Introduction
Osteosarcoma (OS) is the most common mesenchymal
sarcoma in bone, mainly arising from the metaphysis of the long
bones (1). Over the past few decades,
the prognosis of advanced OS has remained poor, mainly due to its
resistance to radiotherapy (2).
Cisplatin [also known as diamminedichloridoplatinum (II) (DDP)] is
commonly used for the clinical treatment of OS (3,4). However,
treatment with DDP may induce tumor chemotherapy resistance
(5). Although the underlying
molecular mechanism has not been fully uncovered yet, it has been
demonstrated that the activation of autophagy plays a key role in
DDP-induced chemotherapy resistance (6).
Autophagy is an evolutionarily conserved
self-catabolic degradation process, which is responsible for the
lysosomal degradation of long-lived proteins and aged or damaged
organelles (7). Autophagy can
generate amino acids and fatty acids, which can be reused for cell
growth and proliferation (7).
Therefore, autophagy is important for sustainable cell survival
(8). Recently, accumulating evidences
have demonstrated that autophagy is involved in DDP-induced
chemotherapy resistance in multiple types of human cancer (9,10).
Additionally, inhibition of autophagy could increase the
chemotherapeutic sensitivity to DDP of OS (11).
MicroRNAs (miRNAs or miRs) are a class of 18–25
nucleotides-long non-coding RNAs that generally lead to messenger
RNA (mRNA) degradation or inhibition of translation via directly
binding to 3′-untranslated regions (UTRs) of the mRNA of their
target genes (12). Through
negatively mediation of their targets, miRs have been implicated in
numerous cellular processes, including cell survival,
proliferation, differentiation, apoptosis and autophagy (13). miR-199a-5p has been observed to be
deregulated and important in a variety of human cancers, including
gastric cancer, non-small cell lung cancer, melanoma and colorectal
cancer (14–18). In addition, miR-199a-5p also has an
inhibitory effect on autophagy (19,20). Xu
et al noticed that miR-199a-5p could suppress DDP-induced
autophagy and chemotherapy resistance in hepatocellular carcinoma
cells (20). Yi et al reported
that overexpression of miR-199a-5p inhibited irradiation-induced
autophagy and sensitized breast cancer cells to irradiation
(19). However, the exact role of
miR-199a-5p in OS, particularly in DDP-induced chemotherapy
resistance, has not been previously studied.
The present study aimed to investigate the role of
miR-199a-5p in DDP-induced chemoresistance in OS cells, as well as
the underlying mechanism.
Materials and methods
Cell culture
The human OS MG63 cell line was obtained from the
Cell Bank of Central South University (Changsha, China) and
cultured in Dulbecco's modified Eagle medium (DMEM; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin (Thermo Fisher Scientific, Inc.) and 100 mg/ml
streptomycin (Thermo Fisher Scientific, Inc.) at 37°C in 5%
CO2.
Cell treatment
MG63 cells were treated with DDP (10 mM;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) for 6 h, and
the following assays were conducted.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
RT-qPCR assay was used to determine the miR
expression. Total RNA was extracted using TRIzol reagent (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
All-in-One™ miRNA Reverse Transcription kit (GeneCopoeia, Inc.,
Rockville, MD, USA) was used to convert RNA into cDNA according to
the manufacturer's protocol. qPCR was then performed using mirVana™
qRT-PCR miRNA Detection kit (Thermo Fisher Scientific, Inc.) on an
ABI 7500 Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The PCR primers were provided by Yearthbio
(Changsa, China). The conditions were 95°C for 10 min, and 40
cycles of denaturation at 95°C for 15 sec and annealing/elongation
at 60°C for 60 sec. U6 was used as an internal reference. The
relative expression was analyzed by the 2−ΔΔCq method
(21).
Transfection
MG63 cells were cultured to 70% confluence and
resuspended in serum-free DMEM. Serum-free DMEM was also used to
dilute Lipofectamine 2000 (Thermo Fisher Scientific, Inc.),
miR-199a-5p mimic, miR-199a-5p inhibitor and scramble miR mimic
[which served as miR-negative control (NC)]. The diluted
Lipofectamine 2000 was then added to the above diluted
oligonucleotides, which were purchased from Guangzhou RiboBio Co.,
Ltd. (Guangzhou, China), and then incubated at room temperature for
20 min prior to addition of the mixture to the cell suspension.
