Introduction
Hepatoblastoma is a highly malignant common primary
liver tumor generally observed in infants and children <3 years
old (1). Hepatoblastoma is the third
most common abdominal neoplasm in this age group after
neuroblastoma and nephroblastoma (2),
and comprises 25–45% of all liver tumors and nearly 60% of all
primary malignant tumors of the liver during childhood, posing a
serious threat to health in the early stages of life. The incidence
of hepatoblastoma is 1.5 times higher in males than in females
(3). The morbidity rate was
1.2–1.7/million in the late 1990s in Europe, and may still be
decreasing (3–5).
Recent innovations in treatment have significantly
increased the survival rate of patients with hepatoblastoma during
the past few decades (6). Before the
1970s, the major method of treating hepatoblastoma was hepatectomy
without the application of effective post-surgical chemotherapy,
resulting in a low survival rate of 20–30% for patients (7). Since the 1980s, however, chemotherapy
with platinum-based anticancer drugs has been used and the 5-year
survival rate of patients with hepatoblastoma has increased to ≥75%
(8,9).
Currently, the primary method of treating hepatoblastoma employs
systemic chemotherapy (including pre-surgical volume reduction and
postoperative chemotherapy), local interventional chemotherapy and
high-dose chemotherapy in combination with autologous peripheral
blood stem cell transplantation (APBSCT) (10). However, despite the wide application
of various chemotherapy measures, the 5-year survival rate for
advanced hepatoblastoma remains low, particularly for subjects with
metastatic tumors in distant tissues or organs (11).
In order to identify the risk factors affecting
disease prognosis and outcome, a single-center retrospective
analysis was conducted on 102 subjects with advanced stages of
hepatoblastoma collected between September 2006 and June 2014. The
present study aimed to identify appropriate treatment for such
subjects, and thus increase the survival rate of patients with
advanced hepatoblastoma.
Patients and methods
Patients
Information on 102 patients, including 55 males and
47 females, was collected by Beijing Tongren Hospital (Beijing,
China) between September 2006 and June 2014. These subjects were
diagnosed to have pediatric hepatoblastoma based on results from
pathobiology and iconography analyses. Prior to surgical operation,
subjects were categorized into different clinical stages according
to the pretreatment extension of disease (PRETEXT) system adopted
by the International Childhood Liver Tumors Strategy Group (SIOPEL)
(12). Following surgical operation,
subjects were recategorized as stage I, II, III or IV based on the
malignancy of the hepatoblastoma, according to the Children's
Oncology Group (COG) staging criteria (13). Additionally, the hepatoblastoma tumor
tissues removed from these subjects were grouped based on pathology
as epithelial (including embryonal and fetal subtypes),
macrotrabecular or mixed type (14).
The detailed categorization and clinical diagnoses of the patients
are presented in Table I. The current
study was approved by the Committee on Human Study of the Chinese
People's Liberation Army General Hospital (Beijing, China) and the
Ethics Review Committee of Beijing Tongren Hospital. All procedures
involving human participants were in accordance with the 1964
Declaration of Helsinki and its later amendments. Written informed
consent was provided by the parents or guardians of the
patients.
 | Table I.Categorization and clinical diagnoses
for all 102 patients with pediatric hepatoblastoma. |
Table I.
Categorization and clinical diagnoses
for all 102 patients with pediatric hepatoblastoma.
| Criteria | Number of
patients | Ratio (%) |
|---|
| COG stage |
|
|
| II | 4 | 4/102 (3.9) |
| III | 48 | 48/102 (47.1) |
| IV | 50 | 50/102 (49.0) |
| Pathological
typea |
|
|
|
Epithelial | 52 | 52/91 (57.1) |
|
Macrotubular | 11 | 11/91 (12.1) |
|
Mixed | 28 | 28/91 (30.8) |
| Primary site |
|
|
| Left
lobe | 13 | 13/102 (12.7) |
| Right
lobe | 47 | 47/102 (46.1) |
|
Diffused | 42 | 42/102 (41.2) |
| Metastatic
siteb |
|
|
|
Lung | 37 | 37/49 (75.5) |
|
Superior vena cava tumor
emboli | 10 | 10/49 (20.4) |
|
Intrahepatic metastasis | 17 | 17/49 (34.7) |
|
Bone | 6 | 6/49 (12.2) |
|
Atria | 2 | 2/49 (4.1) |
|
Encephalic | 2 | 2/49 (4.1) |
| Colon
and small intestine | 2 | 2/49 (4.1) |
|
Pleura | 4 | 4/49 (8.2) |
Treatment
A total of 91 subjects were treated by surgical
operation; the remaining 11 patients were unable to undergo
surgical operation due to the accelerated malignancy of
hepatoblastoma or other reasons (Table
I).
For subjects with stage III hepatoblastoma,
interventional therapy or surgical operation was conducted
following iconographical and serological evaluation. Following
surgery, patients underwent 6–9 cycles of chemotherapy. For stage
IV subjects with solely intrahepatic metastasis, pre-surgical
chemotherapy was adopted for 2–4 cycles. Iconographical and
serological evaluation was conducted every 2 cycles. Following
surgery, chemotherapy was sustained for 9 cycles.
Pre-surgical volume reduction, local interventional
chemotherapy, radiofrequency ablation, and/or minimally invasive
surgery were performed following surgical evaluation of the
disease. For stage III and stage IV subjects with solely
intrahepatic metastasis, liver transplantation was performed
following tumor volume reduction induced by 2–4 cycles of
pre-surgical chemotherapy or radiofrequency ablation.
Pre-surgical chemotherapy consisted of 90
mg/m2 cisplatin on day 1, 1.5 mg/m2
vincristine on day 2 and 600 mg/m2 fluorouracil on day
2, and was sustained for 2–4 cycles. Post-surgical chemotherapy
consisted of the AEP method (90 mg/m2 cisplatin on day
1, 25 mg/m2 pirarubicin on days 1–3 and 100
mg/m2 etoposide on days 1–4) and the ACP method (90
mg/m2 cisplatin on day 1, 25 mg/m2
pirarubicin on days 1–3 and 800–1,000 mg/m2
cyclophosphamide on day 1), alternately. Post-surgical chemotherapy
was sustained for 4–6 cycles.
