Introduction
Bladder cancer is the most common malignancy of the
urinary tract, ranking the first in incidence in China and the
fourth in Western countries (1). At
initial diagnosis, ~95% of bladder cancers are determined to be
transitional cell carcinoma (TCC), which results in significant
morbidity and mortality (2). Among
them, ~70% are superficial (3), and
>50% of these cancers recur after surgery, with 15–20% of the
recurrences progressing to a more invasive form (4). This high rate of disease recurrence
requires lifelong surveillance. Currently, the most standard
diagnostic approaches for identification and monitoring of
recurrence and progression of bladder cancer are cystoscopy
(5) and urine cytology (6). However, the application of these
approaches is limited in routine clinical practice due to the
invasive nature, expensive cost and inconvenience of cystoscopy,
and the low sensitivity of urine cytology (7). Therefore, there is a requirement for
alternative approaches and therapeutic targets for better
management of bladder TCC patients.
Therapeutic approaches to bladder cancer are often
limited to radiation therapy and chemotherapy, with poor overall
clinical outcome (8). Recently,
immunotherapy has emerged as a promising treatment modality due to
its low side effects and high specificity, which results in an
improved quality of life for patients (9). Cancer testis (CT) antigens are named
after their pattern of expression, as they are abundantly detected
in various types of cancers, including melanoma, lung cancer,
bladder cancer, liver cancer and breast cancer (10), but generally not in normal tissue,
with the exception of the testis (11–13).
Furthermore, CT antigens are not expected to induce autoimmune
damage to any normal tissues due to the testes being
immune-privileged (14). Due to these
unique expression patterns, CT antigens have been the focus of
attention as potential targets in immunotherapy for cancer
(15).
Sperm acrosome associated 5 (SPACA5), also named
lysozyme-like (LYZL) 5, encodes a putative protein of 159 amino
acids that has been mapped to p11.23 of the X chromosome (16). SPACA5 is a sperm acrosome-associated,
lysozyme-like protein that was first identified by searching for
transcript clusters that map to multiple locations on the
chromosome, followed by in silico analysis of its gene
expression profile (16). Of the ~100
CT genes or gene families identified thus far, ~30 belong to
multigene families on the X chromosome (17). To date, a number of CT antigens,
including melanoma-associated antigen (MAGE)-A1 (18), MAGE-A3 (19,20),
MAGE-A9 (4), MAGE-A12 (18), cutaneous T-cell-lymphoma-associated
antigen (cTAGE-1) (18), cTAGE-2
(18), cancer/testis antigen 2
(21) and New York esophageal
squamous cell carcinoma 1 (NY-ESO-1) (21–23), have
been confirmed to express a prolific and specific profile in TCC,
providing a possibility of early detection, antigen-specific
immunotherapy and polyvalent vaccination. In a previous study,
SPACA5 messenger RNA (mRNA) expression was detected mainly
in the testes and was designated as an attractive candidate CT
antigen (16). Following a Basic
Local Alignment Search Tool analysis, it was determined that mouse
Spaca5 is 81% identical to human SPACA5. However, to
the best of our knowledge, little is known regarding the properties
and function of Spaca5 in mouse testes, and SPACA5
has not been studied in TCC in the literature. The current study is
therefore designed to investigate the temporal and spacial
expression of SPACA5/Spaca5 in mouse and human testes
in order to explore the possible correlation between the expression
of the SPACA5 antigen and the clinicopathological
characteristics of patients with TCC, and to evaluate whether
SPACA5 could be applied as a specific immunotherapy target
in these patients.
Materials and methods
Ethics statements
Human tissues were collected between 2010 and 2013
under the approval of the Ethics Committee of Shenzhen Second
People's Hospital (Shenzhen, China), and informed consent forms
were signed by the patients. The present study was approved by the
Institutional Review Board of Shenzhen Second People's
Hospital.
Animals and patients samples
A total of 30 female and 10 male C57BL/6J mice
(18–22 g, 6-weeks-old) were acquired from the Laboratory Animal
Center of Southern Medical University (Guangzhou, China) and were
maintained in a humidity and temperature-controlled room. All
animals had free access to standard water and mouse chow. Female
and male mice (3:1) were allowed to mate naturally, and the day of
birth was termed day 1. Testes from C57BL/6J mice were collected on
days 9 (n=10), 14 (n=8), 18 (n=5), 20 (n=5), 22 (n=5), 25 (n=3), 28
(n=3), 30 (n=3) and 35 (n=3), as well as at 6 weeks (n=2). Other
tissues containing brain, heart, liver, lung, spleen, skeletal
muscle, esophagus, stomach, small intestine, bladder, epididymis,
kidney and pancreas were also collected from adult mice (n=2).
The TCC specimens and adjacent normal bladder
tissues were retrospectively recruited from 65 TCC patients (aged
35–80 years) at the Clinical Data and Specimen Bank of Biological
Resources of Major Diseases in Shenzhen (Shenzhen, China). All
patients provided informed consent to participate in the
experiments prior to using their tissues. None of the TCC cases
have received any therapy, including chemotherapy, radiotherapy or
other treatment, prior to surgery. Pathologic grading (G1-G4) and
staging (Tis, Ta and T1-T4) were
determined according to the 2004 World Health Organization bladder
tumor classification criteria (24)
by two experienced pathologists (with >10 years of clinical
experience) under optical microscopes. In addition, total RNA from
human normal tissues, including brain, heart, spleen, lung, thymus,
skeletal muscle, stomach, small intestine, testis, bladder, kidney,
liver, placenta, thyroid gland, prostate, ovary and colon, was
purchased from Takara Biotechnology Co., Ltd. (Dalian, China). Upon
surgical resection, all specimens were initially stored in
RNAlater® (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and immediately frozen at −80°C in liquid nitrogen until
further analysis.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA from the tissue samples was extracted with
the RNeasy Plus Mini kit (Qiagen GmbH, Hilden, UK). RT-PCR was
conducted to analyze and confirm the expression of the
SPACA5/Spaca5 genes. Total RNA (2 µg) was reverse transcribed into
complementary DNA in a total volume of 10 µl with MuLV Reverse
Transcriptase (Thermo Fisher Scientific, Inc.). Forward and reverse
oligonucleotide primers specific to SPACA5/Spaca5,
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and
Gapdh were designed using Primer Express 3.0 software
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and were
synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The
primer sequences for each gene analyzed were summarized in Table I. The genes GAPDH and
Gapdh were used as the internal controls.
 | Table I.Primers for reverse
transcription-polymerase chain reaction analysis. |
Table I.
