Pasquet G, Lohmann JJ, Warin N, Renaud F, De Savi C, Roberts NJ, et
al: Discovery, synthesis, and in vivo activity of a new class of
pyrazoloquinazolines as selective inhibitors of aurora B kinase. J
Med Chem. 50:2213–2224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lorusso D, Petrelli F, Coinu A,
Raspagliesi F and Barni S: A systematic review comparing cisplatin
and carboplatin plus paclitaxel-based chemotherapy for recurrent or
metastatic cervical cancer. Gynecol Oncol. 133:117–123. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Viswanathan AN: Advances in the use of
radiation for gynecologic cancers. Hematol Oncol Clin North Am.
26:157–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun C, Chan F, Briassouli P and
Linardopoulos S: Aurora kinase inhibition downregulates NF-kappaB
and sensitises tumour cells to chemotherapeutic agents. Biochem
Biophys Res Commun. 352:220–225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akiyama M, Izumi H, Wang KY, Yamaguchi T,
Kuma A, Kitamura N, Harada Y, Oya R, Yamaguchi K, Iwai Y and Kohno
K: Hypersensitivity to aurora kinase inhibitors in cells resistant
against platinum- containing anticancer agents. Anticancer Agents
Med Chem. 14:1042–1050. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reynolds CP and Maurer BJ: Evaluating
response to antineoplastic drug combinations in tissue culture
models. Methods Mol Med. 110:73–183. 2005.
|
|
17
|
Yamaguchi T, Kurita T, Nishio K, Tsukada
J, Hachisuga T, Morimoto Y, Iwai Y and Izumi H: Expression of BAF57
in ovarian cancer cells and drug sensitivity. Cancer Sci.
106:359–366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu S, Li Y, Huang J, Liu T, Hong Z, Chen
A, Bast RC, Kavanagh JJ, Gershenson DM, Sood AK and Hu W: Aurora
kinase inhibitor VE 465 synergistically enhances cytotoxicity of
carboplatin in ovarian cancer cells through induction of apoptosis
and downregulation of histone 3. Cancer Biol Ther. 13:1034–1041.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fiskus W, Hembruff SL, Rao R, Sharma P,
Balusu R, Venkannagari S, Smith JE, Peth K, Peiper SC and Bhalla
KN: Co-treatment with vorinostat synergistically enhances activity
of Aurora kinase inhibitor against human breast cancer cells.
Breast Cancer Res Treat. 135:433–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang L and Zhang S: ZM447439, the Aurora
kinase B inhibitor, suppresses the growth of cervical cancer SiHa
cells and enhances the chemosensitivity to cisplatin. J Obstet
Gynaecol Res. 37:591–600. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kimura M, Uchida C, Takano Y, Kitagawa M
and Okano Y: Cell cycle-dependent regulation of the human aurora B
promoter. Biochem Biophys Res Commun. 316:930–936. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ianari A, Gallo R, Palma M, Alesse E and
Gulino A: Specific role for p300/CREB-binding protein-associated
factor activity in E2F1 stabilization in response to DNA damage. J
Biol Chem. 279:30830–30835. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Real S, Espada L, Espinet C, Santidrián AF
and Tauler A: Study of the in vivo phosphorylation of E2F1 on
Ser403. Biochim Biophys Acta. 1803:912–918. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hartman J, Müller P, Foster JS, Wimalasena
J, Gustafsson JA and Ström A: HES-1 inhibits 17beta-estradiol and
heregulin-beta1-mediated upregulation of E2F-1. Oncogene.
23:8826–8833. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sorenson CM, Barry MA and Eastman A:
Analysis of events associated with cell cycle arrest at G2 phase
and cell death induced by cisplatin. J Natl Cancer Inst.
82:749–755. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nam C, Doi K and Nakayama H: Etoposide
induces G2/M arrest and apoptosis in neural progenitor cells via
DNA damage and an ATM/p53-related pathway. Histol Histopathol.
25:485–493. 2010.PubMed/NCBI
|
|
28
|
Ling YH, el-Naggar AK, Priebe W and
Perez-Soler R: Cell cycle-dependent cytotoxicity, G2/M phase
arrest, and disruption of p34cdc2/cyclin B1 activity induced by
doxorubicin in synchronized P388 cells. Mol Pharmacol. 49:832–841.
1996.PubMed/NCBI
|
|
29
|
Wang JG, Barsky LW, Davicioni E, Weinberg
KI, Triche TJ, Zhang XK and Wu L: Retinoic acid induces leukemia
cell G1 arrest and transition into differentiation by inhibiting
cyclin-dependent kinase-activating kinase binding and
phosphorylation of PML/RARalpha. FASEB J. 20:2142–2144. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakazato T, Okudaira T, Ishikawa C, Nakama
S, Sawada S, Tomita M, Uchihara JN, Taira N, Masuda M, Tanaka Y, et
al: Anti-adult T-cell leukemia effects of a novel synthetic
retinoid, Am80 (Tamibarotene). Cancer Sci. 99:2286–2294. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fujimoto K, Hosotani R, Doi R, Wada M, Lee
JU, Koshiba T, Miyamoto Y, Tsuji S, Nakajima S and Imamura M:
Induction of cell-cycle arrest and apoptosis by a novel
retinobenzoic-acid derivative, TAC-101, in human pancreatic-cancer
cells. Int J Cancer. 81:637–644. 1999. View Article : Google Scholar : PubMed/NCBI
|