In order to remove the postoperative residual, the
IEA method of chemotherapy treatment (300 mg/m2
ifosfamide on days 1- 2, 400 mg/m2 carboplatin on day 3,
25 mg/m2 pirarubicin on days 4–5 and 100
mg/m2 etoposide on days 1–5) was applied and repeated
for 6–8 cycles.
Follow-up
Follow-up was conducted monthly until August 1,
2014. Subjects were regarded as censored if they abandoned the
treatment or were uncontactable.
Statistical analysis
Data were expressed as the mean ± standard
deviation. Normally distributed data were compared using the t
test. The χ2 test was used to compare the number of
patients. Comparison of data that did not follow a normal
distribution was performed according to the Mantel-Cox log-rank
test. Kaplan-Meier analysis was used for survival analysis. SPSS
software version 19.0 (IBM, SPSS, Armonk, NY, USA) was used for
statsitical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient clinical features
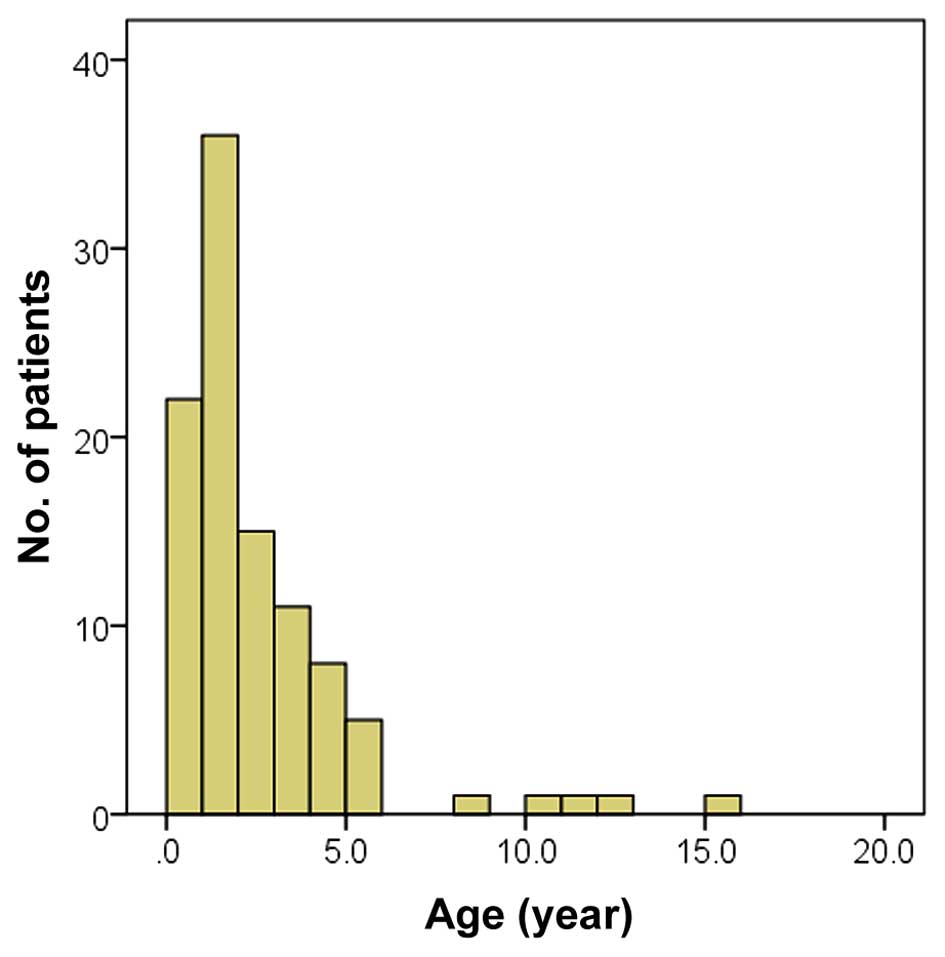
The median age of the 102 subjects was 1.5 years
(range, 1 month-15 years, Fig. 1).
The majority of subjects were <3 years old. Upon first
diagnosis, 74 subjects (72.5%) had developed an abdominal mass; 10
subjects (9.8%) had abdominal pain, fever and jaundice; 11 subjects
(10.8%) had anorexia and weight loss; and 6 (5.9%) had fever and
cough. Interestingly, 1 case of hepatoblastoma was detected during
a physical examination performed during the pregnancy of the
patient's mother.
Application of surgical operations and
chemotherapy
Surgical resection of primary hepatoblastoma was
conducted for 92 out of the 102 subjects. Radiofrequency ablation
was performed to reduce the size of the tumor for 3 patients with
primary hepatoblastoma and 1 patient with metastatic tumor.
Minimally invasive surgical resection of a metastatic tumor located
at the lung was performed for 1 subject.
Pre-surgical chemotherapy was applied to 72 subjects
(71.3%), and the mean number of chemotherapy cycles performed was
2.97±1.69 (range, 1–8; median, 3 cycles). Out of the patients
undergoing pre-surgical chemotherapy, pre-liver-puncture
chemotherapy was conducted for 68 subjects (67.3%), and
post-liver-puncture chemotherapy was conducted for 4 subjects
(4.0%). Post-surgical chemotherapy was administered to 86 patients
(84.3%), and the mean number of cycles performed was 8.50±5.23
(range, 4–32; median, 7 cycles).
Pre-surgical interventional therapy was received by
17 patients. The average times of interventional therapy were
2.00±1.17 times (range, 1–5 times; median, 2). Radiotherapy was
performed twice for a stage IV subject with progressive metastatic
tumor at the lung and once for a stage III subject at the
hepatoblastoma tissue. Liver transplantation was performed for 2
stage IV patients. In one of these subjects, a multiple
space-occupying lesion was diagnosed. Chemotherapy was performed
following hepatoblastoma relapse and partial remission (PR) was
subsequently observed. For the other subject, high-dose
chemotherapy and APBSCT was applied and the survival time for this
subject was 6 months following liver transplantation.