Primers for reverse
transcription-polymerase chain reaction analysis.
| Genes | Primer sequence
(5′-3′) | Annealing
temperature (°C) | Product size
(bp) |
|---|
| SPACA5 | F:
GGCAGCAGTGAATATGGCATT | 60 | 188 |
|
| R:
CTCCAAGAAGTCCAGGCAGAA | 60 |
|
| GAPDH | F:
AGAAGGCTGGGGCTCATTTG | 60 | 258 |
|
| R:
AGGGGCCATCCACAGTCTTC | 60 |
|
| Spaca5 | F:
GCAGCATTGTGGTAGTGATCTT | 60 | 308 |
|
| R:
GCGGTTGAGCAGGTCATTAC | 60 |
|
| Gapdh | F:
CCGGGGCTGGCATTGCTCTC | 60 | 150 |
|
| R:
GTCCTTGCTGGGGTGGGTGGTC | 60 |
|
The following PCR conditions were used: 95°C for 3
min; 40 cycles of 95°C for 30 sec, 65°C for 30 sec and 72°C for 30
sec; and 72°C for 5 min. The PCR products were subjected to
electrophoresis by 1.5% agarose gels and monitored under
ultraviolet light. Each sample was analyzed in triplicate. Relative
levels of SPACA5/Spaca5 mRNA expression were
normalized to GAPDH/Gapdh mRNA expression.
RT-quantitative (q) PCR
RT-qPCR analysis was performed with a
SYBR-Green Chemistry kit (Qiagen GmbH) on an ABI PRISM 7500
Sequence Detection System (Applied Biosystems; Thermo Fisher
Scientific, Inc.), with each sample analyzed in triplicate. The
following qPCR conditions were used: 95°C for 3 min; 40 cycles of
95°C for 30 sec, 65°C for 30 sec and 72°C for 30 sec; and 72°C for
5 min, followed by a typical dissociation stage. GAPDH was
used as a reference gene in parallel (Table I). The results were presented as the
levels of expression following normalization to GAPDH using
the 2−ΔΔCq method (25).
Immunohistochemistry
Sections of 5-µm thickness were sectioned from the
selected paraffin blocks, mounted on silane-coated slides and dried
at 37°C overnight. The sections were deparaffinized using xylene,
rehydrated through descending grades of alcohol to distilled water
and incubated in 3% H2O2 solution for 10 min
to quench endogenous peroxidase activity. Subsequently, the slides
were heated for 10 min in a microwave oven for antigen retrieval
and washed three times in phosphate-buffered saline (PBS). Upon
nonspecific binding blocking with 5% normal goat serum (Fuzhou
Maixin Biotech Co., Ltd., Fuzhou, China) for 20 min at room
temperature, sections were incubated with a primary rabbit
polyclonal anti-SPACA5/Spaca5 antibody (dilution 1:1,000; cat. no.
ab111474; Abcam, Cambridge, UK) overnight at 4°C. Following three
washes in PBS, the sections were incubated for 1 h at 37°C with
biotin-conjugated goat anti-rabbit secondary antibody (dilution
1:5,000; cat. no. KIT-9710; Fuzhou Maixin Biotech Co., Ltd.). After
three washes in PBS, the sections were incubated at room
temperature for 10 min with streptavidin-anti-biotin peroxidase.
Following three washes in PBS, the sections were incubated at room
temperature for 5 min with 3,3′-diaminobenzidine, which generated a
brown color at the site of peroxidase activity. Sections were then
washed (3×5 min) in deionized water, and the nuclei were
counterstained with hematoxylin. Finally, the sections were
dehydrated and mounted. In the negative control slides, the primary
antibody was replaced by normal immunoglobulin G (IgG) (dilution
1:500; cat. no. ab172730; Abcam). The results were observed under a
microscope (Leica Microsystems GmbH, Wetzlar, Germany).
Statistical analysis
All statistical tests were performed with SPSS
version 13.0 software (SPSS, Inc., Chicago, IL, USA). The
correlations between SPACA5 expression and clinical parameters,
including gender, tumor stage, tumor grade and staining score, were
evaluated by Pearson's χ2 test. All analysis were
two-tailed, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Tissue distribution of SPACA5/Spaca5
mRNA in humans and mice
The tissue expression pattern of Spaca5 was
evaluated by RT-PCR in 14 different mouse tissues, including brain,
heart, liver, lung, spleen, skeletal muscle, esophagus, stomach,
small intestine, bladder, testis, epididymis, kidney and pancreas.
The gene was specifically expressed in testis and not in the other
normal tissues (Fig. 1A). Having
certificated the unique distribution of Spaca5 in mouse
normal tissues, the expression pattern of SPACA5 was
subsequently investigated in different human tissues to decide
whether SPACA5 possesses CT antigen expression
characteristics. RT-PCR consisting of 40 amplification cycles was
performed on total RNA isolated from 17 human normal tissues.
SPACA5 mRNA was not expressed in any of the normal tissues
with the exception of testes (Fig.
1B), which parallels the results of multi-tissue RT-PCR in
mice. Thus, the expression pattern of SPACA5/Spaca5,
similar to that of other CT antigens, is restricted to testis
(11–13).
