Introduction
Gastric cancer is a complex and heterogeneous
disease and one of the most frequently diagnosed types of cancer in
Korea (1). Multiple unknown factors
contribute to its pathogenesis, progression, metastasis and relapse
(1,2).
Currently, the main treatment options for gastric cancer are
surgery, radiation therapy and chemotherapy (1,2). There
have been numerous improvements in the diagnosis and treatment of
gastric cancer (2). However, the
development of novel therapeutic strategies is still required to
reduce the debilitating side effects of the drugs currently used in
chemotherapy and radiotherapy (2).
The majority of mammals are able to synthesize their
own vitamin C in the liver by the action of the enzyme
L-gulonolactone oxidase (3). However,
certain primates, including humans, cannot produce vitamin C
themselves due to a mutation in the gene encoding L-gulonolactone
oxidase (3). It has been demonstrated
that vitamin C has various beneficial effects, including
anti-inflammatory and anti-oxidative activity (4). In cancer therapy, however, vitamin C
exhibits pro-oxidant activity that often enhances its cytotoxic
effects, including cellular damage through the accumulation of
hydrogen peroxide (H2O2), which leads to the
arrest of tumor cell growth and the induction of tumor cell death
(4).
Apoptosis and necrosis are the two major mechanisms
of cell death (5,6). Apoptosis is a method of programmed cell
death that under normal conditions occurs as a homeostatic
mechanism to maintain the population of cells in tissues and as a
defense mechanism to remove cells damaged by noxious agents or
disease (5,6). The apoptotic process is initiated by
recognizing the death signal either from outside the cell or from
mitochondria within the cell, leading to the activation of various
caspases that results in DNA/RNA fragmentation, nuclear chromatin
condensation, protein degradation, cell shrinkage and ultimately
the shedding of apoptotic bodies (5,6).
Phagocytic cells remove the released apoptotic bodies by engulfment
without inflammation (5,6). By contrast, necrosis is a non-programmed
mechanism of cell death initiated by a high level of toxic
materials (5,6). The necrotic process begins with the cell
swelling instead of shrinking and is accompanied by the formation
of large vacuoles (5,6). It ends with complete cell lysis and
diffusion of disrupted intracellular contents, eliciting
inflammatory responses in the adjacent tissue (5,6). In
addition, as an alternative form of programmed cell death,
necroptosis is a form of regulated necrosis induced by signals
received by death receptors, including tumor necrosis factor
receptor (TNFR) 1, TNFR2 and FASR, or pattern recognition receptors
(6). Necrosis and necroptosis are
typically not associated with caspase activation (6).
A previous report demonstrated that vitamin C
produces H2O2, thus inducing the apoptosis of
human adenocarcinoma gastric cancer cells from the AGS cell line
(7). A concentration of vitamin C
>1 mM can produce H2O2, causing the death
of cancer cells by producing the ascorbate radical (4). However, H2O2
generally causes cell swelling, which does not usually occur during
necrosis (8). Therefore, it is
necessary to examine whether reactive oxygen species (ROS)
production induced by vitamin C mediates the apoptosis or necrosis
of AGS cells. The present study aimed to confirm that a high
concentration of vitamin C induces cell death in the AGS cell line
and to determine whether vitamin C-induced cell death is triggered
by apoptosis or necrosis.
Materials and methods
Cell line and cytotoxic assays
The human gastric cancer cell line AGS was purchased
from the Korean Cell Line Bank (Cancer Research Institute, Seoul
National University of Medicine, Seoul, Korea). AGS cells were
grown in RPMI 1640 medium (Lonza, Walkersville, MD, USA)
supplemented with 5% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and a 1%
penicillin-streptomycin mixture (Gibco; Thermo Fisher Scientific,
Inc.). Cells were incubated at 37°C in 5% CO2. A total
of 7×104 AGS cells were plated in RPMI 1640 medium for
24 h in a 24-well plate. Cells were subsequently treated with
incremental doses (0, 0.5 and 1.5 mM) of vitamin C (Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) for 4 or 24 h. Cell viability
was determined by a colorimetric method using
3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide
(MTT) as described previously (9).