Retrospective study on the
prognoses
The follow-up lasted until August 31, 2014. The
average time length of follow-up was 25.9 months (range, 1–94
months; median, 21 months). Complete remission (CR) was achieved in
52 subjects (51.0%) and PR in 20 cases (19.6%). The recurrence of
hepatoblastoma was observed in 3 subjects (2.9%) and 27 subjects
succumbed to the disease (26.5%). The effective rate (CR + PR) was
69.6%.
Advanced hepatoblastoma is associated
with a low survival rate
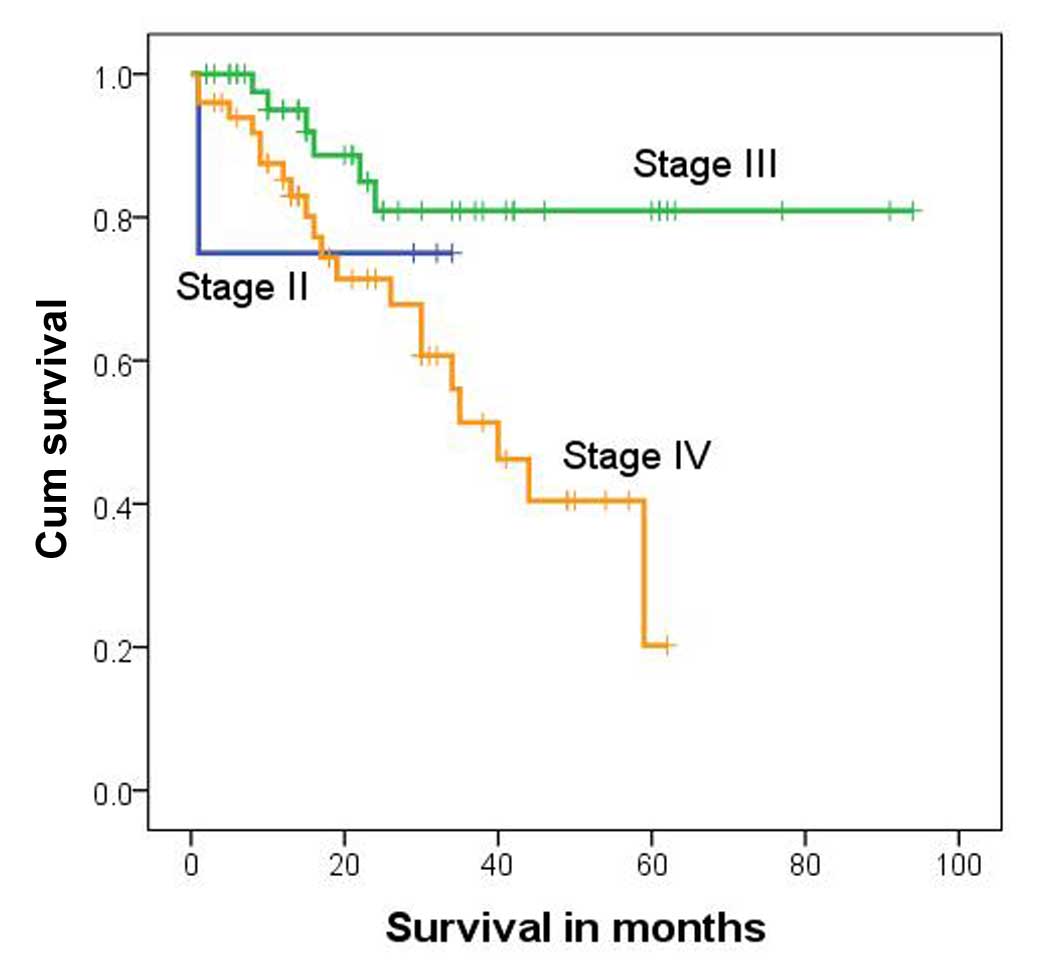
Total patient survival rates were 75.0, 80.9 and
20.2% for subjects with stage II, III and IV hepatoblastoma,
respectively. The survival rate differed significantly among these
different groups (P=0.015, χ2=8.359, log-rank test;
Table II). The median survival time
for stage IV subjects was 40 months [95% confidence interval
(CI)=27.8–52.2 months; Fig. 2].
 | Table II.Survival rate of subjects with
different stages of hepatoblastoma. |
Table II.
Survival rate of subjects with
different stages of hepatoblastoma.
|
| Outcomes |
|
|---|
|
|
|
|
|---|
| COG stage | Complete remission
(%) | Partial remission
(%) | Mortality (%) | Relapse (%) | Total (%) |
|---|
| IIa | 3 (75.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 4 (100.0) |
| III | 32 (66.7) | 10 (20.8) | 6 (12.5) | 0 (0.0) | 48 (100.0) |
| IV | 17 (34.0) | 10 (0.0) | 20 (40.0) | 3 (6.0) | 50 (100.0) |
| Total | 52 (51.0) | 20 (19.6) | 27 (26.5) | 3 (2.9) | 102 (100.0) |
Intrahepatic metastasis is associated
with high mortality
In order to identify the major factor associated
with the low survival rate of the stage IV subjects, the rate of
hepatoblastoma metastasis among such patients was evaluated. At
first, subjects with intrahepatic metastasis were assessed.