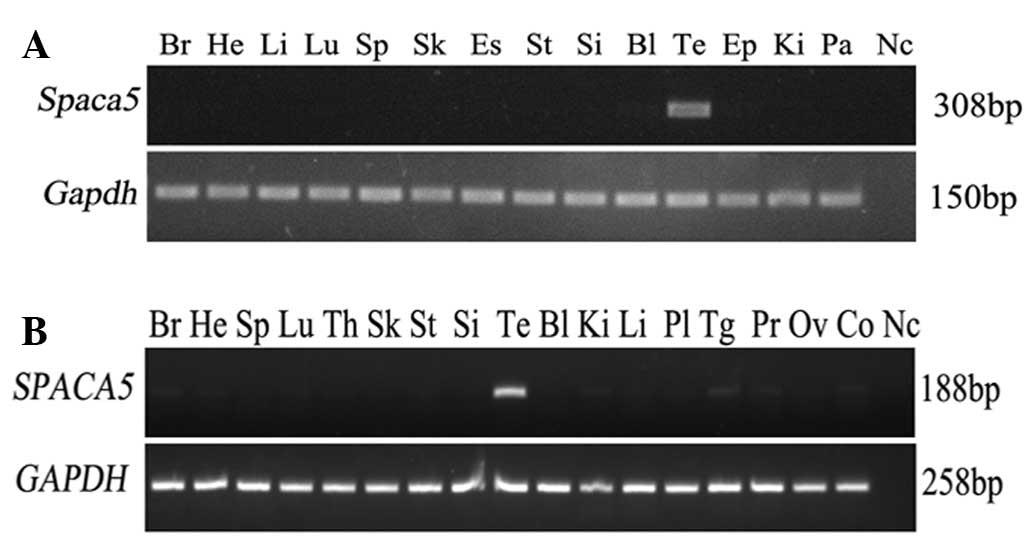 | Figure 1.Tissue distribution of
SPACA5/Spaca5 mRNA in humans and mice. (A)
Distribution of Spaca5 mRNA in 14 diverse mouse tissues.
Expression of Spaca5 was detected only in the testis.
Gapdh gene expression was used as an internal control. (B)
Expression pattern of SPACA5 mRNA in 17 different human
tissues. The gene was not detected in any of the normal tissues
with the exception of the testis. GAPDH gene expression was
used as an internal control. Br, brain; He, heart; Li, liver; Lu,
lung; Sp, spleen; Sk, skeletal; Es, esophagus; St, stomach; Si,
small intestine; Bl, bladder; Te, testis; Ep, epididymis; Ki,
kidney; Pa, pancreas; Th, thymus; Pl, placenta; Tg, thyroid gland;
Pr, prostate; Ov, ovary; Co, colon; Nc, negative control;
Spaca5, sperm acrosome associated 5; Gapdh,
glyceraldehyde 3-phosphate dehydrogenase; mRNA, messenger RNA. |
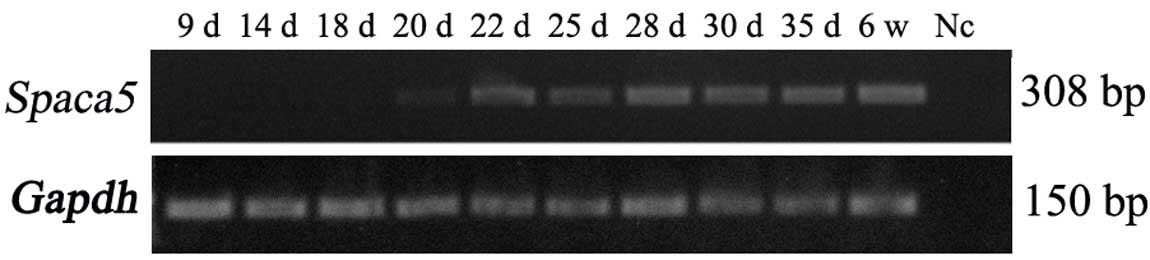
Temporal expression analysis of Spaca5
during testis development
To further examine the temporal expression of
Spaca5, a time-course study was performed during testis
development. Our results revealed that Spaca5 mRNA was not
detected on day 9 through day 18 in mice testes, but a low
expression level of Spaca5 could be detected on day 20, when
late pachytene spermatocytes appeared (26). Its expression increased gradually from