Analysis of cell death mechanism
(apoptosis vs. necroptosis)
To determine which mechanism is induced by vitamin C
and causes the death of AGS cells, an inhibitor of
caspase-dependent apoptosis, z-VAD-FMK (z-VAD; Tocris Bioscience,
Bristol, UK) and a necroptosis inhibitor, necrostatin-1 (Nec-1;
BioVision, Inc., Milpitas, CA, USA) were used. A total of
7×104 AGS cells were cultured in RPMI 1640 medium for 24
h in a 24-well plate. AGS cells were divided into four groups. Two
groups were treated with either 0 or 1.5 mM vitamin C for 4 h,
while the other two groups were pretreated with 20 mM nec-1 or 10
mM z-VAD for 1 h prior to co-incubation with vitamin C. Cell
viability was determined by MTT assay.
Analysis of apoptosis with annexin
V-propidium iodide (PI)
Apoptosis was quantified using PI (MBL International
Co., Woburn, MA, USA) and an Annexin V-FITC Apoptosis Detection kit
(BD Biosciences, San Jose, CA, USA) according to the manufacturer's
protocol. Briefly, following centrifugation at 400 × g for 5
min at room temperature, the cells were double stained with annexin
V-fluorescein isothiocyanate (FITC) and PI as recommended by the
manufacturer. The apoptotic cell population was determined using a
FC 500 flow cytometry system (BD Biosciences) and analyzed with CXP
2.2 Acquisition Software CXP 2.2 Analysis Software (Beckman
Coulter, Inc., Brea, CA, USA). For each sample, ≥10,000 cells were
measured.
Measurement of calcium
The level of intracellular calcium was measured
using the fluorescent calcium indicator Fluo-3 (Invitrogen; Thermo
Fisher Scientific, Inc.), as described previously (10). Briefly, cells were incubated with 1 µM
Fluo-3 calcium indicator for 1 h. The cell intracellular calcium
distributions were determined by using a FACSCalibur™ flow
cytometer (BD Biosciences) and analyzed using the CellQuest Pro
software program (BD Biosciences). For each sample, ≥10,000 cells
were analyzed. To examine the role of intracellular calcium in the
cytotoxicity and apoptosis induced by vitamin C, 10 µM calcium
inhibitor 1,2-bis (2-aminophenoxy) ethane N,N,N',N'-tetraacetic
acid (BAPTA; Tocris Bioscience) was incubated prior to
co-incubation with vitamin C at the indicated concentrations.
Cytotoxicity was determined by MTT assays, and apoptosis was
analyzed by flow cytometry following double staining with annexin
V-FITC and PI.
Measurement of ROS
The level of intracellular ROS was measured using a
ROS detection reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's guidelines. ROS levels were
measured with a FACSCalibur™ flow cytometer. To examine the role of
intracellular ROS in the cytotoxicity and apoptosis induced by
vitamin C, 5 mM antioxidant N-acetylcysteine (NAC; Tocris
Bioscience) was incubated prior to co-incubation with vitamin C.
Cytotoxicity was determined by MTT assays, and apoptosis was
analyzed by flow cytometry following double staining with annexin
V-FITC and PI.
Measurement of adenosine triphosphate
(ATP)
The production of ATP was measured using the ATP
Colorimetric/Fluorometric Assay kit (BioVision, Inc.) according to
the manufacturer's guidelines.
Western blot analysis
AGS cells were cultured in 6-well plates and
incubated with vitamin C at different concentrations for 4 h.