According to the criteria adopted by COG (11), out of the 50 stage IV subjects, 16
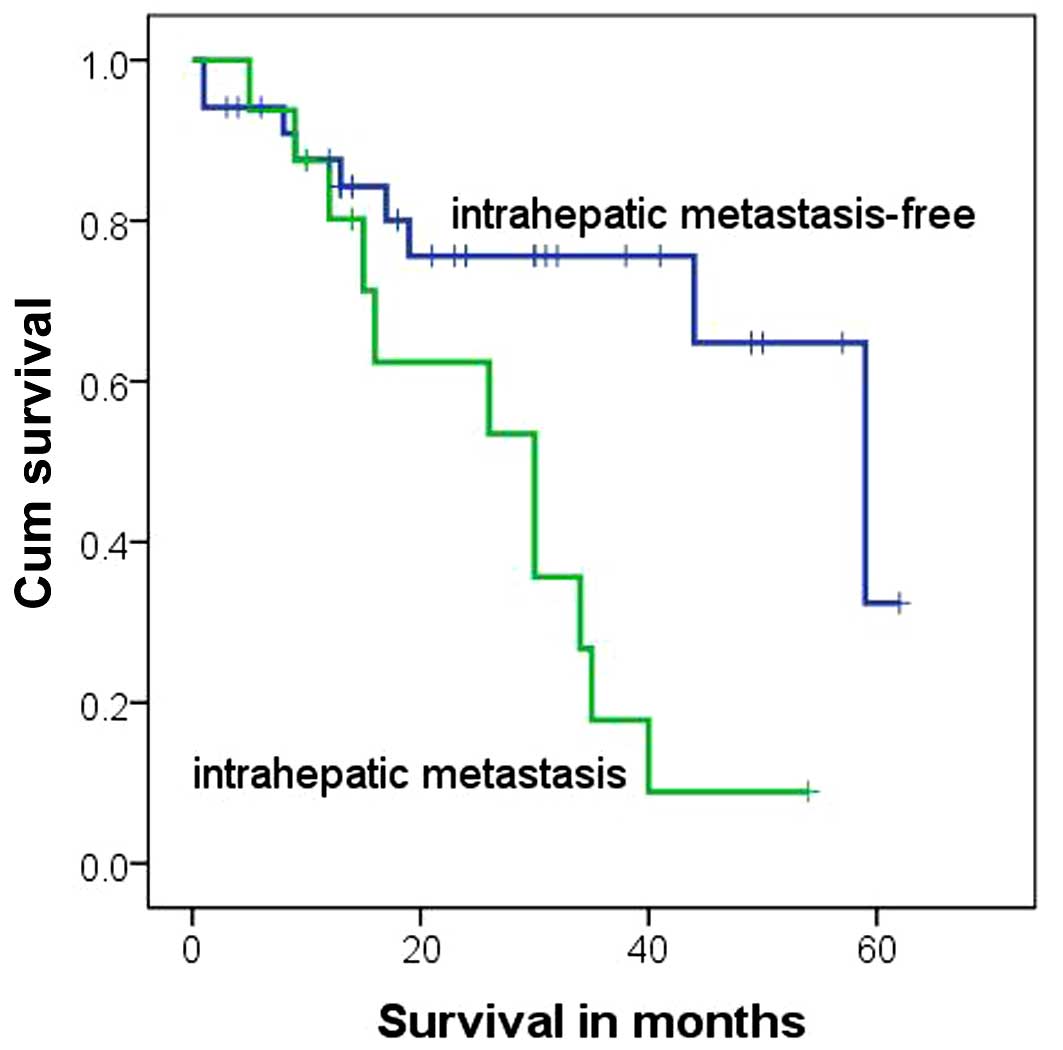
were diagnosed with intrahepatic metastasis. The mortality rate of
stage IV subjects with intrahepatic metastasis was significantly
higher than that of stage IV patients without intrahepatic
metastasis (P=0.004; Table III). In
addition, the survival time was significantly shorter for stage IV
subjects with intrahepatic metastasis compared with stage IV
patients without intrahepatic metastasis (P=0.006,
χ2=7.467, log-rank test; Table IV and Fig.
3).
 | Table III.Difference in mortality rates between
stage IV subjects with and without intrahepatic metastasis. |
Table III.
Difference in mortality rates between
stage IV subjects with and without intrahepatic metastasis.
| Intrahepatic
metastasis | Alivea | Succumbed | P-value (Fisher's
exact test) |
|---|
| Yes | 5 | 11 | 0.004b |
| No | 25 | 9 |
|
 | Table IV.Survival time of stage IV subjects
with and without intrahepatic metastasis. |
Table IV.
Survival time of stage IV subjects
with and without intrahepatic metastasis.
| Intrahepatic
metastasis | Median survival
time, months | 95% confidence
interval | 3-year survival
rate, % | 5-year survival
rate, % |
|---|
| Yes | 30 | 15.6–44.4 | 17.8 | 8.9 |
| No | 59 | 37.5–80.5 | 75.6 | 32.4 |
Due to the existence of postoperative residual in
stage IV subjects, the high mortality rate associated with
intrahepatic metastasis implies that postoperative residual may
affect the outcome of stage IV hepatoblastoma patients.