day 20 to day 28, when round spermatids developed (Fig. 2). The current study indicated that the
expression profile of Spaca5 is developmental-stage
specific.
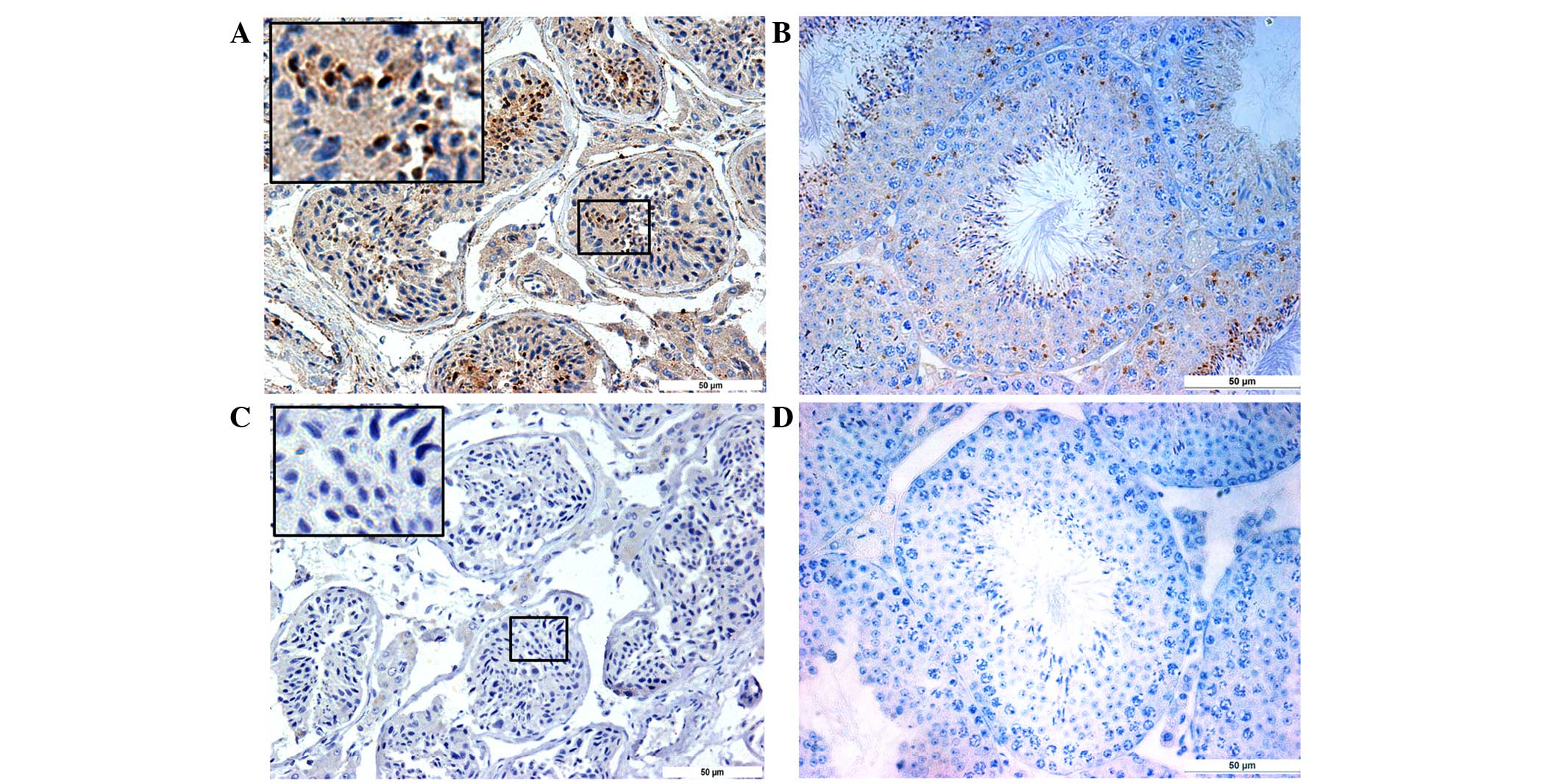
Localization of SPACA5/Spaca5 protein
in normal human and mouse testes by immunostaining
In the normal testes, all stages of spermatogenic
cells were present in the seminiferous epithelia. As shown in
Fig. 3A and B, SPACA5/Spaca5 protein
was mainly located in elongated spermatids, with no background
signal in Leydig cells or basal membranes. No staining was observed
in the tissue sections where the anti-SPACA5/Spaca5 antibody was
replaced by normal IgG (Fig. 3C and
D).
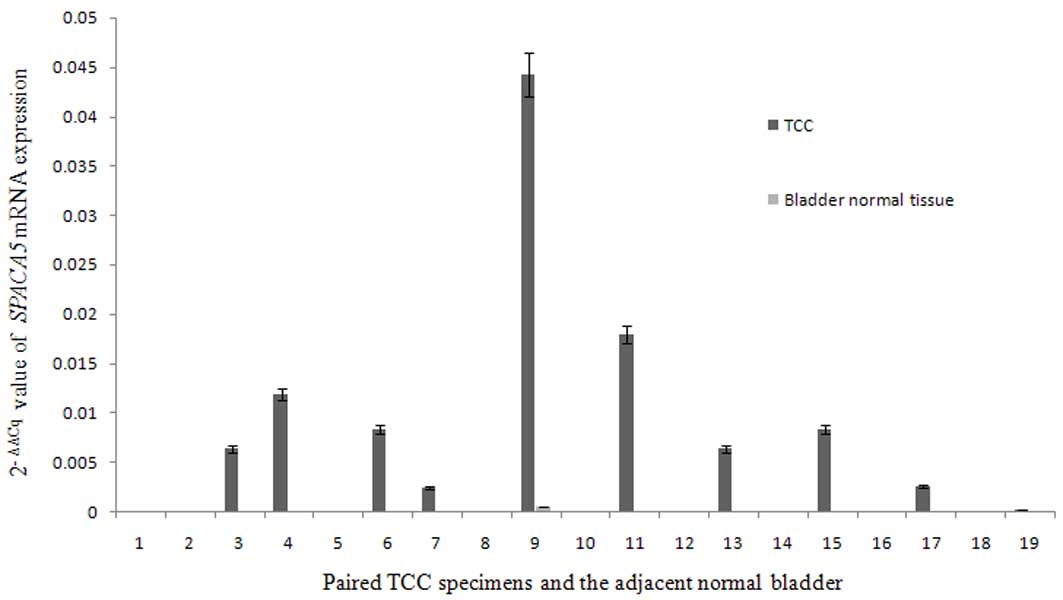
RT-qPCR analysis of SPACA5 transcripts
in TCC
RT-qPCR was used to determine whether low copy
numbers of SPACA5 transcripts could be detected in certain
normal tissues, and to compare the levels of transcript expression
in tumor cells with those of normal tissues. Analysis of 20 paired
TCC specimens and adjacent normal bladder tissues revealed that
SPACA5 was ectopically expressed in TCC tissues, while it
was not expressed in normal bladder tissues (Fig. 4). The frequency of SPACA5
expression in TCC was 45% (9/20). Together with the findings of
SPACA5 expression in normal tissues, which revealed that
SPACA5 expression is restricted to the testis, our results
indicate that SPACA5 is a novel CT antigen in TCC.