Upon incubation at 37°C in the presence of 5% CO2 for 6
h, the medium was replaced by DMEM supplemented with 10% FBS. After
transfection for 48 h, the following assays were performed.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
MG63 cells (100,000) were plated in a 96-well plate
and incubated for 48 h at 37°C with 5% CO2. MTT (5
mg/ml; Thermo Fisher Scientific, Inc.) was added to each well and
incubated for 4 h at 37°C with 5% CO2. The supernatant
was removed, and 100 µl dimethylsulfoxide (Thermo Fisher
Scientific, Inc.) was added to dissolve the precipitate. The
absorbance was detected at 492 nm using the ELX-800™ Absorbance
Microplate Reader (BioTek Instruments, Inc., Winooski, VT,
USA).
Western blotting
MG63 cells were solubilized in cold
radioimmunoprecipitation assay lysis buffer (Thermo Fisher
Scientific, Inc.) to extract the proteins, which were separated by
10% sodium dodecyl sulfate polyacrylamide gel electrophoresis
(Pierce Biotechnology, Inc., Rockford, USA), and transferred onto a
polyvinylidene difluoride (PVDF) membrane (Pierce Biotechnology,
Inc.). The PVDF membrane was incubated with rabbit anti-Beclin1
monoclonal antibody (1:50; ab55878; Abcam, Cambridge, MA, USA),
rabbit anti-light chain 3 (LC3)-II polyclonal antibody (1:50;
ab48394; Abcam), rabbit anti-LC3-I polyclonal primary antibody
(1:50; ab62721; Abcam) and rabbit anti-GAPDH polyclonal primary
antibody (1:50; ab9484; Abcam) at 4°C overnight. Upon being washed
three times with PBS containing Tween 20, the PVDF membrane was
then incubated with mouse anti-rabbit secondary antibody (1:20,000;
ab99702; Abcam) at room temperature for 40 min. Chemiluminescent
detection was conducted using the ECL Western Blotting Substrate
kit (Pierce Biotechnology, Inc.). Protein expression was analyzed
by Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville,
MD, USA), and represented as the density ratio vs. GAPDH.
Dual luciferase reporter assay
Bioinformatics analysis was conducted to predict the
putative target genes of miR-199a-5p using TargetScan 3.1 online
software (http://www.targetscan.org/). The
predicted seed sequence of miR-199a-5p in the Beclin1 3′-UTR as
well as the mutant seed sequence in the Beclin1 3′-UTR were cloned
downstream of the luciferase gene in the pGL3 Luciferase Reporter
Vector (Promega Corporation, Madison, WI, USA), generating vectors