Following incubation, cells were washed with ice-cold
phosphate-buffered saline and lysed with lysis buffer [50 mM
Tris-HCl (pH 8.0), 150 mM NaCl, 0.5% sodium deoxycholate, 0.1%
sodium dodecyl sulfate (SDS) and 1% NP-40] containing a protease
inhibitor cocktail (Calbiochem; Merck Millipore). Cell debris was
removed by centrifugation at 18,210 × g for 30 min, and
protein concentration was determined using a Bradford Protein Assay
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Proteins were
separated by 10% SDS-polyacrylamide gel electrophoresis and
transferred to an Immobilon-P nitrocellulose membrane (0.45 µm;
Merck Millipore) using the TE 77 Semi-Dry Transfer Unit (GE
Healthcare Life Sciences, Chalfont, UK). The membrane was blocked
with 5% non-fat milk in Tris-buffered saline containing 1% Tween-20
(pH 7.4) at room temperature for 1 h, and blots were probed with
rabbit monoclonal antibodies for pro-caspase-3 (1:200 dilution;
Cat# 7148; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
cleaved caspase-3 (1:200 dilution; Cat# 22171-R; Santa Cruz
Biotechnology, Inc.) and light chain 3 (LC3) I and LC3 II (1:3,000
dilution; Cat# 51520; Abcam, Cambridge, UK), or with mouse
monoclonal antibody for β-actin (1:5,000 dilution; Cat# 47778;
Santa Cruz Biotechnology, Inc.) at 4°C overnight, and then
incubated at room temperature for 1 h with goat anti-rabbit or
anti-mouse monoclonal antibodies (1:5,000 and 1:10,000 dilution,
respectively; Cat# 2004 and 2055, respectively; Santa Cruz
Biotechnology, Inc.). The proteins were visualized using an
enhanced chemiluminescence kit and western blotting detection
reagents (GE Healthcare Life Sciences, Pittsburgh, PA, USA),
followed by exposure to X-ray film (Fujifilm, Tokyo, Japan). Each
band was determined quantitatively using ImageJ software
(http://rsb.info.nih.gov). The densitometry
reading of the bands was normalized to β-actin expression.
Statistics
Statistical analyses were performed using GraphPad
Prism version 5.03 software (GraphPad Software, Inc., La Jolla, CA,
USA). Differences among groups were analyzed using a two-tailed
t-test. P<0.05 was considered to indicate a statistically
significant difference. Data were represented as the mean ±
standard deviation.
Results
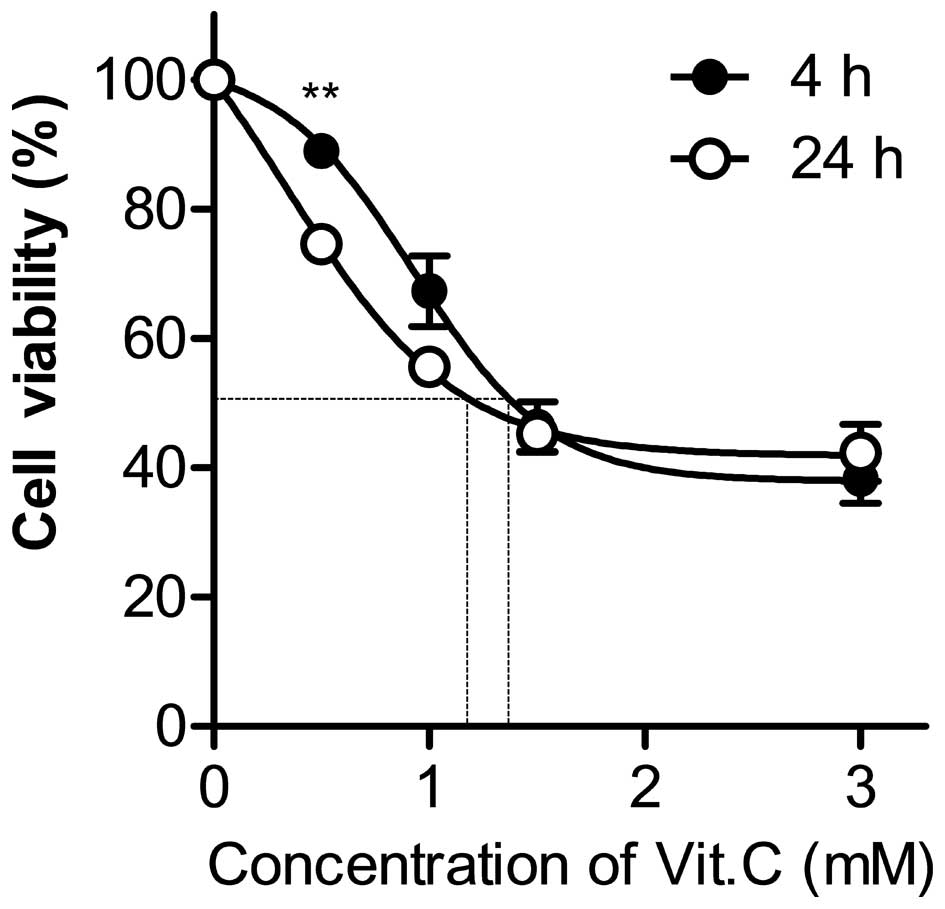
Vitamin C decreases the viability of
human gastric cancer cells
To examine whether treatment of high-dose vitamin C
affects the growth of AGS cells, cells were treated with various
concentrations of vitamin C for 4 or 24 h, and their cytotoxicity
was subsequently analyzed using an MTT assay. Vitamin C treatment
decreased the number of AGS cells in a dose-dependent manner
(Fig. 1). Although a longer
incubation time slightly increased the susceptibility to cell death
induced by vitamin C treatment, there was no significant difference
between cell viability at 4 and 24 h, except when cells were
incubated with 0.5 mM vitamin C. The half maximal effective
concentration (EC50) values of the cells incubated for 4
and 24 h were 1.2 and 1.1 mM, respectively (Fig. 1). This result indicates that high-dose
vitamin C has an anti-tumor effect in the AGS cell line.