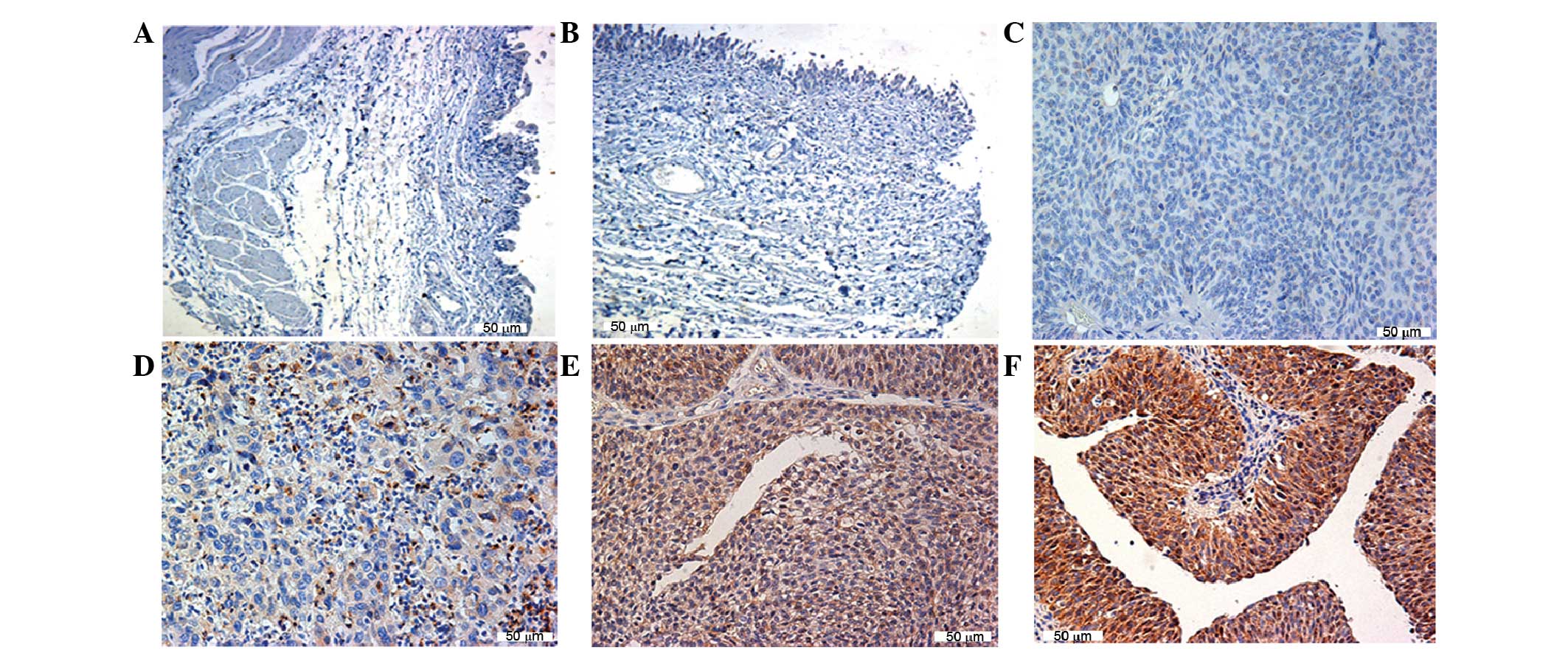
SPACA5 protein expression in TCC is
confirmed by immunohistochemistry
To examine whether SPACA5 mRNA was translated
into protein in TCC cells, paraffin sections were prepared.
Specimens from a group (n=65) of TCC patients and normal bladder
tissue were investigated for SPACA5 protein expression by
immunohistochemical analysis. SPACA5 protein was specifically
detected in TCC cells but not in normal bladder tissue. Positive
cytoplasmic staining in TCC cells was detectable, with weak to
strong intensity (Fig. 5). SPACA5
protein was detected in 25 of 65 (38.46%) tumor specimens (Table II).
 | Table II.Correlation between SPACA5 protein
expression in TCC and clinicopathological variables. |
Table II.
Correlation between SPACA5 protein
expression in TCC and clinicopathological variables.
|
Characteristics | Patients, n
(%) | SPACA5
protein+, n (%) | P-value |
|---|
| All cases | 65
(100.00) | 25 (38.46) |
|
| Gender |
|
|
|
|
Male | 51 (78.46) | 21 (41.18) | 0.390 |
|
Female | 14 (21.54) | 4
(28.57) |
| Tumor grade |
|
|
|
| Low
(G1+G2) | 50 (76.92) | 15 (30.00) | 0.035 |
| High
(G3+G4) | 13 (20.00) | 8
(61.54) |
|
|
Unknown | 2 (3.08) | 2
(100.00) |
|
| Tumor stage |
|
|
|
| NMIBC
(Tis, Ta, T1) | 19 (29.23) | 10 (52.63) | 0.121 |
| MIBC
(T2, T3, T4) | 41 (63.08) | 13 (31.71) |
|
Unknown | 5 (7.69) | 2
(40.00) |
Correlation between SPACA5 protein
expression and clinicopathological variables in TCC
Patients with primary bladder cancer stage are
categorized into two groups with different performance
characteristics: Non-muscle-invasive bladder cancer (NMIBC) or
muscle-invasive bladder cancer (MIBC) (27). At initial diagnosis, NMIBC (which
consist of stages Ta, Tis and T1) accounts
for 75–80% of bladder cancer patients, whereas the remaining 20–25%
of primary tumors are already MIBC (which consists of stages
T2, T3 and T4)or metastatic at the
time of initial presentation (21).
TCC comprises nearly 95% of all primary malignant tumors of the
bladder, showing a broad biological spectrum ranging from
superficial to invasive tumors (28).
Tumor grade is based on cellular dysplasia and architectural
abnormalities in tumor tissue, and is usually employed to
categorize TCC in terms of malignant potential (29). High-grade (G3) TCC progresses to
muscle invasion more frequently than low-grade (G1 and G2) tumors
(30). Although G1 and G2 tumors
often recur, they are less likely to become invasive (30).
In the present study, the frequency of SPACA5
protein expression in TCC tumors was 38.46% (25/65). SPACA5
expression was not significantly associated with gender (males vs.
females, P=0.390) or tumor stage (NMIBC vs. MIBC, P=0.121), but the
frequency of SPACA5 expression was significantly higher in
high-grade (G3+G4) than in low-grade (G1+G2) (61.54 vs. 30.00%,
P=0.035) tumors (Table II).
The intensity of staining was semiquantitatively
evaluated (score 0, 1, 2 and 3), and the association with
clinicopathological features was assessed (Table III): Staining detectable in <10%
of tumor cells was defined as score 1; staining detectable in
10–50% of tumor cells was defined as score 2; and staining
detectable in >50% of tumor cells was defined as score 3. Score
0 was attributed to negative samples. The high SPACA5 staining
scores were observed to be significantly associated with high tumor
grade (n=65, R=0.279, P=0.027) (Table
III).
 | Table III.Sample classification based on
anti-SPACA5 staining score. |
Table III.
