Introduction
Cervical cancer (CC) is a major public health
concern, representing the fourth most commonly diagnosed cancer in
women and the seventh overall, with an estimated 528,000 new cases
worldwide in 2012 (1). Globally,
~266,000 mortalities from CC occurred in 2012, accounting for 7.5%
of all female cancer mortalities; this number is expected to
increase to 410,000 by the year 2030 (2).
Although the systemic treatment of cervical squamous
cell carcinoma (CSCC) has advanced into an era of targeted drugs,
such as erlotinib (3) and bevacizumab
(4), the antitumor efficacies of
current therapies are limited, most likely due to the high degree
of cancer clonal heterogeneity, intratumoral genetic heterogeneity
and cell signal complexity (5). In
this context, there is an urgent necessity for more active
treatment and rationally designed targeted therapies (6).
More than 95% of CSCC patients are positive for
oncogenic human papillomavirus (HPV) DNA. HPV infection plays a
central role in the development of this cancer, particularly
infection with the high-risk subtypes, HPV 16 and 18 (7). The HPV infection has multiple
intracellular effects in different signaling pathways. The
phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian
target of rapamycin (mTOR) pathway is often dysregulated in
gynecological cancers, particularly in HPV-associated tumors
(6). Alterations that cause the
activation and dysregulation of the PI3K/AKT/mTOR pathway may act
as potentially drug-treatable targets (8).
The PI3K/AKT/mTOR signaling pathway, which functions
in mammal cells, acts to coordinate important cell activities
(6). Tumor cells may have a greater
sensitivity to mTOR inhibitors than normal cells as a consequence
of the dysregulation of mTOR and other proteins associated with
this pathway in solid tumors (9).
Mechanisms for pathway activation include loss of function of the
tumor suppressor gene phosphatase and tensin homolog (PTEN),
amplification or mutation of PI3K, amplification or mutation
of AKT, activation of growth factor receptors and exposure
to carcinogens (10,11).
Rapamycin was the first mTOR inhibitor to be
defined; however, other analogs of rapamycin have been developed,
including temsirolimus and everolimus (12). Certain natural compounds also possess
mTOR inhibitor properties, such as curcumin, resveratrol and
epigallocatechin gallate (13).
Temsirolimus and everolimus have already been incorporated into
clinical practice to treat kidney and breast cancer (14–16).
Therefore the present systematic review was conducted to verify the
potential effect of mTOR inhibitors on CSCC, and thereby provide
support for future rationally designed strategies that may involve
this pathway.
Materials and methods
Protocol and registration
This systematic review followed the the Preferred
Reporting Items for Systematic Reviews and Meta-Analyses checklist
(17). The protocol was registered in
PROSPERO (no. CRD42015016329) (18).
Eligibility criteria
Randomized and non-randomized clinical trials that
evaluated women of any age with CSCC who received mTOR inhibitors
alone or in association with other treatments (drugs or
radiotherapy) were included.
Studies were excluded for the following reasons: i)
Different target conditions, such as studies that did not use mTOR
inhibitors to treat CSCC or did not verify the association between
mTOR inhibitors and CSCC; ii) study assessed associations between
mTOR inhibitor treatment and CSCC in vitro or in vivo
in animal studies; iii) insufficient information provided regarding
histological type, response or treatment.
Information sources and search
strategies
Detailed individual search strategies were developed
for each of the following bibliographic electronic databases:
Cochrane Library (http://www.cochranelibrary.com), Google Scholar
(https://scholar.google.com.br), LILACS
(http://lilacs.bvsalud.org), PMC
(https://www.ncbi.nlm.nih.gov/pmc/),
PubMed (https://www.ncbi.nlm.nih.gov/pubmed/), ScienceDirect
(http://www.sciencedirect.com), Scopus
(https://www.scopus.com) and Web of Science
(http://login.webofknowledge.com/). The
search strategy for Pubmed included the following terms: ‘Cervical
cancer’ or ‘uterine cancer’ or ‘cervix cancer’ or ‘cervical
neoplasm’ or ‘cervix neoplasm’; and ‘mTOR’. The reference lists in
the selected articles were also searched to identify any additional
references that may have been missed in the electronic databases
searches. The search was conducted through January 19th, 2015,
across all databases, without date and language restrictions. The
references were managed and the duplicates removed using
appropriate software (EndNote; Thomson Reuters, New York, NY,
USA).
Study selection
Studies were considered for inclusion in two phases.
In the first phase, two reviewers (D.X.A. and S.T.E.) independently
reviewed the titles and abstracts of all references. These authors
selected articles that met the inclusion criteria based on their
titles and abstracts. In the second phase, the two authors read the
full text of all selected articles and excluded studies that did
not meet the inclusion criteria. The same two authors independently
reviewed all full text articles. Disagreements were resolved by
consensus of the authors or by a third reviewer (E.N.S.G.).
Data collection process and data
items
One reviewer (D.X.A.) collected the required
information from the selected articles, including the following:
Author, year, country, study design, treatment agents, number of
patients with CC and CSCC included, patient population with number
of prior treatments, maximum tolerated dose (MTD) of treatment,
recommended dose of treatment (RD), number of partial responses
(PRs), percentage of patients with stable disease (SD) lasting ≤6
months, time to treatment failure (TTF) or duration of
progression-free survival (PFS), complications, main conclusions
and clinical application. A second reviewer (S.T.E.) crosschecked
all retrieved information. Disagreements were resolved by author
consensus or by a third reviewer (E.N.S.G.).
Risk of bias in individual
studies
The Grades of Recommendation, Assessment,
Development and Evaluation (GRADE) approach was used to assess the
quality of evidence (19). Two
authors (D.X.A. and S.T.E.) completed the required criteria
necessary to qualify the selected articles, which were categorized
as ‘high’, ‘moderate’, ‘low’ or ‘very low’, according to the
analysis of each study. The third reviewer (E.N.S.G.) was involved
when required to make a final decision.