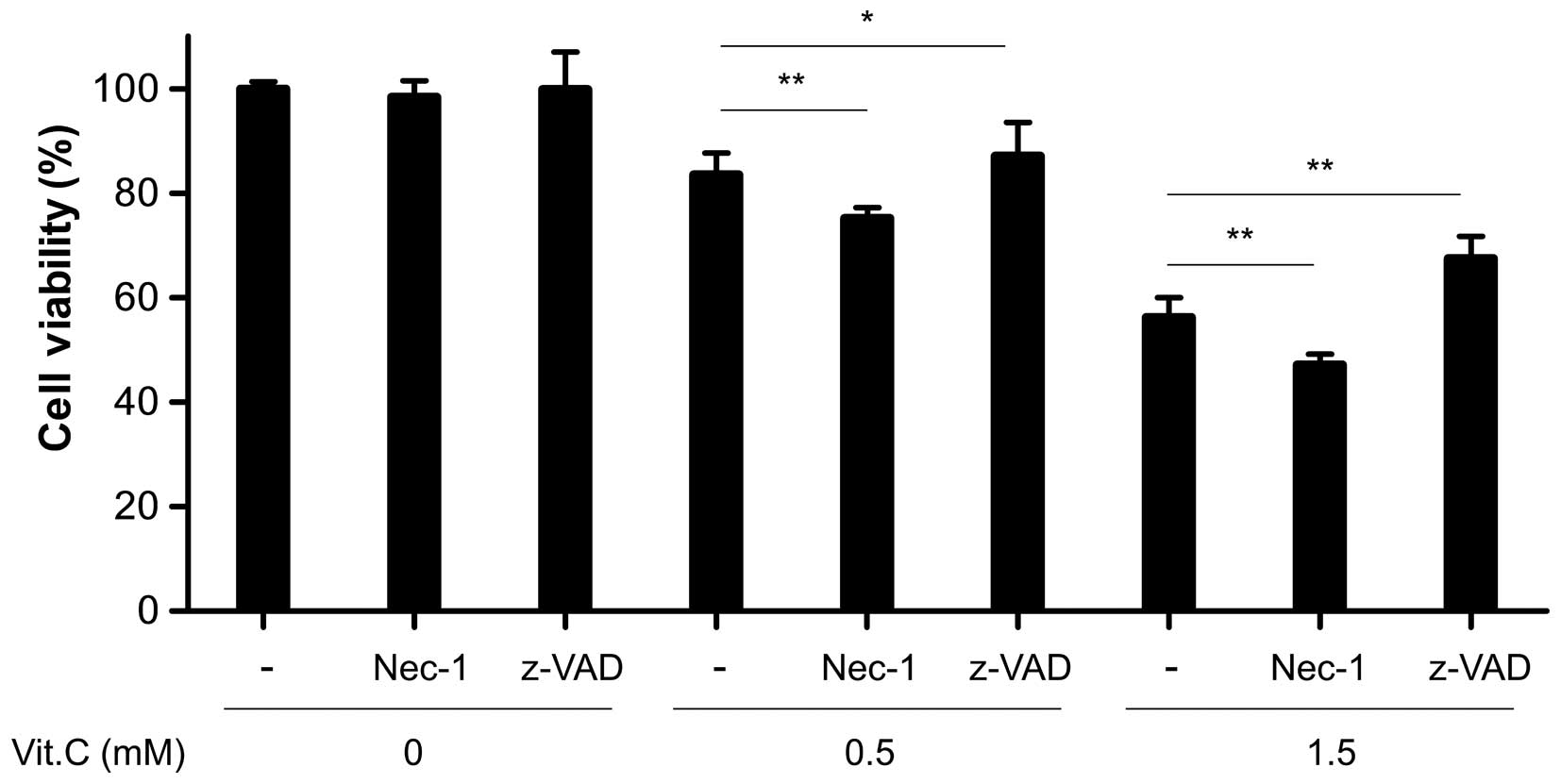
Vitamin C-induced cell death is
dependent on apoptosis, not necroptosis
Apoptosis and necrosis (or the form of necrosis
known as necroptosis) may occur simultaneously depending on certain
factors, including stimulus intensity and duration, caspase
availability and the extent of ATP depletion (5). To examine whether the death of AGS cells
by vitamin C was dependent on apoptosis or necroptosis, AGS cells
were incubated with an apoptosis-specific inhibitor or
necroptosis-specific inhibitor prior to vitamin C treatment.