Sample classification based on
anti-SPACA5 staining score.
| Score | Positive tumor
cells (%) | Grade | Number of cases
(%) |
|---|
| 0–3 | 0–100 | Total | 65 (100.0) |
|
|
| Low | 50 (76.9) |
|
|
| High | 13 (20.0) |
|
|
| Undefined | 2 (3.1) |
| 0 | 0 | Total | 40/65 (61.5) |
|
|
| Low | 35/50 (70.0) |
|
|
| High | 5/13 (38.5) |
| 1 | <10 | Total | 11/65 (16.9) |
|
|
| Low | 7/50 (14.0) |
|
|
| High | 3/13 (23.1) |
|
|
| Undefined | 1/2 (50.0) |
| 2 | 10–50 | Total | 9/65 (13.8) |
|
|
| Low | 6/50 (12.0) |
|
|
| High | 3/13 (23.1) |
| 3 | >50 | Total | 5/65 (7.7) |
|
|
| Low | 2/50 (4.0) |
|
|
| High | 2/13 (15.4) |
|
|
| Undefined | 1/2 (50.0) |
Discussion
C-type lysozyme is expressed in the majority of
species, and due to its ability to act on microbial membranes, it
is considered to be important in innate immune defense (31). In the human male reproductive system,
four c-type lysozyme genes (LYZL2, LYZL3/SPACA3,
LYZL4 and LYZL6) have been identified, which are highly
expressed in the testes or epididymis (32,33). Mouse
Lyzl3/Spaca3 and Lyzl4 can block sperm-egg binding or
fusion in the hamster oocyte penetration assay, which indicates
their possible role in fertilization (34,35).
SPACA3 is a novel CT antigen in hematological malignancies,
and is immunogenic in cancer-bearing patients in vivo and a
target for tumor immunotherapy (36).
Among the SPACA family, only SPACA5 and SPACA3 have strong amino
acid homology, containing conserved c-lysozyme-like domains
(37). SPACA5 is also a c-type
lysozyme gene named LYZL5; therefore, we presume that
SPACA5/Spaca5 participates in male spermatogenesis
and is a CT antigen in TCC. However, the expression pattern of
SPACA5/Spaca5 and its association with
clinicopathological characteristics with TCC are not clear. In the
present study, we report a unique expression pattern of
SPACA5/Spaca5 in humans and mice, and determine
whether SPACA5 is a potential CT antigen in TCC.
Spermatogenesis is a complicated biological event
that includes the mitotic proliferation of spermatogonia, the
meiotic division of spermatocytes and the morphogenic
differentiation of spermatids to mature spermatozoa (38). In previous studies, it has been
reported that different CT antigens are involved in different
stages of spermatogenesis. Certain CT antigens are expressed
exclusively in one stage, including synaptonemal complex protein 1,
which is expressed in the synapsed regions of meiotic prophase
spermatocytes only, and sperm protein 17, which is restricted to
the mature spermatozoa (39,40). Other CT antigens such as NY-ESO-1 and
preferentially expressed antigen in melanoma-like 1 (PRAMEL1) are
expressed predominantly in several stages of spermatogenesis.
NY-ESO-1 is strongly expressed in spermatogonia and in primary
spermatocytes (41), whereas PRAMEL1
is expressed in spermatocytes through elongated spermatids
(42). In the present study, RT-PCR
was performed to detect the expression profile of Spaca5 in
different developmental stages of mouse testis. It has been
reported that, in C57BL/6J mice, primitive type A spermatogonia
occurs at postnatal days 4–5; type A and type B spermatogonia occur
at day 9; preleptotene and leptotene spermatocytes appear at day
14; zygotene spermatocytes are detected at day 18; pachytene
spermatocytes are present at day 20; round spermatids production
and elongated spermatid formation occur at day 28–35; and normal
postpubertal spermatogenesis occurs at week 6 (26). Similar to the PRAMEL1 gene, we
confirmed that Spaca5 is broadly expressed in different
types of germ cells during spermatogenesis. Spaca5 mRNA was
expressed at a weak level in mouse testes on day 20, and its
expression was increased after day 20 and remained stably at a high
level after day 28. According to the aforementioned developmental
stages of mouse testis, we propose that Spaca5 may
participate in the generation of round and elongated
spermatids.
Having demonstrated the expression of Spaca5
in mouse spermatogenesis, SPACA5/Spaca5 expression
characteristics were then investigated in different tissues to
determine whether SPACA5 possesses CT antigen expression
characteristics. RT-PCR consisting of 40 amplification cycles was
performed on total RNA obtained from 17 and 14 normal tissues in
humans and mice, respectively. The results of multi-tissue RT-PCR
confirmed that, similar to other CT antigens, the expression of
SPACA5/Spaca5 is restricted to the testis, which
provides evidence supporting the testis-specific expression of
SPACA5/Spaca5, and makes it a potentially useful
target for tumor-specific immunotherapy.
To examine whether SPACA5 mRNA was also
present in TCC cells, 20 paired bladder cancer specimens and
adjacent normal bladder tissues were investigated by RT-qPCR.
SPACA5 mRNA was expressed in 45.00% of bladder TCC
specimens, but not in the adjacent normal tissues. This predominant
SPACA5 gene expression in bladder TCC patients was similar
to other known potential CT antigens. For example, the mRNA
expression of the well-characterized CT antigen NY-ESO-1 was
detected in 45.10% of bladder TCC specimens (18), and MAGE-A10 expression was detected in
32.43% of bladder carcinomas (43).
The specificity of SPACA5 protein expression in
normal and TCC tissues was then validated using an
immunohistochemical assay. The results revealed that SPACA5 protein
is expressed in TCC tissues, but not in normal bladder tissue.
Specimens from a group (n=65) of TCC tissues, of which 13 were
high-grade tumors, 50 were low-grade tumors and 2 were unknown. The
present study is the first report to determine that SPACA5 protein
is exclusively present in the cytoplasm of TCC cells, and to
demonstrate an association between SPACA5 expression and tumor
grade, since 15 of 50 (30.00%) grade 1 and grade 2 (G1+G2), and 8
of 13 (61.54%) grade 3 and grade 4 (G3+G4) tumors were
SPACA5-protein positive. In addition, specific staining was
observed more frequently in high-grade compared with low-grade
tumors (61.54 vs. 30.00%, P=0.035). In previous studies on
urothelial carcinoma of the bladder, Sharma et al documented
that NY-ESO-1 was highly expressed in high-grade carcinoma, forming
the basis of a vaccine clinical trial in which the NY-ESO-1 protein
was used as an adjuvant treatment following complete resection of
urothelial carcinoma (22).