Summary measures
Any reported outcome or efficacy measurements were
considered, including MTD, RD, response rate (RR), percentage of
patients with SD lasting ≥6 months, PFS time, TTF and
complications.
Synthesis of results
A meta-analysis was planned since the data from the
included studies was considered relatively homogeneous.
Results
Study selection
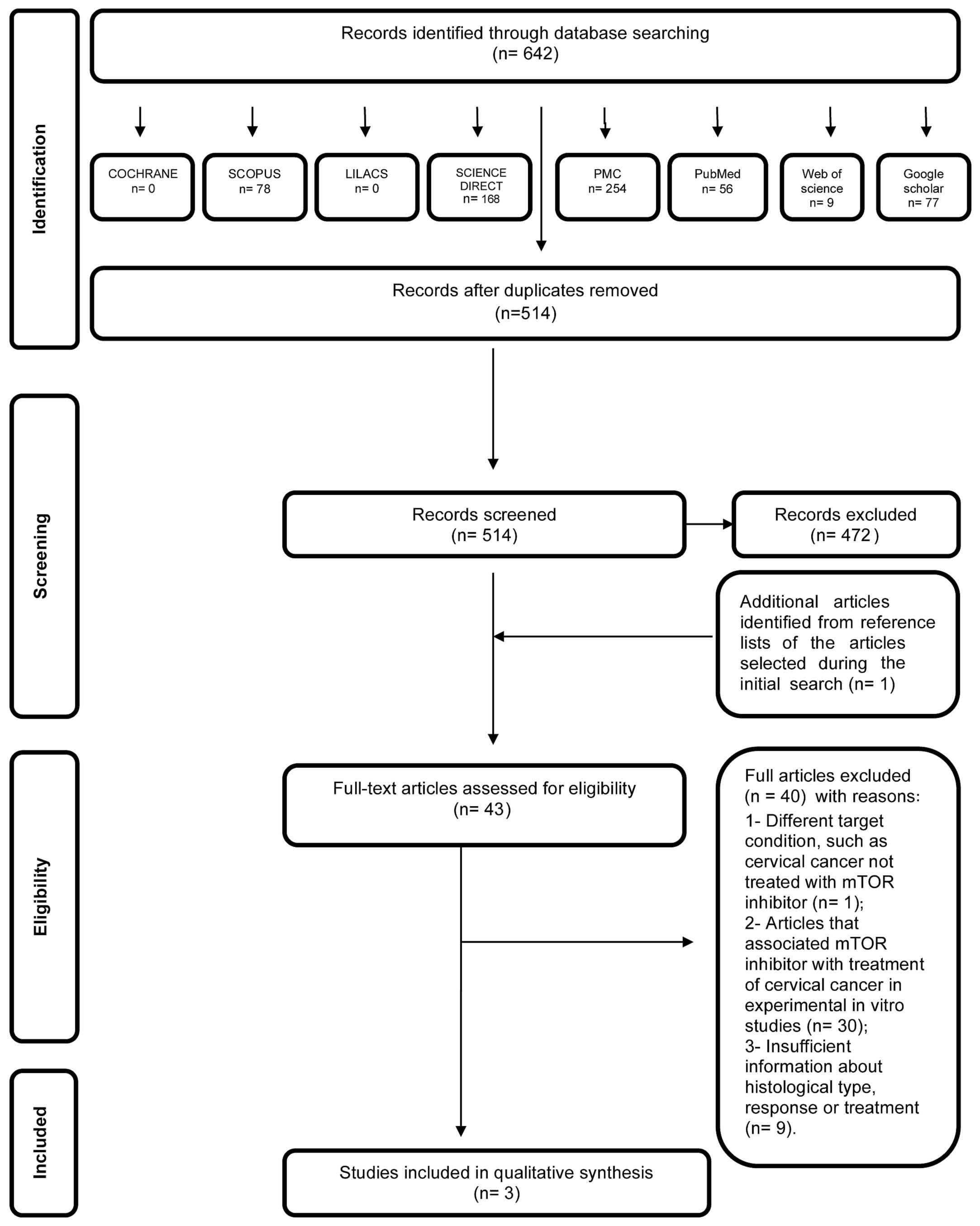
In the first phase of study selection, 642 citations
were identified across the seven electronic databases and Google
Scholar. Following the removal of duplicates, 514 citations
remained. Comprehensive evaluation of the title and abstracts was
completed and 472 articles were excluded; thus, 42 articles
remained after the first phase. One additional study was included
from the reference lists of the identified studies. From the 43
articles retrieved, full text reviews were conducted. This process
excluded 40 studies (20–59). Finally, 3 studies were selected
(60–62). A flow chart detailing the process of
identification, inclusion and exclusion of studies is shown in
Fig. 1.
Study characteristics
The selected studies were conducted in two
countries: The USA (60,61) and Canada (62). All 3 studies were published recently,
in 2011 (60), 2013 (62), 2014 (61), and all were written in English. All
included articles were non-randomized clinical trials; two studies
were phase 1 and one was phase 2. A summary of the descriptive
characteristics of the included studies is given in Table I.
 | Table I.Summary of descriptive
characteristics of clinical studies in included studies. |
Table I.