Pretreatment with z-VAD significantly inhibited vitamin C-induced
cell death, regardless of vitamin C concentration (Fig. 2). By contrast, pretreatment with Nec-1
enhanced the reduction in cell viability induced by vitamin C
(Fig. 2). This unexpected decrease is
consistent with the results of a previous study demonstrating that
Nec-1 treatment strongly inhibits programmed cellular necrosis
while somewhat increasing apoptotic cell death (11). This result suggests that apoptosis,
not necrosis, primarily mediates the AGS cell death that occurs
following treatment with high-dose vitamin C.
Increased intracellular calcium is
partially responsible for AGS cell apoptosis induced by vitamin
C
Several previous studies have reported that
releasing calcium from the endoplasmic reticulum (ER) into the
cytosol induces apoptosis in cancer cells (12–14). The
present study demonstrated that treatment of AGS cells with 0.5 and
1.5 mM vitamin C for 4 h significantly increased the amount of
intracellular calcium, by 5.78 and 36.00%, respectively (Fig. 3A and B). To examine whether the
increased calcium concentration induced by vitamin C affects the
viability of AGS cells, cells were treated with 1.5 mM vitamin C
following pretreatment with BAPTA. The inhibition of calcium
accumulation by BAPTA pretreatment significantly suppressed the
cytotoxic activity of vitamin C (Fig.
3C). To confirm the effect of calcium accumulation on vitamin
C-induced apoptosis, AGS cells treated in the same way were stained
with annexin V and PI, and subsequently analyzed by flow cytometry.
Consistent with the cytotoxicity results, vitamin C markedly
increased the rate of late apoptosis by 16.29%, whereas BAPTA
pretreatment reduced vitamin C-induced late apoptosis by 11.99%
(Fig. 3D and E). These data indicate
that the accumulation of intracellular calcium, at least in part,
contributes to the apoptotic effect of vitamin C in AGS cells.
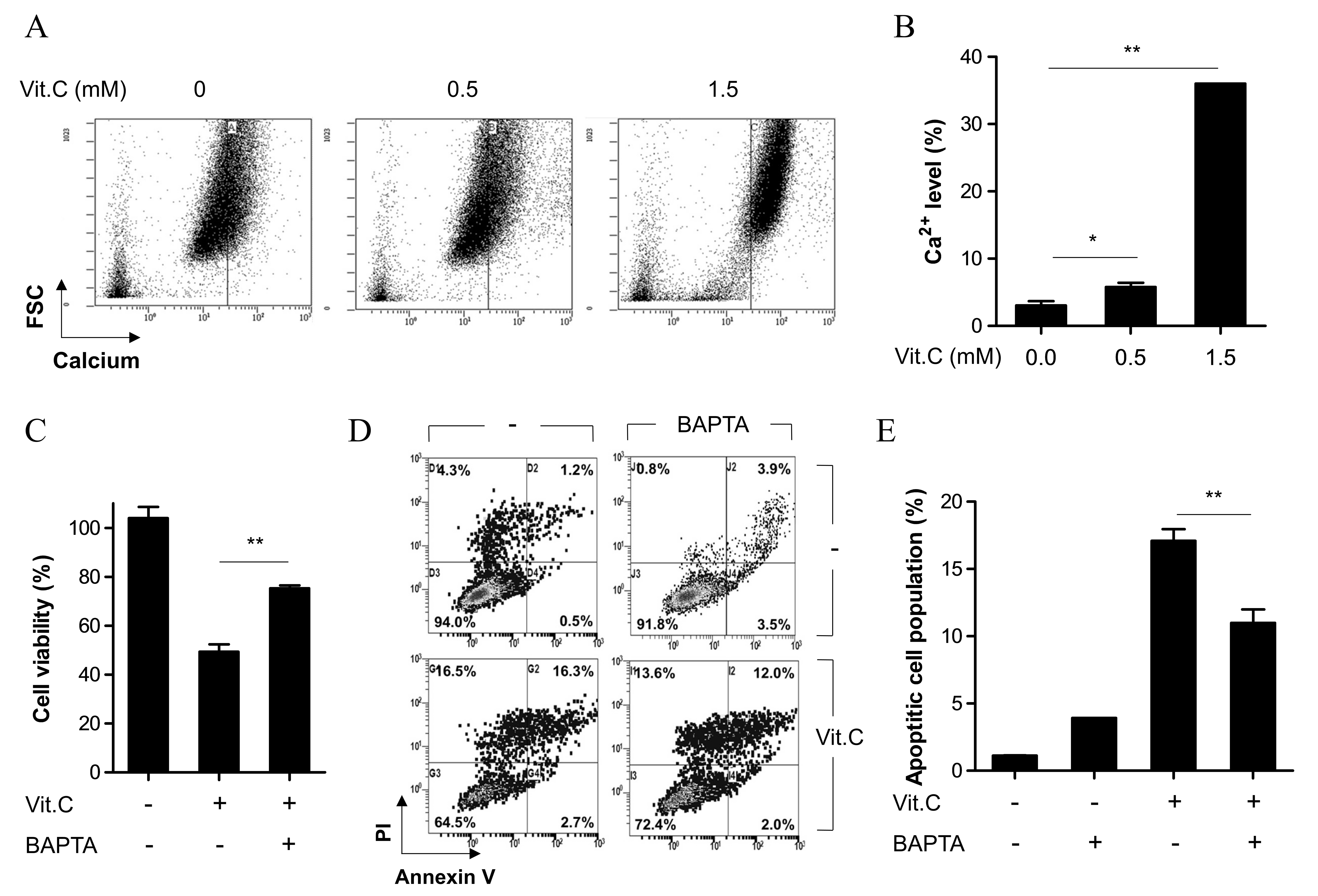 | Figure 3.Calcium concentrations increased by
Vit. C serve a role in AGS cell apoptosis. AGS cells were treated