containing wild type Beclin1 3′-UTR (Luc-Beclin1 vector) or mutant
Beclin1 3′-UTR (Luc-mutant Beclin1 vector). MG63 cells were
co-transfected with miR-199a-5p mimics and Luc-Beclin1 or
Luc-mutant Beclin1 vector. Luciferase activity was determined after
transfection for 24 h using the ELX-800™ Absorbance Microplate
Reader.
Statistical analysis
All data were represented as the mean of at least
triplicate samples ± standard deviation. Statistical analysis of
differences was performed by one-way analysis of variance using
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
DDP treatment leads to decreased
expression of miR-199a-5p and activation of autophagy in OS MG63
cells
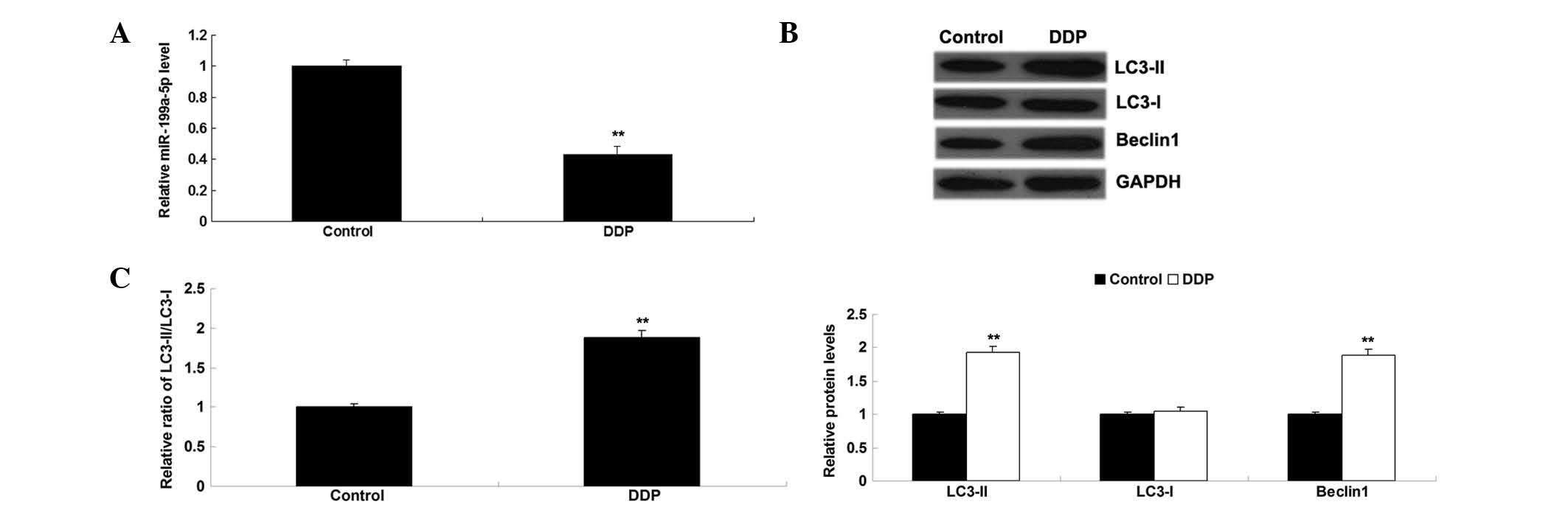
MG63 cells were treated with DDP (30 µg/ml). After
treatment for 6 h, the expression level of miR-199a-5p was
significantly reduced in MG63 cells treated with DDP, compared with
the control group (Fig. 1A). Next,
the autophagy-related protein levels were determined in MG63 cells
treated with DDP. Western blotting revealed that treatment with DDP
upregulated the protein levels of LC3-II and Beclin1 as well as the
ratio of LC3-II vs. LC3-I in MG63 cells (Fig. 1B and C).
Enforced expression of miR-199a-5p
suppresses DDP-induced autophagy in MG63 cells
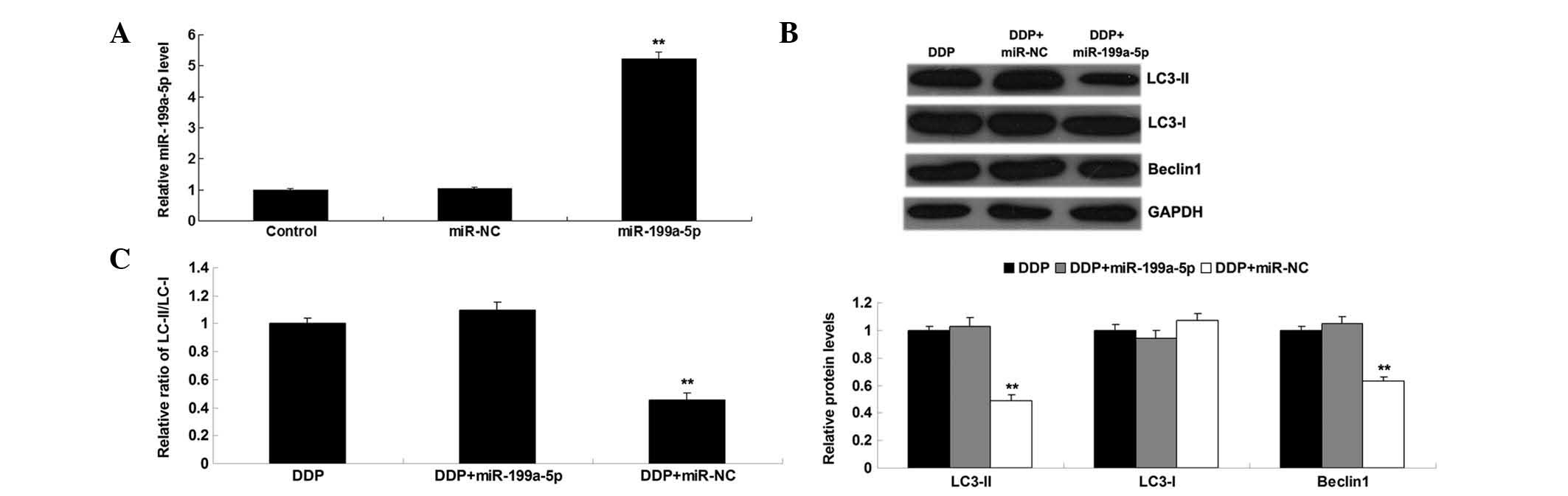
The role of miR-199a-5p in DDP-induced autophagy in
OS cells was further studied. MG63 cells were transfected with
miR-199a-5p mimic or miR-NC prior to DDP treatment. Upon
transfection, RT-qPCR was conducted to determine the miR-199-5p
levels in MG63 cells, and it was observed that transfection with
miR-199a-5p mimic led to a significant increase in miR-199a-5p
levels, when compared with the control group (Fig. 2A). Next, it was further studied
whether enforced expression of miR-199a-5p could suppress
DDP-induced autophagy in MG63 cells. MG63 cells were treated with
DDP for 6 h, and the levels of autophagy-related proteins were
examined by western blot assay. As shown in Fig. 2B and C, the protein levels of LC3-II
and Beclin1 as well as the ratio of LC3-II vs. LC3-I were lower in
DDP-treated MG63 cells overexpressing miR-199a-5p compared with
those in the control group. These findings indicate that enforced
miR-199a-5p expression suppresses DDP-induced autophagy in OS MG63
cells.