Summary of descriptive
characteristics of clinical studies in included studies.
| Author, year
(ref.), country | Study design | Treatment
agents | No. of patients
with CC/CSCC | Patient
population | MTD | RD | PR, n
(%a) | SD ≥6 months | TTF or PFS | Complications | Main
conclusions | Clin.
app.b |
|---|
| Moroney et
al, 2011 (60), USA | Phase 1, NR | Liposomal
doxorubicin, bevacizumab and temsirolimus | 13 CC/10 CSCC | Metastatic disease;
median 4 prior regimens | Level 6:
Bevacizumab, 15 mg/kg, d1; liposomal doxorubicin 30
mg/m2, d1; and temsirolimus, 25 mg IV, d1, 8 and 15 | Bevacizumab, 15
mg/kg, d1; liposomal doxorubicin, 20–30 mg/ m2, d1; and
temsirolimus, 25 mg IV, d1, 8 and 15 | 2 among CSCC
patients (15.4%) | 17.6% (among all
patients) | 112 days (among all
patients)b; 172 days
(among patients with PR)b | Grade 3 and 4
toxicities: Thrombocytopenia (9.5%), mucositis (6.7%), cardiac
(4.1%), genitourinary (1.4%), bowel perforation (2.7%) | Combination is well
tolerated with manageable side effects; 2 PRs among 13 CC
patients | 2 |
| Piha-Paul et
al, 2014 (61), USA | Phase 1, NR | Bevacizumab and
temsirolimus | 6 CC/4 CSCC | Metastatic disease;
median 4 prior regimens | Dose level 13:
Temsirolimus, 25 mg IV, d1, 8 and 15; bevacizumab, 15 mg/kg IV was
reached and no MTD was obtained | N/A | 2 among CSCC
patients (33.3%) | 20% (among all
patients) | N/A | Grade 3 and 4
toxicities: Thrombocytopenia (10%), mucositis (2%), hypertension
(2%), hypercholesterolemia (2%), fatigue (7%), increased AST (2%),
neutropenia (2%) | Bevacizumab and
temsirolimus were well tolerated; 2 PRs among 6 CC patients | 2 |
| Tinker et
al, 2013 (62), Canada | Phase 2, NR | Temsirolimus | 38 CC/22 CSCC | Metastatic disease;
≤1 prior therapy permitted | N/A | N/A | 0 | 28% (95% confidence
interval, 14–43%) among all patients | 3.52 months (among
all patients) | Grade 3 toxicity:
fatigue (5.4%), mucositis (5.4%), rash (2.7%), lymphopenia (43.2%),
anemia (16.2%), leucopenia (2.7%), hyponatremia (16.2%),
hypertriglyceridemia (5.4%), hypokalemia (10.8%) | Temsirolimus was
not active in this population as defined by RECIST. SD rates were
notably high. | 3 |
Risk of bias within studies
The GRADE approach (19) was used to assess the quality of
evidence of the included studies, as outlined in Table II. All studies were categorized as
having a low quality level of evidence (60–62). All
had serious issues with regard to the design, none had control
groups, and all lacked blinding. Imprecision occurred in one study
due to the extremely small number of patients included (61).
 | Table II.Assessment of the quality of evidence
for intervention. |
Table II.
Assessment of the quality of evidence
for intervention.
|
| GRADE factors |
|---|
|
|
|
|---|
| Author, year
(ref.) | Study design | Limitations in
study design and/or executiona | Inconsistency | Indirectness | Imprecision | Publication
bias | Moderate/large
effect size | Dose effect | Overall
quality |
|---|
| Moroney et
al, 2011 (27) | Phase 1,
non-randomized | P | N | N | N | N | Not present | Not present | ++ |
| Piha-Paul et
al, 2014 (28) | Phase 1,
non-randomized | P | N | N | P (very few
patients were included) | N | Not present | Not present | + |
| Tinker et
al, 2013 (29) | Phase 2,
non-randomized | P | N | N | N | N | Not present | Not present | ++ |
The clinical application of the studies was also
evaluated. The mTOR inhibitor application in CC was classified as 1
(potential effect in CSCC treatment), 2 (inconclusive or 3
(evidence not supportive of mTOR inhibitors a drug for CSCC
treatment). A summary of descriptive characteristics of the studies
is given in Table I. All studies were
classified as inconclusive with regard to the effect of mTOR
inhibitors in CSCC (Table I).
Synthesis of results
Study 1
The study by Moroney et al (60) evaluated 74 patients with gynecological
and breast malignancies who were treated with liposomal
doxorubicin, bevacizumab and temsirolimus. This included 13 CC
patients, of whom 10 had CSCC. The study was a non-randomized,
phase 1 clinical trial, for which the primary endpoints were to
establish the MTD and characterize dose-limiting toxicities.
Secondary endpoints included a preliminary assessment of antitumor
efficacy. All 74 patients were heavily pretreated with a median of
4 previous lines of chemotherapy. There were two PRs in the group
of patients with CSCC [treated with dose level 6: Bevacizumab, 15
mg/kg intravenously (IV), day 1; liposomal doxorubicin, 30
mg/m2 IV, day 1; and temsirolimus, 25 mg IV, days 1, 8
and 15]. The MTD for the study was reached at level 6. The RD for
the corresponding phase 2 clinical trial study was as follows:
Bevacizumab, 15 mg/kg, day 1; liposomal doxorubicin, 20–30
mg/m2, day 1; and temsirolimus, 25 mg IV, days 1, 8 and
15. The overall RR in this heavily pretreated population was 20.3%.
Among all 74 patients included, 17.6% had SD lasting ≥6 months. The
TTFs were 112 days [95% confidence interval (CI), 89–147 days]
among all patients, and 172 among patients with PRs. The median
overall survival (OS) time was 214 days (95% CI, 185–312 days).
All 74 patients (100%) experienced ≥1 adverse event
that was at least possibly drug-related. These events were
predominantly grades 1 or 2 and reversible. The treatment
combination was relatively safe and well tolerated. Among the 15
responders (complete response plus PR), PI3K catalytic subunit α
(PIK3CA) and PTEN statuses were known in 9 (60%) and
5 (33.3%), respectively. Of the 9 responders for whom PIK3CA
mutational status was known, 4 (44.4%) were positive. Of the 5
responders for whom PTEN status was known, 3 (60%) were
found to have PTEN loss. The tumor molecular analysis is
listed in Table III. As the
molecular alterations were not reported for each type of cancer
separately, it is not possible to conclude anything regarding
treatment responses and mutations in CSCC.
 | Table III.Tumor molecular analysis of the
included studies. |
Table III.