with 0.5 or 1.5 mM Vit. C for 4 h. Cells were subsequently treated
with Fluo-3, a fluorescent calcium indicator, and analyzed using
flow cytometry. (A) Representative plots of intracellular calcium
analysis are shown. (B) The calcium-positive cell population is
shown as the mean ± standard deviation. AGS cells were pretreated
with 10 µM BAPTA, and 1 h later, 1.5 mM Vit. C was added to the
cells. Following 4 h of treatment with Vit. C, cell viability and
apoptotic cell population were analyzed by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
and by annexin V/PI staining, respectively. (C) Cell viability is
shown as the mean ± standard deviation. (D) Representative plots of
apoptotic cell analysis are shown. (E) The ratio of apoptotic cell
population is shown as the mean ± standard deviation. *P<0.05,
**P<0.01. Vit., vitamin; FSC, forward scatter; PI, propidium
iodide; BAPTA, 1,2-bis (2-aminophenoxy) ethane
N,N,N',N'-tetraacetic acid. |
Enhanced generation of ROS is
indispensable for AGS cell apoptosis induced by vitamin C
Several studies have reported that vitamin C has
numerous anti-oxidative activities, whereas other studies argue its
pro-oxidative properties (4,15,16). To
determine whether the apoptotic activity of vitamin C in the AGS
cells is associated with its anti-oxidant or pro-oxidant
properties, the amount of ROS in the cells was measured. When AGS
cells were treated with 0.5 and 1.5 mM vitamin C, ROS levels
increased by 13.56 and 22.97%, respectively (Fig. 4A and B). To examine whether enhanced
ROS generation contributes to the cytotoxic activity of vitamin C,
AGS cells were pretreated with the antioxidant NAC prior to the
addition of vitamin C. NAC pretreatment significantly reduced the
rate of vitamin C-induced cell death (Fig. 4C). In addition, NAC pretreatment
markedly suppressed the late apoptosis induced by vitamin C to
1.29% (Fig. 4D and F). These data
suggest that the generation of intracellular ROS by vitamin C
treatment is involved in the induction of apoptosis. Therefore, the
apoptotic activity of vitamin C is associated with its pro-oxidant
properties.