Enforced expression of miR-199a-5p
enhances the inhibitory effect of DDP on MG63 cell
proliferation
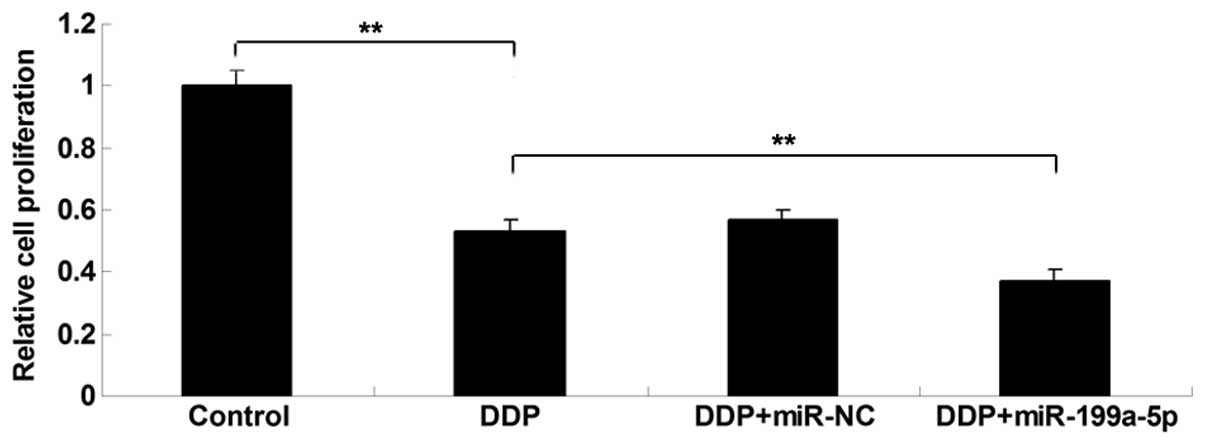
Next, the role of miR-199a-5p in mediating the
proliferation of MG63 cells treated with DDP was investigated. MTT
assay revealed that enforced expression of miR-199a-5p
significantly suppressed the proliferation of DDP-treated MG63
cells compared with the control group, indicating that miR-199a-5p
enhances the inhibitory effect of DDP on MG63 cell proliferation
(Fig. 3).
Beclin1 is a direct target of
miR-199a-5p
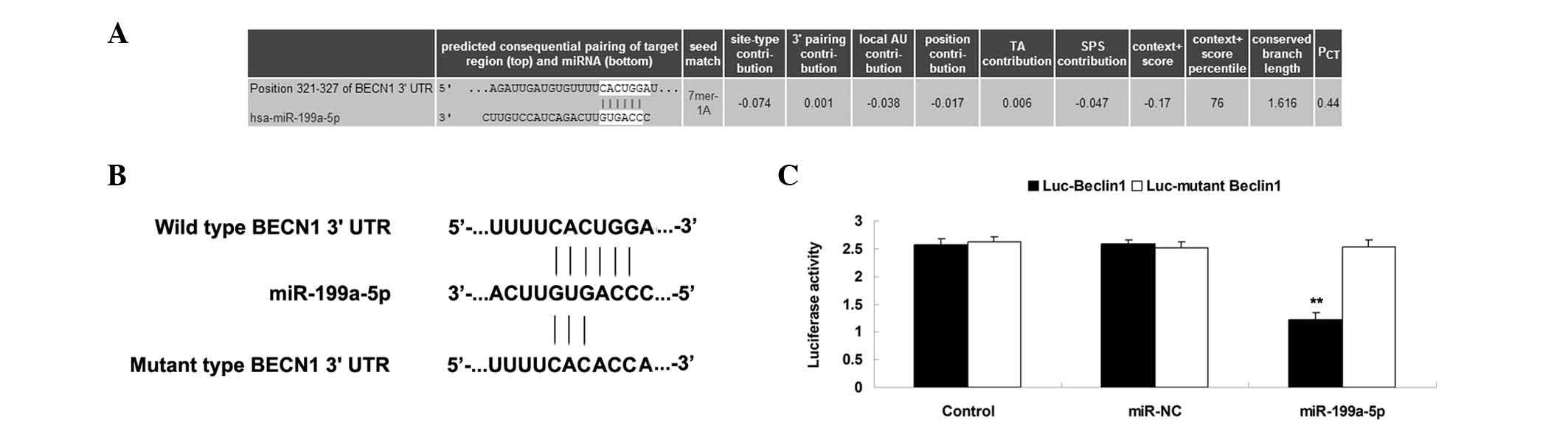
Bioinformatics prediction was further conducted to
analyze the putative target genes of miR-199a-5p. As shown in
Fig. 4A, Beclin1 was a putative
target of miR-199a-5p. Next, the predicted seed sequence of
miR-199a-5p in the Beclin1 3′-UTR as well as a mutant seed sequence
in the Beclin1 3′-UTR were cloned downstream of the luciferase gene
driven by the cytomegalovirus promoter, generating Luc-Beclin1
vector or Luc-mutant Beclin1 vector, respectively (Fig. 4B). MG63 cells were co-transfected with
miR-199a-5p mimics and Luc-Beclin1 or Luc-mutant Beclin1 vector.