Tumor molecular analysis of the
included studies.
|
| Moroney et
al (60) | Piha-Paul et
al (61) | Tinker et al
(62) |
|---|
|
|
|
|
|
|---|
| Variable | n (%) | Response
comments | n (%) | Response
comments | n (%) | Response
comments |
|---|
| Total patients
included | 74 | N/A | 41 | N/A | 38 | N/A |
| KRAS
mutation status |
|
|
|
|
|
|
| Number
tested | 49 | N/A | 17 | N/A | 0 | N/A |
| Number
with mutation | 8 (16.3%) | 8 KRAS
mutation-positive patients (100%) achieved a response | 1 (5.9%) | KRAS
mutation-positive patient did not achieve SD ≥6 months/PR | N/A | KRAS
mutation was not tested |
| NRAS
mutation status |
|
| Number
tested | 0 | N/A | 17 | N/A | 0 | N/A |
| Number
with mutation | N/A | NRAS
mutation was not tested | 1 (5.9%) | NRAS
mutation-positive patient did not achieve SD ≥6 months/PR | N/A | NRAS
mutation was not tested |
| PIK3CA
mutation status |
|
| Number
tested | 57 | N/A | 25 | N/A | 33 | N/A |
| Number
with mutation | 16 (28.1%) | 4 PIK3CA
mutation-positive patients (25%) achieved a response | 1 (4.0%) | The PIK3CA
mutation-positive patient achieved a PR | 8
(24.2%)a | The single patient
with PR did not have PIK3CA mutation |
| PTEN
status |
|
| Number
tested | 25 | N/A | 2 | N/A | 33 | N/A |
| Number
with loss | 11 (44.0%) | 5 PTEN
loss-positive patients (45.5%) achieved a response | 0 (0.0%) | No PTEN loss
was detected | 3
(9.1%)a | The single patient
with PR did not have PTEN loss |
Study 2
Piha-Paul et al (61) evaluated 41 patients with advanced
gynecological malignancies who were treated with bevacizumab and
temsirolimus. There were 6 patients with CC included, of whom 4 had
CSCC. This study was a non-randomized, phase 1 clinical trial. The
primary endpoints were to establish the MTD and to characterize
dose-limiting toxicities. Secondary endpoints included a
preliminary assessment of antitumor efficacy. All patients were
heavily pretreated with a median of 4 previous lines of
chemotherapy. Among all patients included, 20% had SD lasting ≥6
months. Analysis of the mutational statuses of PTEN, PIK3CA,
RAS and RAF was not performed for all included patients.
Of the 2 patients who achieved PRs, the mutational status was not
determined in 1, while the other patient was negative for
PIK3CA, RAS and RAF mutations. The 5
responders for whom PTEN status was known were found to have
PTEN loss. Tumor molecular analysis is listed in Table III. As the molecular alterations
were not reported for each type of cancer, conclusions regarding
the association of responses and mutations in CSCC are not
possible.
Grade 1 and 2 toxicities were described in 71% of
the patients. The highest dose escalation was obtained (dose level
13: Bevacizumab, 15 mg/kg IV, day 1; and temsirolimus, 25 mg/kg IV,
days 1, 8 and 15), and the MTD was not reached. All 41 patients
experienced ≥1 adverse event that was possibly drug-related. These
events were predominantly grades 1 or 2 and reversible.
Study 3
Tinker et al (62) evaluated 38 patients with CC, of whom
22 had CSCC. The study was a non-randomized, phase 2 clinical
trial. The primary endpoint was the objective RR, as determined by
Response Evaluation Criteria in Solid Tumors (version 1.1). Up to 1
prior line of chemotherapy for metastatic or recurrent disease was
permitted. Patients were treated with temsirolimus (25 mg IV,
weekly) in 4-week cycles. Only 1 PR occurred, and this patient had
cervical adenocarcinoma. The median duration of SD was 6.5 months
(range, 2.4–12.0 months) and the proportion of patients with SD
lasting ≥6 months was 28% (95% CI, 14–43%). The median PFS time was
3.52 months (95% CI, 1.81–4.7 months). There were 11 serious
adverse events among 7 patients that were possibly related to the
therapy protocol. Original diagnostic material was available for
molecular analysis of 33 patients. No significant association was
found between any of the markers and response to temsirolimus
therapy. The 5 responders for whom PTEN status was known had
PTEN loss. Tumor molecular analysis is presented in Table III.
Risk of bias across studies
The selected studies used similar methods, which
reduced the possibility of misinterpretation. The studies selected
for this analysis were considered to be relatively homogeneous;
however, they did not provide compatible data that would allow a
meta-analysis.
Discussion
The present study reviewed the available evidence
regarding the potential impact of mTOR inhibitors in the treatment
of CSCC. Palliation with platinum-based chemotherapy remains the
standard of care for inoperable patients who have advanced disease
(63). Few advances in medical
management have occurred in recent years in the treatment of
advanced recurrent gynecological malignancies, and a poor prognosis
remains (12). Rationally designed
molecularly targeted therapy is an emerging and important option in
this setting (12).
mTOR is a serine/threonine protein kinase of the
PI3K/AKT signaling pathway, with a critical role in controlling
cancer cell growth, metabolism and cell cycle progression. Aberrant
PI3K-dependent signaling occurs frequently in a wide range of tumor
types, including ovarian, endometrial and cervical cancers
(6,12).