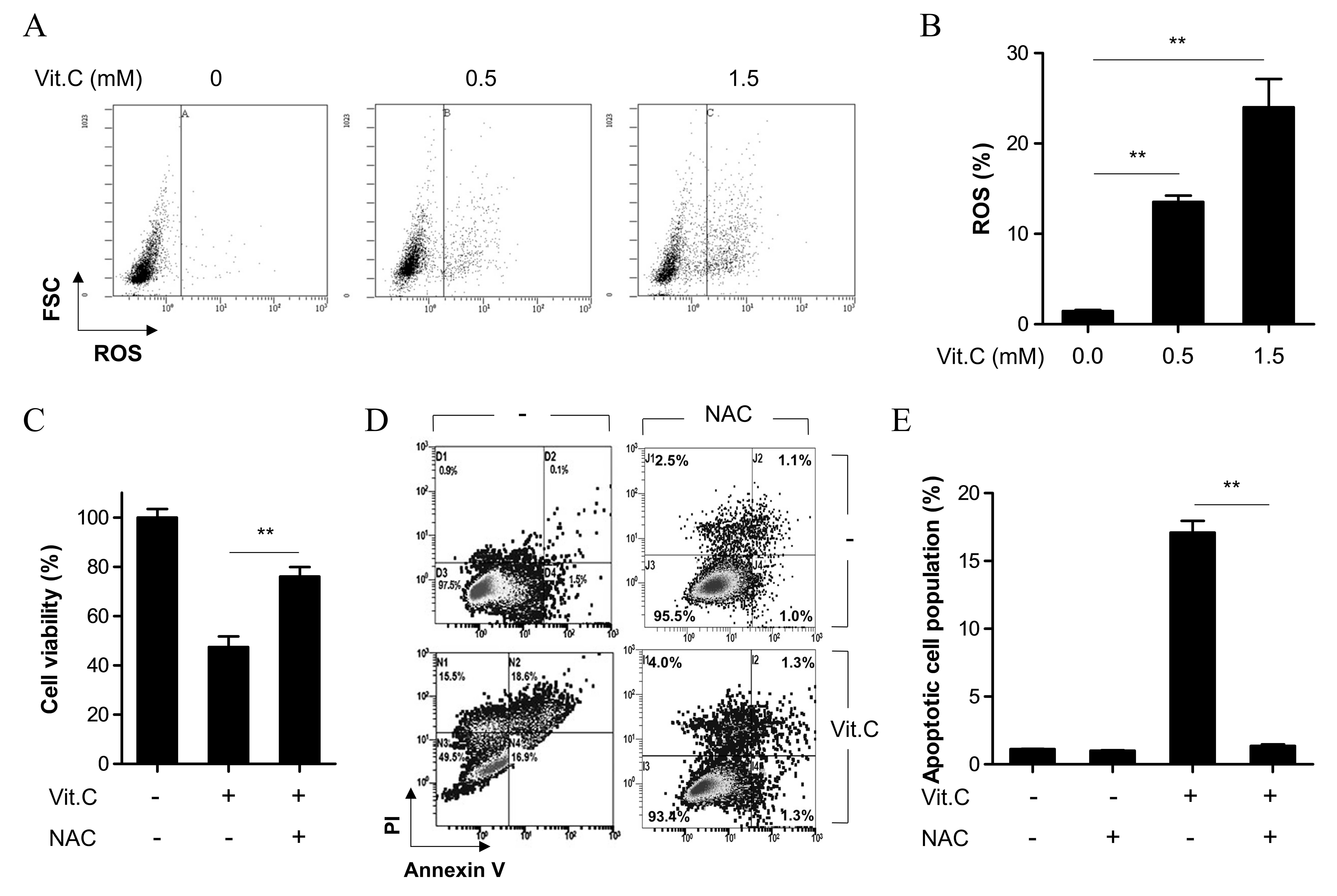 | Figure 4.Vit. C increases the levels of ROS,
which is critical in the apoptosis of AGS cells. AGS cells were
treated with 0.5 or 1.5 mM Vit. C for 4 h and the levels of
intracellular ROS were analyzed using a ROS detection kit and flow
cytometry. (A) Representative plots of intracellular ROS analysis
are shown. (B) The ratio of ROS-positive cell population is shown
as the mean ± standard deviation. AGS cells were pretreated with 5
mM NAC, and 1 h later, 1.5 mM Vit. C was added to the cells.