Luciferase reporter assay demonstrated that the luciferase activity
was significantly reduced in MG63 cells co-transfected with
Luc-Beclin1 vector and miR-199a-5p mimics, compared with the
control group. However, the luciferase activity exhibited no
difference in MG63 cells co-transfected with Luc-mutant Beclin1
vector and miR-199a-5p mimics, when compared with that in the
control group (Fig. 4C). These data
indicate that miR-199a-5p can directly bind to the seed sequences
in the Beclin1 3′-UTR in MG63 cells. Therefore, Beclin1 was
identified as a target of miR-199a-5p in MG63 cells.
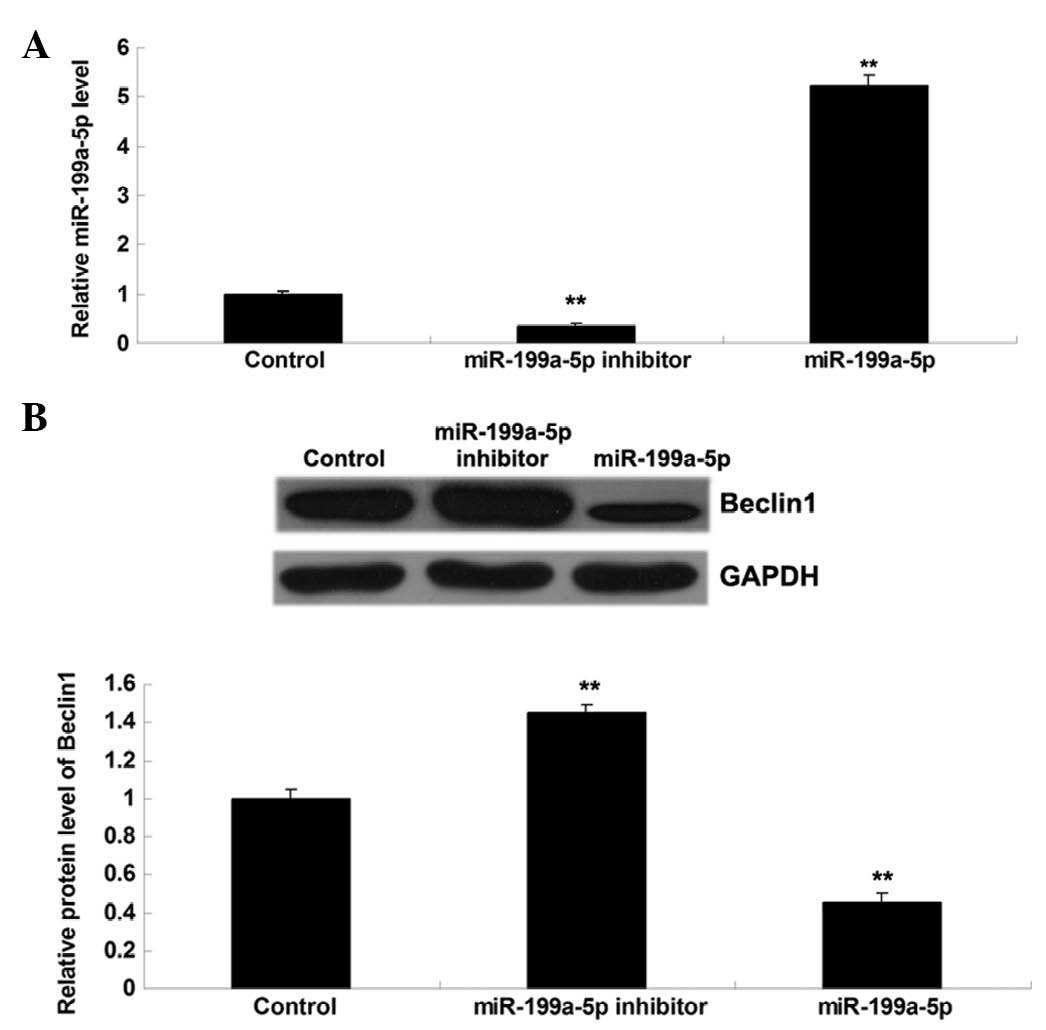
miR-199a-5p negatively mediates the
protein expression of Beclin1 in MG63 cells
The effect of miR-199a-5p on the expression of
Beclin1 in MG63 cells was further investigated. MG63 cells were
transfected with miR-199a-5p mimic or inhibitor. Upon transfection,
RT-qPCR was performed to determine the miR-199a-5p level. As shown
in Fig. 5A, transfection with
miR-199a-5p mimic enhanced the miR-199a-5p level, while
transfection with miR-199a-5p inhibitor decreased the miR-199a-5p
level, compared with the control group. Subsequently, western blot
assay was conducted to examine the Beclin1 protein level. As shown
in Fig. 5B, overexpression of
miR-199a-5p suppressed the Beclin1 protein level, while knockdown
of miR-199a-5p enhanced the Beclin1 protein level, indicating that
miR-199a-5p negatively mediates the protein expression of Beclin1
in MG63 cells.
Discussion
miRs have been identified to play key roles in the
regulation of tumorigenesis and chemotherapy resistance via
directly inhibition of their targets expression (22). In the present study, it was observed
that treatment with DDP not only led to a significant decrease in
miR-199a-5p levels but also induced an activation of autophagy in
OS MG63 cells. Enforced expression of miR-199a-5p inhibited
DDP-induced autophagy and enhanced DDP-induced inhibition of MG63
cell proliferation. In addition, it was demonstrated that Beclin1,
a key autophagy inducer, was a direct target of miR-199a-5p, which
suggested that the suppressive effect of miR-199a-5p on DDP-induced
autophagy in MG63 cells may occur through the mediation of Beclin1
expression.
Autophagy is an evolutionarily conserved function,
responsible for the reuse of degraded components to sustain
metabolic homeostasis and the prevention of the toxic accumulation
of damaged components (23).