HPV infection of the uterine cervix is linked to the
pathogenesis of CC. Preclinical in vitro and in vivo
studies using HPV-containing human cervical carcinoma cell lines
have demonstrated that rapamycin is able to induce growth delay of
xenografts. Activation of Akt and mTOR in CSCC, and expression of
phosphorylated mTOR have been reported to serve as a markers to
predict response to chemotherapy and survival of CC patients
(8).
Several studies have provided evidence of the
association between the activated PI3K/AKT/mTOR pathway and CC
(8,12,64,65);
however, few studies have analyzed the effect of treatment with
mTOR inhibitors in CC patients (26,33–36,
44, 46,47,
53,60–62).
Research has also been conducted in the field of combined treatment
with mTOR inhibitors, chemotherapy and radiotherapy for locally
advanced CC (66).
The present review identified only two phase 1
(60,61) and one phase 2 (62) clinical trials that met the inclusion
criteria. All studies were non-randomized and included treatment
with temsirolimus as the mTOR inhibitor agent. No control groups
were included. The toxicities were manageable, and the predominant
grade 3 and 4 toxicities included hematological and hepatic side
effects.
The analysis of responses in these three studies was
compromised due to the design of the studies and the lack of
control groups. The two phase 1 studies had RR as secondary
endpoints, and were thus not powered to detect differences in
response. These studies revealed that a number of patients treated
with chemotherapy or bevacizumab in association with mTOR
inhibitors achieved PRs (15.4–33.3% of cases) or SD lasting ≥6
months (17.6–28% of cases). Patients were heavily pretreated in two
studies (60,61), thus it is possible that RRs could be
improved with these agents if used in earlier lines of
treatment.
One serious limitation in determining the activity
of temsirolimus in these two phase 1 studies, aside from the lack
of statistical power to analyze RR, is the lack of a control arm.
In the 22 CSCC patients included in the phase 2 study, there was no
PR following treatment, only SD, and this study also did not
include a control arm (62). The two
phase 1 studies used temsirolimus combined with bevacizumab.
Therefore, it cannot be affirmed whether the benefit obtained was
due to the mTOR inhibitor, the combination of agents, or
bevacizumab alone. In the study by Moroney et al (60), liposomal doxorubicin was also
included, making this analysis further complicated. The other
important limitation is that only the phase 2 trial included >20
CSCC patients, but this study reported no responses to temsirolimus
in CSCC patients (62). The phase 1
studies included ≤10 CSCC patients (60,61). Thus,
it is not possible to conclude the effectiveness of temsirolimus in
the treatment of CSCC based on the phase 1 studies, and the phase 2
study indicates that treatment is inactive.
There is evidence that tumor PIK3CA mutation
status may predict response to PI3K/AKT/mTOR inhibitors (34). Only the study by Tinker et al
(62) could be used to evaluate
molecular alterations and responses, as the studies by Piha-Paul
et al (61) and Moroney et
al (60) did not report the
results of molecular analysis in a separate manner with regard to
the different types of tumor. No established association between
PI3K pathway activating mutations, loss of PTEN and
treatment response could be determined in the study by Tinker et
al (62).
In another study, Moroney et al (67) at the MD Anderson Cancer Center
(University of Texas, Houston, TX, USA) described their experience
of treatment with mTOR inhibitors in a phase 1 clinical trials of
solid tumors (67). Patients with
PIK3CA mutations were treated, whenever possible, with
agents targeting the PI3K/AKT/mTOR pathway (36). In patients with CSCC, the presence of
PIK3CA mutations was associated with a significantly longer
OS time (median, 9.4 months) than the absence of PIK3CA
mutations (median, 4.2 months; P=0.019). Identifying patients who
may, or more importantly may not, benefit from a molecularly
targeted agent is highly desirable. Furthermore, evaluation of
PI3K/AKT/mTOR pathway-targeted therapy is warranted, particularly
in metastatic or recurrent CSCC (33).
The study of genomic and molecular characteristics
of cervical tumors is underway by The Cancer Genome Atlas (TGCA;
http://cancergenome.nih.gov/cancersselected). This
will confirm the genomic and molecular alterations in the disease
and provide rationale for specific targeted therapies. The
evaluation of the PI3K/AKT/mTOR pathway by the TCGA is of great
importance, as new classes of drugs targeted to this pathway are in
the process of development, including PI3K inhibitors.
In summary, the current study is the first
systematic review of the potential impact of mTOR inhibitors on
CSCC treatment. Some serious methodological limitations of this
review should be considered, such as that the studies were
non-randomized, had only one arm, included a limited number of
patients and lacked a control group. All studies were categorized
as having a low or very low quality level of evidence. The
currently available evidence is inconclusive with regard to the
effects of mTOR inhibitors on CSCC. The phase 2 study in women with
recurrent, unresectable, locally advanced or metastatic carcinoma
of the cervix indicated that temsirolimus was inactive in this
population. Investigation of PI3K/AKT/mTOR pathway-targeted
therapies is warranted, and future studies should include
information regarding mutations. Randomized, high-quality clinical
trials are necessary to confirm the efficacy of mTOR inhibitors in
the treatment of CSCC patients.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forman DBF, Brewster DH, Mbalawa C Gombe,
Kohler B, Piñeros M, Steliarova-Foucher E, Swaminathan R and Ferlay
J: GLOBOCAN 2012: Estimated Cancer Incidence. Mortality and
Prevalence Worldwide in 2012. accessed. 6th–March. 2015, Available
at. http://globocan.iarc.fr
|
|
3
|
Nogueira-Rodrigues A, Moralez G,
Grazziotin R, Carmo CC, Small IA, Alves FV, Mamede M, Erlich F,
Viegas C, Triginelli SA and Ferreira CG: Phase 2 trial of erlotinib
combined with cisplatin and radiotherapy in patients with locally
advanced cervical cancer. Cancer. 120:1187–1193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tewari KS, Sill MW, Long HJ III, Penson
RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao
MM, et al: Improved survival with bevacizumab in advanced cervical
cancer. N Engl J Med. 370:734–743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Movva S, Rodriguez L, Arias-Pulido H and
Verschraegen C: Novel chemotherapy approaches for cervical cancer.