Consequently, the significant correlation between specific staining
and high tumor grade of TCC suggests that SPACA5 protein expression
may be a characteristic of aggressive TCC, and may pave the way for
successful immunotherapy with high efficiency. However, 52.63% of
superficial tumors expressed SPACA5 protein, while 31.71% of
invasive tumors expressed SPACA5 protein. Since this protein was
not observed in normal bladder tissue, these results make
SPACA5 a highly relevant target for superficial bladder,
which suggests its potential role in early bladder tumorigenesis.
By contrast, no significant association between SPACA5 expression
and tumor stage was observed (P=0.121). In addition, no gender
differences in the expression of SPACA5 in TCC were observed.
Together with the findings of SPACA5/Spaca5
testicular-specific expression, our results suggest that
SPACA5 is a potential CT antigen in TCC.
In conclusion, the present study provided the first
evidence that SPACA5/Spaca5 is important in
spermatogenesis and also possesses several features of CT antigens.
In addition, SPACA5 expression is more abundant in high-grade TCC
than low-grade TCC, which indicates its aggressive role in
tumorigenesis. Thus, SPACA5 could be a potential target for
specific immunotherapy in patients suffering from TCC. However,
large prospective studies are required to confirm our preliminary
findings.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (Beijing, China; grant nos.
81170613 and 81270740), the Medical Research Foundation of
Guangdong Province (Guangzhou, China; grant no. B2014426) and the
Shenzhen Science and Technology Project (grant nos. JCYJ
20140416180323426 and JSGG 20160301162913683). The authors would
like to thank Elsevier Language Editing Services (Oxford, UK) for
editing the present manuscript.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patton SE, Hall MC and Ozen H: Bladder
cancer. Curr Opin Oncol. 14:265–272. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amling CL: Diagnosis and management of
superficial bladder cancer. Curr Probl Cancer. 25:219–278. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Picard V, Bergeron A, Larue H and Fradet
Y: MAGE-A9 mRNA and protein expression in bladder cancer. Int J
Cancer. 120:2170–2177. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitropoulos D, Kiroudi-Voulgari A,
Nikolopoulos P, Manousakas T and Zervas A: Accuracy of cystoscopy
in predicting histologic features of bladder lesions. J Endourol.
19:861–864. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karakiewicz PI, Benayoun S, Zippe C,
Lüdecke G, Boman H, Sanchez-Carbayo M, Casella R, Mian C, Friedrich
MG, Eissa S, et al: Institutional variability in the accuracy of
urinary cytology for predicting recurrence of transitional cell
carcinoma of the bladder. BJU Int. 97:997–1001. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanojia D, Garg M, Saini S, Agarwal S,
Parashar D, Jagadish N, Seth A, Bhatnagar A, Gupta A, Kumar R, et
al: Sperm associated antigen 9 plays an important role in bladder
transitional cell carcinoma. PLoS One. 8:e813482013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dyrskjot L, Zieger K, Lildal T Kissow,
Reinert T, Gruselle O, Coche T, Borre M and Ørntoft TF: Expression
of MAGE-A3, NY-ESO-1, LAGE-1 and PRAME in urothelial carcinoma. Br
J Cancer. 107:116–122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agarwal S, Saini S, Parashar D, Verma A,
Sinha A, Jagadish N, Batra A, Suri S, Gupta A, Ansari AS, et al:
The novel cancer-testis antigen A-kinase anchor protein 4 (AKAP4)
is a potential target for immunotherapy of ovarian serous
carcinoma. Oncoimmunology. 2:e242702013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Simpson AJ, Caballero OL, Jungbluth A,
Chen YT and Old LJ: Cancer/testis antigens, gametogenesis and
cancer. Nat Rev Cancer. 5:615–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zendman AJ, Ruiter DJ and Van Muijen GN:
Cancer/testis-associated genes: Identification, expression profile
and putative function. J Cell Physiol. 194:272–288. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scanlan MJ, Gure AO, Jungbluth AA, Old LJ
and Chen YT: Cancer/testis antigens: An expanding family of targets
for cancer immunotherapy. Immunol Rev. 188:22–32. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scanlan MJ, Simpson AJ and Old LJ: The
cancer/testis genes: Review, standardization, and commentary.
Cancer Immun. 4:12004.PubMed/NCBI
|
|
14
|
Suri A, Saini S, Sinha A, Agarwal S, Verma
A, Parashar D, Singh S, Gupta N and Jagadish N: Cancer testis
antigens: A new paradigm for cancer therapy. Oncoimmunology.
1:1194–1196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghafouri-Fard S and Modarressi MH:
Cancer-testis antigens: Potential targets for cancer immunotherapy.
Arch Iran Med. 12:395–404. 2009.PubMed/NCBI
|
|
16
|
Chen YT, Iseli C, Venditti CA, Old LJ,
Simpson AJ and Jongeneel CV: Identification of a new cancer/testis
gene family, CT47, among expressed multicopy genes on the human X
chromosome. Genes Chromosomes Cancer. 45:392–400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Caballero OL and Chen YT: Cancer/testis
(CT) antigens: Potential targets for immunotherapy. Cancer Sci.
100:2014–2021. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin B, Liu G, Wang XS, Zhang H, Song YS
and Wu B: Expression profile of cancer-testis genes in transitional
cell carcinoma of the bladder. Urol Oncol. 30:886–892. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patard JJ, Brasseur F, Gil-Diez S,
Radvanyi F, Marchand M, François P, Abi-Aad A, Van Cangh P, Abbou
CC, Chopin D, et al: Expression of MAGE genes in transitional-cell
carcinomas of the urinary bladder. Int J Cancer. 64:60–64. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishiyama T, Tachibana M, Horiguchi Y,
Nakamura K, Ikeda Y, Takesako K and Murai M: Immunotherapy of
bladder cancer using autologous dendritic cells pulsed with human
lymphocyte antigen-A24-specific MAGE-3 peptide. Clin Cancer Res.