Recently, aberrant activation of autophagy has been detected in
various cancers under administration of chemotherapy drugs such as
DDP, and accumulating evidences have demonstrated that chemotherapy
drug-induced autophagy often causes chemotherapy resistance of
tumor cells (24). In addition,
inhibition of chemotherapy-induced autophagy has been observed to
be beneficial for promoting the efficiency of chemotherapy drug
treatment in human cancers, including OS (25). DDP is one of the most commonly used
chemotherapy drugs for the treatment of OS (25). In the present study, it was observed
that treatment with DDP induced the activation of autophagy in OS
MG63 cells by upregulating the protein levels of Beclin1 and
LC3-II. Notably, DDP treatment also led to a significant decrease
in the expression levels of miR-199a-5p, which has also been
implicated in autophagy in breast cancer and hepatocellular
carcinoma (19,20).
Deregulation of miR-199a-5p has been implicated in
multiple types of human cancer (14,17,18). For
instance, miR-199a-5p was observed to be downregulated in
triple-negative breast cancer, and its levels in plasma were
notably restored upon surgical resection, suggesting that it may be
involved in the development of breast cancer (17). Hu et al reported that
miR-199a-5p was frequently downregulated in colorectal cancer,
which led to the upregulation of discoidin domain receptor 1 and
the activation of epithelial-to-mesenchymal transition-related
signaling (16). In addition, the
expression level of miR-199a-5p was significantly increased in
gastric cancer tissues compared with paired normal tissues, and
higher miR-199a-5p level was associated with increased lymph node
metastasis and higher tumor-node-metastasis stage, suggesting that
miR-199a-5p may act as an oncogene in gastric cancer (26). Recently, miR-199a-5p has been reported
to exhibit inhibitory effects on autophagy (27). For instance, Lee et al observed
that protoporphyrin IX, a photocatalyzer, could increase the
expression of miR-199a-5p, which further inhibited E2F
transcription factor 3 and sensitized mesenchymal tumor cells to
chemotherapy drugs (28). In the
present study, it was observed that miR-199a-5p inhibited
DDP-induced autophagy. Xu et al reported similar findings in
hepatocellular carcinoma, where DDP treatment caused downregulation
of miR-199a-5p and increased drug resistance by activating
autophagy (20).