Cancer. 115:3166–3180. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Husseinzadeh N and Husseinzadeh HD: mTOR
inhibitors and their clinical application in cervical, endometrial
and ovarian cancers: A critical review. Gynecol Oncol. 133:375–381.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bosch FX, Lorincz A, Muñoz N, Meijer CJ
and Shah KV: The causal relation between human papillomavirus and
cervical cancer. J Clin Pathol. 55:244–265. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng W, Duan X, Liu J, Xiao J and Brown
RE: Morphoproteomic evidence of constitutively activated and
overexpressed mTOR pathway in cervical squamous carcinoma and high
grade squamous intraepithelial lesions. Int J Clin Exp Pathol.
2:249–260. 2009.PubMed/NCBI
|
|
9
|
Advani SH: Targeting mTOR pathway: A new
concept in cancer therapy. Indian J Med Paediatr Oncol. 31:132–136.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Courtney KD, Corcoran RB and Engelman JA:
The PI3K pathway as drug target in human cancer. J Clin Oncol.
28:1075–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
LoPiccolo J, Blumenthal GM, Bernstein WB
and Dennis PA: Targeting the PI3K/Akt/mTOR pathway: Effective
combinations and clinical considerations. Drug Resist Updat.
11:32–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Diaz-Padilla I, Duran I, Clarke BA and Oza
AM: Biologic rationale and clinical activity of mTOR inhibitors in
gynecological cancer. Cancer Treat Rev. 38:767–775. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou H, Luo Y and Huang S: Updates of mTOR
inhibitors. Anticancer Agents Med Chem. 10:571–581. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hutson TE, Escudier B, Esteban E,
Bjarnason GA, Lim HY, Pittman KB, Senico P, Niethammer A, Lu DR,
Hariharan S and Motzer RJ: Randomized phase III trial of
temsirolimus versus sorafenib as second-line therapy after
sunitinib in patients with metastatic renal cell carcinoma. J Clin
Oncol. 32:760–767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Motzer RJ, Escudier B, Oudard S, Hutson
TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA,
Hollaender N, et al: Efficacy of everolimus in advanced renal cell
carcinoma: A double-blind, randomised, placebo-controlled phase III
trial. Lancet. 372:449–456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baselga J, Campone M, Piccart M, Burris HA
III, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun
F, et al: Everolimus in postmenopausal hormone-receptor-positive
advanced breast cancer. N Engl J Med. 366:520–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate health care
interventions: Explanation and Elaboration. Ann Intern Med.
151:W65–W94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
The University of York, . Centre for
Reviews and Dissemination. [cited 02/23/2015]. Available at.
http://www.crd.york.ac.uk/PROSPERO/
|
|
19
|
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist
G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, et al:
GRADE guidelines: 1. Introduction-GRADE evidence profiles and
summary of findings tables. J Clin Epidemiol. 64:383–394. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bae-Jump VL, Zhou C, Gehrig PA, Whang YE
and Boggess JF: Rapamycin inhibits hTERT telomerase mRNA
expression, independent of cell cycle arrest. Gynecol Oncol.
100:487–494. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brana I, Berger R, Golan T, Haluska P,
Edenfield J, Fiorica J, Stephenson J, Martin LP, Westin S, Hanjani
P, et al: A parallel-arm phase I trial of the humanised anti-IGF-1R
antibody dalotuzumab in combination with the AKT inhibitor MK-2206,
the mTOR inhibitor ridaforolimus, or the NOTCH inhibitor MK-0752,
in patients with advanced solid tumours. Br J Cancer.
111:1932–1944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brüning A, Rahmeh M and Friese K:
Nelfinavir and bortezomib inhibit mTOR activity via ATF4-mediated
sestrin-2 regulation. Mol Oncol. 7:1012–1018. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen YJ, Kay N, Yang JM, Lin CT, Chang HL,
Wu YC, Fu CF, Chang Y, Lo S, Hou MF, et al: Total synthetic
protoapigenone WYC02 inhibits cervical cancer cell proliferation
and tumour growth through PIK3 signalling pathway. Basic Clin
Pharmacol Toxicol. 113:8–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chi EY, Viriyapak B, Kwack HS, Lee YK, Kim
SI, Lee KH and Park TC: Regulation of paclitaxel-induced programmed
cell death by autophagic induction: A model for cervical cancer.
Obstet Gynecol Sci. 56:84–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choi Y and Lee JH: The combination of
tephrosin with 2-deoxy-D-glucose enhances the cytotoxicity via
accelerating ATP depletion and blunting autophagy in human cancer
cells. Cancer Biol Ther. 12:989–996. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cohen EE, Sharma MR, Janisch L, Llobrera
M, House L, Wu K, Ramirez J, Fleming GF, Stadler WM and Ratain MJ:
A phase I study of sirolimus and bevacizumab in patients with
advanced malignancies. Eur J Cancer. 47:1484–1489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui F, Li X, Zhu X, Huang L, Huang Y, Mao
C, Yan Q, Zhu J, Zhao W and Shi H: MiR-125b inhibits tumor growth
and promotes apoptosis of cervical cancer cells by targeting
phosphoinositide 3-kinase catalytic subunit delta. Cell Physiol
Biochem. 30:1310–1308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dan S, Okamura M, Mukai Y, Yoshimi H,
Inoue Y, Hanyu A, Sakaue-Sawano A, Imamura T, Miyawaki A and Yamori
T: ZSTK474, a specific phosphatidylinositol 3-kinase inhibitor,
induces G1 arrest of the cell cycle in vivo. Eur J Cancer.