There is no difference in the survival
rate between stage IV subjects with and without distant
metastasis
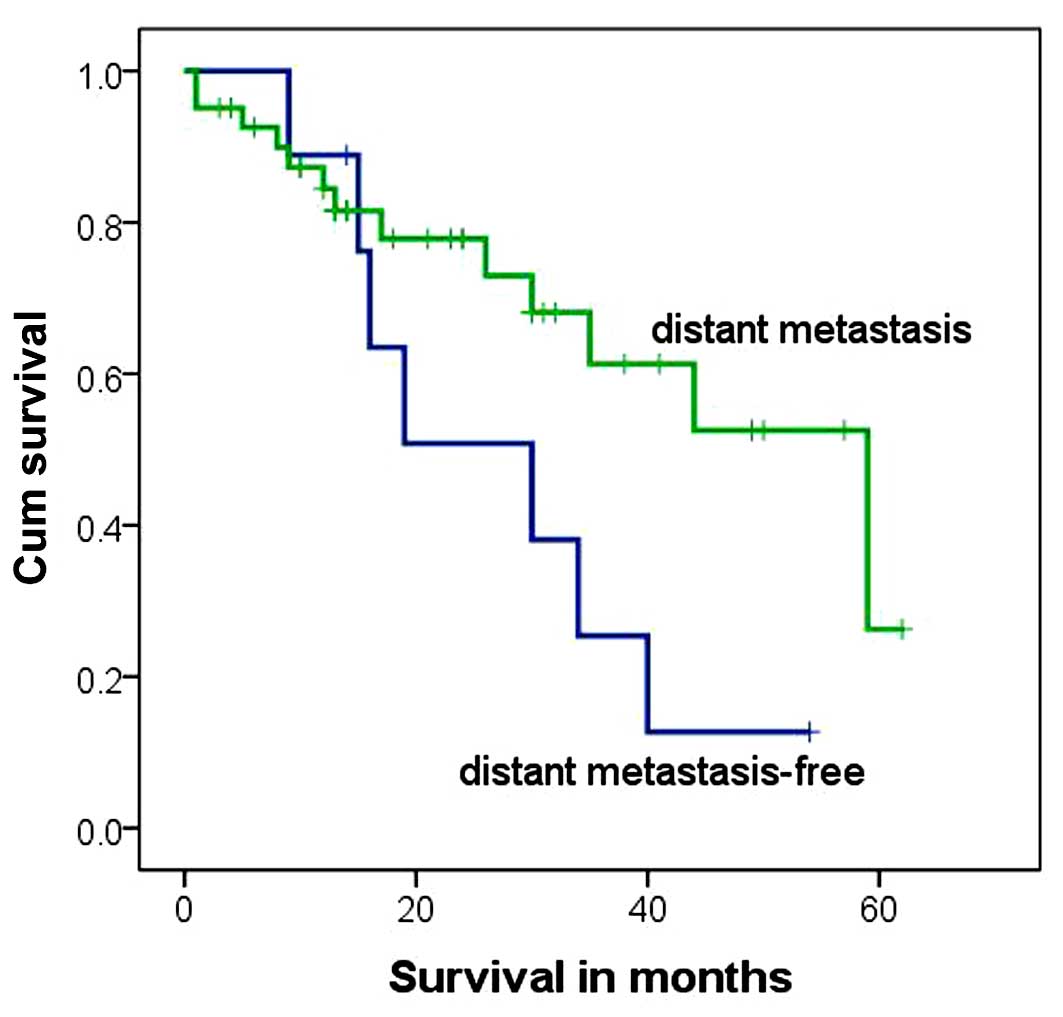
Subsequently, the association between distant
metastasis and survival rate of the 50 stage IV subjects was
investigated. Distant metastasis was detected in 41 cases, and
among them, 10 patients also had intrahepatic metastasis. A
significant difference between the survival rates of stage IV
subjects with distant metastasis and those without distant
metastasis was detected (P=0.021; Table
V). However, according to the results of log-rank test and
Kaplan-Meier survival analysis, the median survival time did not
differ significantly between stage IV subjects with and without
distant metastasis (P=0.063, χ2=3.462; Table VI and Fig.
4), suggesting that distant metastasis does not affect the
outcome of stage IV hepatoblastoma.
 | Table V.Significant difference in survival
rates between stage IV subjects with and without distant
metastasis. |
Table V.
Significant difference in survival
rates between stage IV subjects with and without distant
metastasis.
| Distant
metastasis | Alive | Succubed | P-value (Fisher's
exact test) |
|---|
| Yes | 28 | 13 | 0.021a |
| No | 2 | 7 |
|
 | Table VI.Survival time of stage IV subjects
with or without distant metastasis. |
Table VI.
Survival time of stage IV subjects
with or without distant metastasis.
| Distant
metastasis | Median survival
time, months | 95% confidence
interval | 3-year survival
rate, % | 5-year survival
rate, % | P-value |
|---|
| Yes | 59 | 32.8–85.2 | 61.3 | 26.3 | 0.063 |
| No | 30 | 11.4–48.6 | 25.4 | 12.7 |
High mortality rate for subjects
experiencing relapse who abandon chemotherapy
Hepatoblastoma relapsed was observed in 45 out of
the total 102 subjects. The median survival time for the 45
subjects was 12 months (range, 1–60 months). Hepatoblastoma relapse
was detected in 1 stage II, 9 stage III and 35 stage IV patients.
The stage II subject with relapsed hepatoblastoma experienced tumor
progression to stage IV. Following two surgical operations and
chemotherapy, the patient succumbed to the disease. Intrahepatic
tumor recurrence was detected for all 9 stage III subjects with
relapsed hepatoblastoma, implying that this caused the
postoperative residual in relapse. Following treatment, 3 patients
survived and 6 succumbed to the disease. For the 35 stage IV
subjects with relapsed hepatoblastoma, 20 succumbed to the disease
following chemotherapy.
The results of Fisher's exact test demonstrated that
the relapse rate was significantly higher in stage IV subjects
compared with stage III subjects (P=0.001; Table VII). Furthermore, the mortality rate
for patients with relapsed hepatoblastoma was higher among stage
III subjects compared with stage IV subjects (P=0.041; Table VIII).
 | Table VII.Difference in relapse rate of
hepatoblastoma between stage III and stage IV subjects. |
Table VII.
Difference in relapse rate of
hepatoblastoma between stage III and stage IV subjects.
| Relapse | Stage III | Stage IV | P-value (Fisher's
exact test) |
|---|
| Yes | 9 | 35a | 0.001b |
| No | 39 | 15 |
|
 | Table VIII.Difference in the mortality rates of
stage III and IV subjects following treatment for relapsed
tumor. |
Table VIII.
Difference in the mortality rates of
stage III and IV subjects following treatment for relapsed
tumor.
| Stage | Alive | Succumbed | P-value (Fisher's
exact test) |
|---|
| III | 3 | 6 | 0.041a |
| IV | 15 | 20 |
|
A total of 27 out of the 45 subjects with relapsed
hepatoblastoma succumbed to the disease. Among these 27 subjects,
20 had abandoned the chemotherapy treatment (1 stage II, 4 stage
III and 15 stage IV subjects). Among the 25 relapsed patients that
continued regular chemotherapy, only 7 succumbed to the disease
following treatment. The difference in the mortality rate between
subjects that continued chemotherapy and those that did not
suggested that the mortality rate of patients with relapsed
hepatoblastoma may be affected by chemotherapy.