7:23–31. 2001.PubMed/NCBI
|
|
21
|
Sharma P, Gnjatic S, Jungbluth AA,
Williamson B, Herr H, Stockert E, Dalbagni G, Donat SM, Reuter VE,
Santiago D, et al: Frequency of NY-ESO-1 and LAGE-1 expression in
bladder cancer and evidence of a new NY-ESO-1 T-cell epitope in a
patient with bladder cancer. Cancer Immun. 3:192003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sharma P, Shen Y, Wen S, Bajorin DF,
Reuter VE, Old LJ and Jungbluth AA: Cancer-testis antigens:
Expression and correlation with survival in human urothelial
carcinoma. Clin Cancer Res. 12:5442–5447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kurashige T, Noguchi Y, Saika T, Ono T,
Nagata Y, Jungbluth A, Ritter G, Chen YT, Stockert E, Tsushima T,
et al: Ny-ESO-1 expression and immunogenicity associated with
transitional cell carcinoma: Correlation with tumor grade. Cancer
Res. 61:4671–4674. 2001.PubMed/NCBI
|
|
24
|
Montironi R and Lopez-Beltran A: The 2004
WHO Classification of bladder tumors: A summary and commentary. Int
J Surg Pathol. 13:143–153. 2004. View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eddy EM: Male germ cell gene expression.
Recent Prog Horm Res. 57:103–128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reuter VE: Pathology of bladder cancer:
Assessment of prognostic variables and response to therapy. Semin
Oncol. 17:524–532. 1990.PubMed/NCBI
|
|
28
|
Bane BL, Rao JY and Hemstreet GP:
Pathology and staging of bladder cancer. Semin Oncol. 23:546–570.
1996.PubMed/NCBI
|
|
29
|
Epstein JI, Amin MB, Reuter VR and Mostofi
FK: The world health organization/international society of
urological pathology consensus classification of urothelial
(transitional cell) neoplasms of the urinary bladder. Bladder
consensus conference committee. Am J Surg Pathol. 22:1435–1448.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Spruck CH III, Ohneseit PF,
Gonzalez-Zulueta M, Esrig D, Miyao N, Tsai YC, Lerner SP, Schmütte
C, Yang AS, Cote R, et al: Two molecular pathways to transitional
cell carcinoma of the bladder. Cancer Res. 54:784–788.
1994.PubMed/NCBI
|
|
31
|
Wei J, Li SJ, Shi H, Wang HY, Rong CT, Zhu
P, Jin SH, Liu J and Li JY: Characterisation of Lyzls in mice and
antibacterial properties of human LYZL6. Asian J Androl.
15:824–830. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang K, Gao R, Zhang H, Cai X, Shen C, Wu
C, Zhao S and Yu L: Molecular cloning and characterization of three
novel lysozyme-like genes, predominantly expressed in the male
reproductive system of humans, belonging to the c-type
lysozyme/alpha-lactalbumin family. Biol Reprod. 73:1064–1071. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mandal A, Klotz KL, Shetty J, Jayes FL,
Wolkowicz MJ, Bolling LC, Coonrod SA, Black MB, Diekman AB,
Haystead TA, et al: SLLP1, a unique, intra-acrosomal,
non-bacteriolytic, c lysozyme-like protein of human spermatozoa.
Biol Reprod. 68:1525–1537. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun R, Shen R, Li J, Xu G, Chi J, Li L,
Ren J, Wang Z and Fei J: Lyzl4, a novel mouse sperm-related
protein, is involved in fertilization. Acta Biochim Biophys Sin
(Shanghai). 43:346–353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Herrero MB, Mandal A, Digilio LC, Coonrod
SA, Maier B and Herr JC: Mouse SLLP1, a sperm lysozyme-like protein
involved in sperm-egg binding and fertilization. Dev Biol.
284:126–142. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Z, Zhang Y, Mandal A, Zhang J, Giles
FJ, Herr JC and Lim SH: The spermatozoa protein, SLLP1, is a novel
cancer-testis antigen in hematologic malignancies. Clin Cancer Res.
10:6544–6550. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Korfanty J, Toma A, Wojtas A, Rusin A,
Vydra N and Widlak W: Identification of a new mouse sperm
acrosome-associated protein. Reproduction. 143:749–757. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Grootegoed JA, Siep M and Baarends WM:
Molecular and cellular mechanisms in spermatogenesis. Baillieres
Best Pract Res Clin Endocrinol Metab. 14:331–343. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Meuwissen RL, Offenberg HH, Dietrich AJ,
Riesewijk A, van Iersel M and Heyting C: A coiled-coil related
protein specific for synapsed regions of meiotic prophase
chromosomes. Embo J. 11:5091–5100. 1992.PubMed/NCBI
|
|
40
|
Kong M, Richardson RT, Widgren EE and
O'Rand MG: Sequence and localization of the mouse sperm
autoantigenic protein, Sp17. Biol Reprod. 53:579–590. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Satie AP, Rajpert-De Meyts E, Spagnoli GC,
Henno S, Olivo L, Jacobsen GK, Rioux-Leclercq N, Jégou B and Samson
M: The cancer-testis gene, NY-ESO-1, is expressed in normal fetal
and adult testes and in spermatocytic seminomas and testicular
carcinoma in situ. Lab Invest. 82:775–780. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mistry BV, Zhao Y, Chang TC, Yasue H,
Chiba M, Oatley J, Diaz F and Liu WS: Differential expression of
PRAMEL1, a cancer/testis antigen, during spermatogenesis in the
mouse. PLoS One. 8:e606112013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mengus C, Schultz-Thater E, Coulot J,
Kastelan Z, Goluza E, Coric M, Spagnoli GC and Hudolin T: MAGE-A10
cancer/testis antigen is highly expressed in high-grade
non-muscle-invasive bladder carcinomas. Int J Cancer.
132:2459–2463. 2013. View Article : Google Scholar : PubMed/NCBI
|