48:936–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Farha AK, Dhanya SR, Mangalam SN, Geetha
BS, Latha PG and Remani P: Deoxyelephantopin impairs growth of
cervical carcinoma SiHa cells and induces apoptosis by targeting
multiple molecular signaling pathways. Cell Biol Toxicol.
30:331–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Faried LS, Faried A, Kanuma T, Nakazato T,
Tamura T, Kuwano H and Minegishi T: Inhibition of the mammalian
target of rapamycin (mTOR) by rapamycin increases chemosensitivity
of CaSki cells to paclitaxel. Eur J Cancer. 42:934–947. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guan TJ, Qin FJ, Du JH, Geng L, Zhang YY
and Li M: AICAR inhibits proliferation and induced S-phase arrest,
and promotes apoptosis in CaSki cells. Acta Pharmacol Sin.
28:1984–1990. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hou LL, Gao C, Chen L, Hu GQ and Xie SQ:
Essential role of autophagy in fucoxanthin-induced cytotoxicity to
human epithelial cervical cancer HeLa cells. Acta Pharmacol Sin.
34:1403–1410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hou MM, Liu X, Wheler J, Naing A, Hong D,
Coleman RL, Tsimberidou A, Janku F, Zinner R, Lu K, Kurzrock R and
Fu S: Targeted PI3K/AKT/mTOR therapy for metastatic carcinomas of
the cervix: A phase I clinical experience. Oncotarget.
5:11168–11179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Janku F, Tsimberidou AM, Garrido-Laguna I,
Wang X, Luthra R, Hong DS, Naing A, Falchook GS, Moroney JW,
Piha-Paul SA, et al: PIK3CA mutations in patients with advanced
cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol Cancer
Ther. 10:558–565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Janku F, Wheler JJ, Naing A, Falchook GS,
Hong DS, Stepanek VM, Fu S, Piha-Paul SA, Lee JJ, Luthra R, et al:
PIK3CA mutation H1047R is associated with response to PI3K/AKT/mTOR
signaling pathway inhibitors in early phase clinical trials. Cancer
Res. 73:276–284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Janku F, Wheler JJ, Naing A, Stepanek VM,
Falchook GS, Fu S, Garrido-Laguna I, Tsimberidou AM, Piha-Paul SA,
Moulder SL, et al: PIK3CA mutations in advanced cancers:
Characteristics and outcomes. Oncotarget. 3:1566–1575. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ji J and Zheng P-S: Activation of mTOR
signaling pathway contributes to survival of cervical cancer cells.
Gynecol Oncol. 117:103–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kang S, Dong SM, Kim BR, Park MS, Trink B,
Byun HJ and Rho SB: Thioridazine induces apoptosis by targeting the
PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells.
Apoptosis. 17:989–997. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim J, Park M, Ryu BJ and Kim SH: The
protein kinase 2 inhibitor CX-4945 induces autophagy in human
cancer cell lines. Bulletin of the Korean Chemical Society.
35:2985–2989. 2014. View Article : Google Scholar
|
|
40
|
Kwan HT, Chan DW, Cai PC, Mak CS, Yung MM,
Leung TH, Wong OG, Cheung AN and Ngan HY: AMPK activators suppress
cervical cancer cell growth through inhibition of DVL3 mediated
Wnt/β-catenin signaling activity. PloS One. 8:e535972013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li J, Ping Z and Ning H: MiR-218 impairs
tumor growth and increases chemo-sensitivity to cisplatin in
cervical cancer. Int J Mol Sci. 13:16053–16064. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li SR, Li Y, Hu R, Li W, Qiu H, Cai H and
Wang S: The mTOR inhibitor AZD8055 inhibits proliferation and
glycolysis in cervical cancer cells. Oncol Lett. 5:717–721.
2013.PubMed/NCBI
|
|
43
|
Ma J, Zi Jiang Y, Shi H, Mi C, Li J, Nan J
Xing, Wu X, Lee J Joon and Jin X: Cucurbitacin B inhibits the
translational expression of hypoxia-inducible factor-1α. Eur J
Pharmacol. 723:46–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Martin-Liberal J, Gil-Martín M,
Sáinz-Jaspeado M, Gonzalo N, Rigo R, Colom H, Muñoz C, Tirado OM
and García del Muro X: Phase I study and preclinical efficacy
evaluation of the mTOR inhibitor sirolimus plus gemcitabine in
patients with advanced solid tumours. Br J Cancer. 111:858–865.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Molinolo AA, Marsh C, El Dinali M, Gangane
N, Jennison K, Hewitt S, Patel V, Seiwert TY and Gutkind JS: mTOR
as a molecular target in HPV-associated oral and cervical squamous
carcinomas. Clin Cancer Res. 18:2558–2568. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
O'Donnell A, Faivre S, Burris HA III, Rea
D, Papadimitrakopoulou V, Shand N, Lane HA, Hazell K, Zoellner U,
Kovarik JM, et al: Phase I pharmacokinetic and pharmacodynamic
study of the oral mammalian target of rapamycin inhibitor
everolimus in patients with advanced solid tumors. J Clin Oncol.