No difference exists in the mortality
rate among subjects with distinct pathological types of tumor
Definite tumor pathological types were attributed to
91 out of the 102 cases, including 52 epithelial, 28 mixed and 11
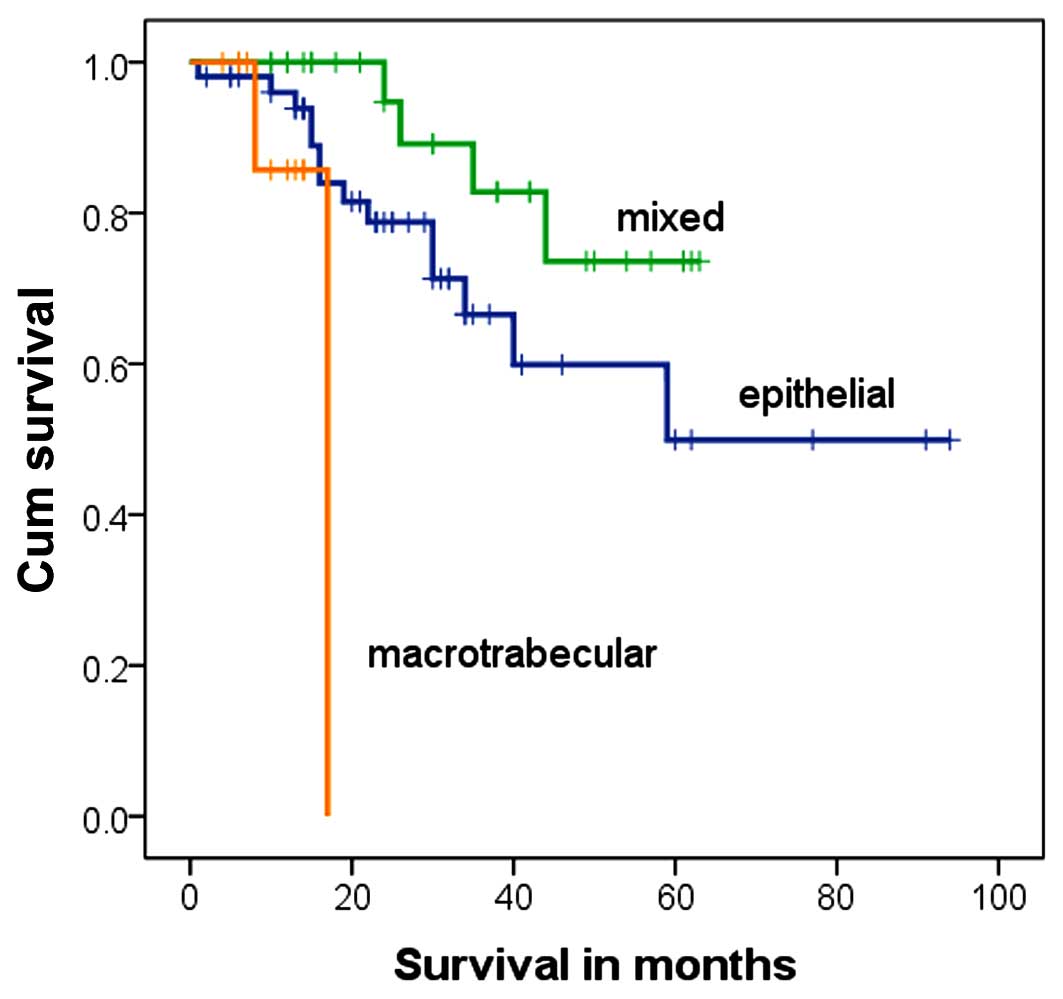
macrotrabecular. No significant difference in the mortality rate
among patients with these different pathological types was detected
(Table IX). However, subjects with
different pathological types had different survival curves,
indicating that the survival times of patients with different
pathological types of tumor differed significantly (P=0.010,
χ2=9.193, log-rank test; Fig.
5). This result implied that, although pathological typing may
effectively demonstrate the degree of differentiation of the tumor
tissue, it is not associated with disease prognosis.
 | Table IX.Mortality rate of subjects with
different pathological types. |
Table IX.
Mortality rate of subjects with
different pathological types.
| Pathological
type | Alive | Succumbed | P-value (Fisher's
exact test) |
|---|
| Epithelial | 38 | 14 | 0.479 |
| Mixed | 24 | 4 |
|
|
Macrotrabecular | 9 | 2 |
|
Discussion
Pediatric hepatoblastoma is usually diagnosed in
infants, particularly among those aged 1–2 years old (15). In the current study, ~70% of subjects
were <3 years old, which was consistent with previous studies
(16,17). The majority of cases of pediatric
hepatoblastoma begin with painless abdominal mass or diffused
lesions (18). At first diagnosis,
abdominal mass was observed in 74 of the subjects included in the
current study and diffused hepatoblastoma was detected in 42
subjects at the primary site. It should be noted that one case was
identified in the physical examination of the fetus prior to birth,
which demonstrates the importance of performing physical
examinations during pregnancy. Additionally, the disease history
during pregnancy for the mothers of patients with pediatric
hepatoblastoma should be emphasized, particularly for cases
diagnosed ≤6 months following birth.
The wildly applied treatment of hepatoblastoma in
the current study included chemotherapy, radiotherapy, surgical
resection and immunotherapy (12,19,20). Liver
transplantation and APBSCT with high-dose chemotherapy were
performed on subjects at an advanced disease stage. The
postoperative residual was difficult to remove solely using
surgical operation due to the complicated structure of pediatric
hepatoblastoma. The postoperative residual may be a cause of
relapse, as suggested in the current study. However, due to the
sensitivity of pediatric hepatoblastoma to chemotherapy, the tumor
tissue may be completely resected following pre-surgical
chemotherapy and reduction of the primary tumor size. Additionally,
post-surgical chemotherapy may effectively decrease the possibility
of relapse and metastasis occurring due to cleaning up the
postoperative residual, thus increasing the survival rate of
subjects. For subjects with intact tumor capsule, intervenient
chemotherapy was frequently applied to reduce the size of
hepatoblastoma (21). A novel method
of adjuvant chemotherapy has been suggested by SIOPEL to reduce
tumor size and clear the postoperative residual (12). Furthermore, the COG has advised that
this new adjuvant chemotherapy should be applied to treat stage III
and IV subjects with vascular invasion or distant metastasis
(13). Following adjuvant
chemotherapy, a satisfactory outcome may be obtained. Therefore,
the combination of pre- and post-surgical chemotherapy and surgical
operation may improve the prognosis of patients with pediatric
hepatoblastoma, even for those with advanced stages of the
disease.