Finally, the present study demonstrated that
Beclin1, a key inducer of autophagy (29), was a direct target of miR-199a-5p, and
that the protein expression of Beclin1 was negatively regulated by
miR-199a-5p in OS cells. These data were consistent with those of a
recent study (19). Yi et al
also identified Beclin1 as a direct target of miR-199a-5p, and
miR-199a-5p negatively mediated Beclin1 expression in breast cancer
cells (19). Therefore, this
regulatory mechanism appears to participate in different
cancers.
In summary, our study demonstrated that treatment
with DDP inhibited the expression of miR-199a-5p and induced the
activation of autophagy in OS cells. Enforced expression of
miR-199a-5p inhibited DDP-induced autophagy and enhanced the
cytotoxicity of DDP in OS cells. Therefore, we suggest that
miR-199a-5p may become a potential candidate for the treatment of
DDP-resistant cancers.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (Beijing, China; grant no.
81402224), the Provincial Science Foundation of Hunan (Changsha,
China; grant no. 2015JJ3139), the Scientific Research Project of
the Development and Reform Commission of Hunan Province [Changsha,
China; grant no. (2014)658-8], the Scientific Research Project of
Science and Technology Bureau of Hunan Province (Changsha, China;
grant no. 2012FJ6001), the Scientific Research Project of Science
and Technology Office of Changsha City (Changsha, China; grant no.
K1203040-31), the Scientific Research Project of Health and Family
Planning Commission of Hunan Province (Changsha, China; grant no.
B2014-12) and the College Student's Innovation and Entrepreneurship
Project of Central South University (Changsha, China; grant no.
DL14505).
References
|
1
|
Thompson LD: Osteosarcoma. Ear Nose Throat
J. 92:288–290. 2013.PubMed/NCBI
|
|
2
|
Liu X, Choy E, Hornicek FJ, Yang S, Yang
C, Harmon D, Mankin H and Duan Z: ROCK1 as a potential therapeutic
target in osteosarcoma. J Orthop Res. 29:1259–1266. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ren XF, Mu LP, Jiang YS, Wang L and Ma JF:
LY2109761 inhibits metastasis and enhances chemosensitivity in
osteosarcoma MG-63 cells. Eur Rev Med Pharmacol Sci. 19:1182–1190.
2015.PubMed/NCBI
|
|
4
|
Yang Q, Zhang S, Kang M, Dong R and Zhao
J: Synergistic growth inhibition by sorafenib and cisplatin in
human osteosarcoma cells. Oncol Rep. 33:2537–2544. 2015.PubMed/NCBI
|
|
5
|
Zhang C, Hong CS, Hu X, Yang C, Wang H,
Zhu D, Moon S, Dmitriev P, Lu J, Chiang J, et al: Inhibition of
protein phosphatase 2A with the small molecule LB100 overcomes cell
cycle arrest in osteosarcoma after cisplatin treatment. Cell Cycle.
14:2100–2108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang R, Wang R, Chen Q and Chang H:
Inhibition of autophagy using 3-methyladenine increases
cisplatin-induced apoptosis by increasing endoplasmic reticulum
stress in U251 human glioma cells. Mol Med Rep. 12:1727–1732.
2015.PubMed/NCBI
|
|
7
|
Su Z, Yang Z, Xu Y, Chen Y and Yu Q:
MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget.
6:8474–8490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang Y, Li M, Wang YL, Threadgill MD, Xiao
M, Mou CF, Song GL, Kuang J, Yang X, Yang L, et al: ART1 promotes
starvation-induced autophagy: A possible protective role in the
development of colon carcinoma. Am J Cancer Res. 5:498–513.
2015.PubMed/NCBI
|
|
9
|
Sun Y, Liu JH, Jin L, Sui YX, Han LL and
Huang Y: Effect of autophagy-related beclin1 on sensitivity of
Cisplatin-resistant ovarian cancer cells to chemotherapeutic
agents. Asian Pac J Cancer Prev. 16:2785–2791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun Y, Liu JH, Jin L, Sui YX, Lai L and
Yang Y: Inhibition of Beclin 1 expression enhances
cisplatin-induced apoptosis through a mitochondrial-dependent
pathway in human ovarian cancer SKOV3/DDP cells. Oncol Res.