26:1588–1595. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Papadopoulos KP, Tabernero J, Markman B,
Patnaik A, Tolcher AW, Baselga J, Shi W, Egile C, Ruiz-Soto R,
Laird AD, et al: Phase I safety, pharmacokinetic, and
pharmacodynamic study of SAR245409 (XL765), a novel, orally
administered PI3K/mTOR inhibitor in patients with advanced solid
tumors. Clin Cancer Res. 20:2445–2456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rashmi R, DeSelm C, Helms C, Bowcock A,
Rogers BE, Rader J, Grigsby PW and Schwarz JK: AKT inhibitors
promote cell death in cervical cancer through disruption of mTOR
signaling and glucose uptake. PloS One. 9:e929482014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Reddy GL, Guru SK, Srinivas M, Pathania
AS, Mahajan P, Nargotra A, Bhushan S, Vishwakarma RA and Sawant SD:
Synthesis of 5-substituted-1H-pyrazolo[4,3-d]pyrimidin-7(6H)-one
analogs and their biological evaluation as anticancer agents: mTOR
inhibitors. Eur J Med Chem. 80:201–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Roy B, Pattanaik AK, Das J, Bhutia SK,
Behera B, Singh P and Maiti TK: Role of PI3K/Akt/mTOR and MEK/ERK
pathway in Concanavalin A induced autophagy in HeLa cells. Chem
Biol Interact. 210:96–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sahin K, Tuzcu M, Basak N, Caglayan B,
Kilic U, Sahin F and Kucuk O: Sensitization of cervical cancer
cells to cisplatin by genistein: The role of NFκB and Akt/mTOR
signaling pathways. J Oncol. 2012:4615622012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shin JM, Jeong YJ, Cho HJ, Park KK, Chung
IK, Lee IK, Kwak JY, Chang HW, Kim CH, Moon SK, et al: Melittin
suppresses HIF-1α/VEGF expression through inhibition of ERK and
mTOR/p70S6K pathway in human cervical carcinoma cells. PloS One.
8:e693802013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Temkin SM, Yamada SD and Fleming GF: A
phase I study of weekly temsirolimus and topotecan in the treatment
of advanced and/or recurrent gynecologic malignancies. Gynecol
Oncol. 117:473–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wan G, Xie W, Liu Z, Xu W, Lao Y, Huang N,
Cui K, Liao M, He J, Jiang Y, et al: Hypoxia-induced MIR155 is a
potent autophagy inducer by targeting multiple players in the MTOR
pathway. Autophagy. 10:70–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang L, Chang L, Li Z, Gao Q, Cai D, Tian
Y, Zeng L and Li M: miR-99a and −99b inhibit cervical cancer cell
proliferation and invasion by targeting mTOR signaling pathway. Med
Oncol. 31:9342014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xiao X, He Q, Lu C, Werle KD, Zhao RX,
Chen J, Davis BC, Cui R, Liang J and Xu ZX: Metformin impairs the
growth of liver kinase B1-intact cervical cancer cells. Gynecol
Oncol. 127:249–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xu QJ, Hou LL, Hu GQ and Xie SQ: Molecular
mechanism of ophiopogonin B induced cellular autophagy of human
cervical cancer HeLa cells. Yao Xue Xue Bao. 48:855–859. 2013.(In
Chinese). PubMed/NCBI
|
|
58
|
Yu SY, Chan DW, Liu VW and Ngan HY:
Inhibition of cervical cancer cell growth through activation of
upstream kinases of AMP-activated protein kinase. Tumor Biol.
30:80–85. 2009. View Article : Google Scholar
|
|
59
|
Zhang C, Yang N, Yang CH, Ding HS, Luo C,
Zhang Y, Wu MJ, Zhang XW, Shen X, Jiang HL, et al: S9, a novel
anticancer agent, exerts its anti-proliferative activity by
interfering with both PI3K-Akt-mTOR signaling and microtubule
cytoskeleton. PloS One. 4:e48812009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Moroney JW, Schlumbrecht MP, Helgason T,
Coleman RL, Moulder S, Naing A, Bodurka DC, Janku F, Hong DS and
Kurzrock R: A Phase I trial of liposomal doxorubicin, bevacizumab,
and temsirolimus in patients with advanced gynecologic and breast
malignancies. Clin Cancer Res. 17:6840–6846. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Piha-Paul SA, Wheler JJ, Fu S, Levenback
C, Lu K, Falchook GS, Naing A, Hong DS, Tsimberidou AM and Kurzrock
R: Advanced gynecologic malignancies treated with a combination of
the VEGF inhibitor bevacizumab and the mTOR inhibitor temsirolimus.
Oncotarget. 5:1846–1855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tinker AV, Ellard S, Welch S, Moens F,
Allo G, Tsao MS, Squire J, Tu D, Eisenhauer EA and MacKay H: Phase
II study of temsirolimus (CCI-779) in women with recurrent,
unresectable, locally advanced or metastatic carcinoma of the
cervix. A trial of the NCIC clinical trials group (NCIC CTG IND
199). Gynecol Oncol. 130:269–274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Serrano-Olvera A, Cetina L, Coronel J and
Dueñas-González A: Emerging drugs for the treatment of cervical
cancer. Expert Opin Emerg Drugs. 20:165–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chan S: Targeting the mammalian target of
rapamycin (mTOR): A new approach to treating cancer. Br J Cancer.
91:1420–1424. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Molinolo AA, Marsh C, El Dinali ME,
Gangane N, Jennison K, Hewitt S, Patel V, Seiwert TY and Gutkind
JS: mTOR as a molecular target in HPV-associated oral and cervical
squamous carcinomas. Clin Cancer Res. 18:2558–2568. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ferreira CG, Alves FVG, Grazziotin R,
Erlich F, Moralez G, Carneiro MP, et al: Abstract CT403: A Phase I
study of oral administration of mTOR inhibitor everolimus (E) in
association with cisplatin (C) and radiotherapy (R) for the
treatment of locally advanced cervix cancer (LACC)-PHOENIX I.
Cancer Res. 74(Suppl 19): CT4032014. View Article : Google Scholar
|
|
67
|
Moroney J, Wheler J, Hong D, Naing A,
Falchook G, Bodurka D, Coleman R, Lu K, Xiao L and Kurzrock R:
Phase I clinical trials in 85 patients with gynecologic cancer: The
M. D. Anderson Cancer Center experience. Gynecol Oncol.
117:467–472. 2010. View Article : Google Scholar : PubMed/NCBI
|