Although hepatoblastoma can undoubtedly be
identified using iconography analysis and serum alpha-fetoprotein
examination, pre-surgical chemotherapy should be applied following
pathological diagnosis (20,22–25). If it
is not feasible to conduct a liver puncture and confirm the
existence of hepatoblastoma, one of the following criteria should
be met to justify the application of pre-surgical chemotherapy: A
significantly higher level of serum alpha-fetoprotein compared with
normal controls; a tumor size that is too large for first resection
or subject unendurable to surgical operation; diffused
hepatoblastoma with calcification; distant metastasis; tumor
hemorrhage; or stage III or IV hepatoblastoma (26).
In patients, 90% of the blood supply for
hepatoblastoma flows through the hepatic artery (27). However, ~75% of blood supplied to the
healthy liver flows through the portal vein (28). Reducing the blood supply to
hepatoblastoma without affecting blood supply to normal liver
tissue may result in the selective necrosis of tumor cells.
Furthermore, given the differences in blood supply, local
interventional chemotherapy may be an ideal and specific measure of
hepatoblastoma treatment.
In the present study, resection of primary
hepatoblastoma was performed in 91 subjects. Radiofrequency
ablation was applied to 4 subjects, including 3 with primary
hepatoblastoma and 1 with a lung metastatic tumor. Additionally,
minimally invasive surgery was conducted for the subject with a
lung metastatic tumor. Systemic pre-surgical chemotherapy was
performed in 72 subjects, and 86 subjects were treated with
post-surgical chemotherapy. A total of 17 subjects were treated
using the hepatic arterial chemoembolization method. CR was
observed in 52 subjects and PR in 20. The effective rate (CR + PR)
was 69.6%. This rate was slightly lower compared with that of a
previous study. This inconsistency may have been caused by the high
percentage of subjects with advanced hepatoblastoma (98 subjects
were stages III and IV) and the abandonment of treatment following
relapse of hepatoblastoma by 20 subjects in the current study.
It has previously been reported that the combination
of liver transplantation and APBSCT with high-dose chemotherapy is
effective in treating PRETEXT IV hepatoblastoma, hepatoblastoma
invading the portal vein, micrometastatic hepatoblastoma and
postoperative residual-induced relapse (19). Otte et al (29) demonstrated that 43 out of 49
hepatoblastoma subjects achieved tumor-free survival following
liver transplantation. However, liver transplantation may be
inappropriate for subjects with lung metastatic tumors or those
unable to undergo surgical operation. For these subjects, APBSCT
with high-dose chemotherapy may at least partially relieve the
symptoms of the disease. In the current study, the stage IV subject
with diffused primary hepatoblastoma relapsed following liver
transplantation. Another stage IV subject achieved PR following
liver transplantation; however, the patient only survived for 6
months. This suggests that, although liver transplantation
partially relieves hepatoblastoma, it has little effect on the
final outcome for subjects with advanced stages of the disease. The
stage of hepatoblastoma, state of subjects and other issues should
be taken into account prior to conducting liver
transplantation.
A total of 27 subjects succumbed to the disease in
the current study. All experienced a relapse of hepatoblastoma
during treatment, indicating that tumor relapse may be the major
cause of mortality among patients with hepatoblastoma. However,
only 7 out of the 25 subjects who experienced relapse but underwent
regular chemotherapy succumbed to the disease by the end of
follow-up, suggesting the importance of chemotherapy in treating
relapsed hepatoblastoma. The postoperative residual was regarded as
a major cause of intrahepatic metastasis and tumor relapse. Distant
metastasis and pathological type may have little effect on the
outcome of hepatoblastoma. Therefore, in order to improve the
survival rate of subjects with pediatric hepatoblastoma, efforts
should be made to clear the postoperative residual and reduce the
relapse rate.
Acknowledgements
The present study was supported by grants the
Capital Health Research and Development of Special (Beijing, China;
grant no. 2014-4-2054).
References
|
1
|
Devi LP, Kumar R, Handique A and Kumar M:
Hepatoblastoma-a rare liver tumor with review of literature. J
Gastrointest Cancer. 45(Suppl 1): 261–264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spyridakis I, Kepertis C, Lampropoulos V,
Mouravas V and Filippopoulos A: Embryonal/Fetal subtype
hepatoblastoma: A case report. J Clin Diagn Res. 8:ND01–ND02.
2014.PubMed/NCBI
|
|
3
|
Stiller CA, Pritchard J and
Steliarova-Foucher E: Liver cancer in European children: Incidence
and survival, 1978–1997. Report from the automated childhood cancer
information system project. Eur J Cancer. 42:2115–2123. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Fine Licht S, Schmidt LS, Rod NH,
Schmiegelow K, Lähteenmäki PM, Kogner P, Träger C, Stokland T and
Schüz J: Hepatoblastoma in the Nordic countries. Int J Cancer.
131:E555–E561. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gatta G, Ferrari A, Stiller CA, Pastore G,
Bisogno G, Trama A and Capocaccia R: RARECARE Working Group:
Embryonal cancers in Europe. Eur J Cancer. 48:1425–1433. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aronson DC, Czauderna P, Maibach R,
Perilongo G and Morland B: The treatment of hepatoblastoma: Its
evolution and the current status as per the SIOPEL trials. J Indian
Assoc Pediatr Surg. 19:201–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Evans AE, Land VJ, Newton WA, Randolph JG,
Sather HN and Tefft M: Combination chemotherapy (vincristine,
adriamycin, cyclophosphamide and 5-fluorouracil) in the treatment
of children with malignant hepatoma. Cancer. 50:821–826. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tiao GM, Bobey N, Allen S, Nieves N,
Alonso M, Bucuvalas J, Wells R and Ryckman F: The current
management of hepatoblastoma: A combination of chemotherapy,
conventional resection and liver transplantation. J Pediatr.