21:261–269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Z, Shao Z, Xiong L, Che B, Deng C
and Xu W: Expression of Beclin1 in osteosarcoma and the effects of
down-regulation of autophagy on the chemotherapeutic sensitivity.
29. 737–740. 2009.
|
|
12
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yates LA, Norbury CJ and Gilbert RJ: The
long and short of microRNA. Cell. 153:516–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou J, Liu R, Wang Y, Tang J, Tang S,
Chen X, Xia K, Xiong W, Xu D, Wang S, et al: miR-199a-5p regulates
the expression of metastasis-associated genes in B16F10 melanoma
cells. Int J Clin Exp Pathol. 7:7182–7190. 2014.PubMed/NCBI
|
|
15
|
Zhao X, He L, Li T, Lu Y, Miao Y, Liang S,
Guo H, Bai M, Xie H, Luo G, et al: SRF expedites metastasis and
modulates the epithelial to mesenchymal transition by regulating
miR-199a-5p expression in human gastric cancer. Cell Death Differ.
21:1900–1913. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu Y, Liu J, Jiang B, Chen J, Fu Z, Bai F,
Jiang J and Tang Z: MiR-199a-5p loss up-regulated DDR1 aggravated
colorectal cancer by activating epithelial-to-mesenchymal
transition related signaling. Dig Dis Sci. 59:2163–2172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shin VY, Siu JM, Cheuk I, Ng EK and Kwong
A: Circulating cell-free miRNAs as biomarker for triple-negative
breast cancer. Br J Cancer. 112:1751–1759. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang F, Chan LW, Law HK, Cho WC, Tang P,
Yu J, Shyu CR, Wong SC, Yip SP and Yung BY: Exploring
microRNA-mediated alteration of EGFR signaling pathway in non-small
cell lung cancer using an mRNA: MiRNA regression model supported by
target prediction databases. Genomics. 104:504–511. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yi H, Liang B, Jia J, Liang N, Xu H, Ju G,
Ma S and Liu X: Differential roles of miR-199a-5p in
radiation-induced autophagy in breast cancer cells. FEBS Lett.
587:436–443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu N, Zhang J, Shen C, Luo Y, Xia L, Xue F
and Xia Q: Cisplatin-induced downregulation of miR-199a-5p
increases drug resistance by activating autophagy in HCC cell.
Biochem Biophys Res Commun. 423:826–831. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kotsopoulos J, Zhang S, Akbari M, Salmena
L, Llacuachaqui M, Zeligs M, Sun P and Narod SA: BRCA1 mRNA levels
following a 4-6-week intervention with oral 3,3′-diindolylmethane.
Br J Cancer. 111:1269–1274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fang Y, Fang D and Hu J: MicroRNA and its
roles in esophageal cancer. Med Sci Monit. 18:RA22–RA30. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rebecca VW and Amaravadi RK: Emerging
strategies to effectively target autophagy in cancer. Oncogene.
35:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pan B, Chen Y, Song H, Xu Y, Wang R and
Chen L: Mir-24-3p downregulation contributes to VP16-DDP resistance
in small-cell lung cancer by targeting ATG4A. Oncotarget.
6:317–331. 2015.PubMed/NCBI
|
|
25
|
Shen C, Wang W, Tao L, Liu B, Yang Z and
Tao H: Chloroquine blocks the autophagic process in
cisplatin-resistant osteosarcoma cells by regulating the expression
of p62/SQSTM1. Int J Mol Med. 32:448–456. 2013.PubMed/NCBI
|
|
26
|
He XJ, Ma YY, Yu S, Jiang XT, Lu YD, Tao
L, Wang HP, Hu ZM and Tao HQ: Up-regulated miR-199a-5p in gastric
cancer functions as an oncogene and targets klotho. BMC Cancer.
14:2182014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Z, Song Y, Liu L, Hou N, An X, Zhan D,
Li Y, Zhou L, Li P, Yu L, et al: miR-199a impairs autophagy and
induces cardiac hypertrophy through mTOR activation. Cell Death
Differ. Jul 10–2015.(Epub ahead of print). View Article : Google Scholar
|
|
28
|
Lee JM, Heo MJ, Lee CG, Yang YM and Kim
SG: Increase of miR-199a-5p by protoporphyrin IX, a photocatalyzer,
directly inhibits E2F3, sensitizing mesenchymal tumor cells to
anti-cancer agents. Oncotarget. 6:3918–3931. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu J, Chen X, Song Y, Zhang Y, Zhou L and
Wan L: Deficit of RACK1 contributes to the spatial memory
impairment via upregulating BECLIN1 to induce autophagy. Life Sci.
151:115–121. 2016. View Article : Google Scholar : PubMed/NCBI
|