146:204–211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pritchard J and Stringer M: Outcome and
complications after resection of hepatoblastoma. J Pediatr Surg.
39:1744–1745. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Zhang WL, Huang DS, Hong L, Wang
YZ, Zhu X, Hu HM, Zhang PW, Yi Y and Han T: Clinical effectiveness
of multimodality treatment on advanced pediatric hepatoblastoma.
Eur Rev Med Pharmacol Sci. 18:1018–1026. 2014.PubMed/NCBI
|
|
11
|
Trobaugh-Lotrario AD and Katzenstein HM:
Chemotherapeutic approaches for newly diagnosed hepatoblastoma:
Past, present and future strategies. Pediatr Blood Cancer.
59:809–812. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Czauderna P, Otte JB, Roebuck DJ, von
Schweinitz D and Plaschkes J: Surgical treatment of hepatoblastoma
in children. Pediatr Radiol. 36:187–191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Malogolowkin MH, Katzenstein HM, Meyers
RL, Krailo MD, Rowland JM, Haas J and Finegold MJ: Complete
surgical resection is curative for children with hepatoblastoma
with pure fetal histology: A report from the children's oncology
group. J Clin Oncol. 29:3301–3306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schnater JM, Aronson DC, Plaschkes J,
Perilongo G, Brown J, Otte JB, Brugieres L, Czauderna P, MacKinlay
G and Vos A: Surgical view of the treatment of patients with
hepatoblastoma: Results from the first prospective trial of the
international society of pediatric oncology liver tumor study
group. Cancer. 94:1111–1120. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan XJ, Wang HM, Jiang H, Tang MJ, Li ZL,
Zou X, Fang YJ, Pan C, Tou JF, Zhang KR, et al: Multidisciplinary
effort in treating children with hepatoblastoma in China. Cancer
Lett. 375:39–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsunaga T, Sasaki F, Ohira M, Hashizume
K, Hayashi A, Hayashi Y, Mugishima H and Ohnuma N: Japanese Study
Group for Pediatric Liver Tumor: Analysis of treatment outcome for
children with recurrent or metastatic hepatoblastoma. Pediatr Surg
Int. 19:142–146. 2003.PubMed/NCBI
|
|
17
|
Brugières L, Branchereau S and Laithier V:
Paediatric malignant liver tumours. Bull Cancer. 99:219–228.
2012.(In French). PubMed/NCBI
|
|
18
|
Honeyman JN and La Quaglia MP: Malignant
liver tumors. Semin Pediatr Surg. 21:245–254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gupta AA, Gerstle JT, Ng V, Wong A,
Fecteau A, Malogolowkin MH, Meyers RL, Grant D and Grant RM:
Critical review of controversial issues in the management of
advanced pediatric liver tumors. Pediatr Blood Cancer.
56:1013–1018. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
von Schweinitz D: Management of liver
tumors in childhood. Semin Pediatr Surg. 15:17–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karski EE, Dvorak CC, Leung W, Miller W,
Shaw PJ, Qayed M, Katsanis E and Feusner JH: Treatment of
hepatoblastoma with high-dose chemotherapy and stem cell rescue:
The pediatric blood and marrow transplant consortium experience and
review of the literature. J Pediatr Hematol Oncol. 36:362–368.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perilongo G, Maibach R, Shafford E,
Brugieres L, Brock P, Morland B, de Camargo B, Zsiros J, Roebuck D,
Zimmermann A, et al: Cisplatin versus cisplatin plus doxorubicin
for standard-risk hepatoblastoma. N Engl J Med. 361:1662–1670.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eicher C, Dewerth A, Ellerkamp V, Fuchs J,
Schott S and Armeanu-Ebinger S: Effect of duplex drugs linking
2′-deoxy-5-fluorouridine (5-FdU) with 3′-C-ethynylcytidine (ECyd)
on hepatoblastoma cell lines. Pediatr Surg Int. 29:121–127. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
von Schweinitz D: Hepatoblastoma: Recent
developments in research and treatment. Semin Pediatr Surg.
21:21–30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zsiros J, Maibach R, Shafford E, Brugieres
L, Brock P, Czauderna P, Roebuck D, Childs M, Zimmermann A,
Laithier V, et al: Successful treatment of childhood high-risk
hepatoblastoma with dose-intensive multiagent chemotherapy and
surgery: Final results of the SIOPEL-3HR study. J Clin Oncol.
28:2584–2590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Ioris M, Brugieres L, Zimmermann A,
Keeling J, Brock P, Maibach R, Pritchard J, Shafford L, Zsiros J,
Czaudzerna P and Perilongo G: Hepatoblastoma with a low serum
alpha-fetoprotein level at diagnosis: The SIOPEL group experience.
Eur J Cancer. 44:545–550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guérin F, Gauthier F, Martelli H, Fabre M,
Baujard C, Franchi S and Branchereau S: Outcome of central
hepatectomy for hepatoblastomas. J Pediatr Surg. 45:555–563. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moreira RK, Chopp W and Washington MK: The
concept of hepatic artery-bile duct parallelism in the diagnosis of
ductopenia in liver biopsy samples. Am J Surg Pathol. 35:392–403.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Otte JB, Pritchard J, Aronson DC, Brown J,
Czauderna P, Maibach R, Perilongo G, Shafford E and Plaschkes J:
International Society of Pediatric Oncology (SIOP): Liver
transplantation for hepatoblastoma: Results from the international
society of pediatric oncology (SIOP) study SIOPEL-1 and review of
the world experience. Pediatr Blood Cancer. 42:74–83. 2004.
View Article : Google Scholar : PubMed/NCBI
|