Following 4 h of Vit. C treatment, the cell viability and apoptotic
cell population were analyzed by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
and by annexin V/PI staining, respectively. (C) The cell viability
is shown as the mean ± standard deviation. (D) Representative plots
of apoptotic cell analysis are shown. (E) The ratio of apoptotic
cell population is shown as the mean ± standard deviation.
**P<0.01. PI, propidium iodide; Vit., vitamin; FSC, forward
scatter; ROS, reactive oxygen species; NAC, N-acetylcysteine. |
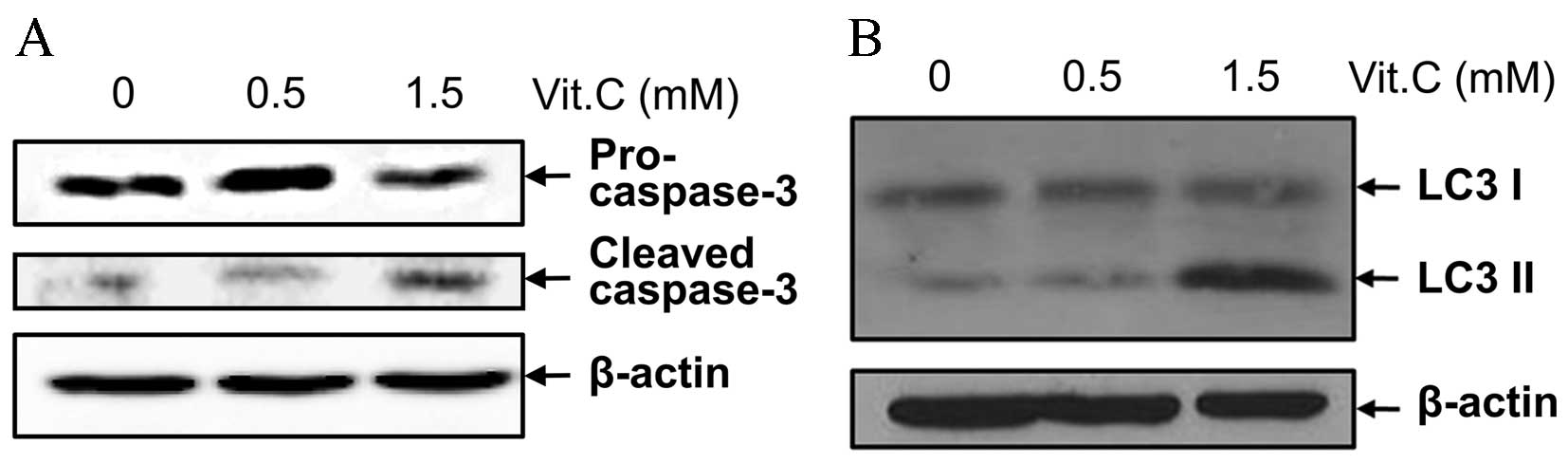
Apoptosis, autophagy and mitochondrial
dysfunction are involved in the cell death process induced by
vitamin C
To identify the molecular mechanisms underlying
vitamin C-induced cell death, the expression of pro-caspase-3 and
its cleaved form, which are indicators for apoptosis (5), as well as that of two types of the
microtubule-associated protein LC3 (LC3 I and LC3 II), which are
indicators for the autophagy pathway (17), were assessed. Although there were no
differences in the expression of any of the above proteins at low
doses of vitamin C, higher concentrations of vitamin C increased
the cleavage of caspase-3 and increased LC3 II expression (Fig. 5A and B). These results support that
apoptosis may be the primary mechanism by which vitamin C-induced
cell death occurs, and that autophagy may be associated with the
apoptosis pathway.
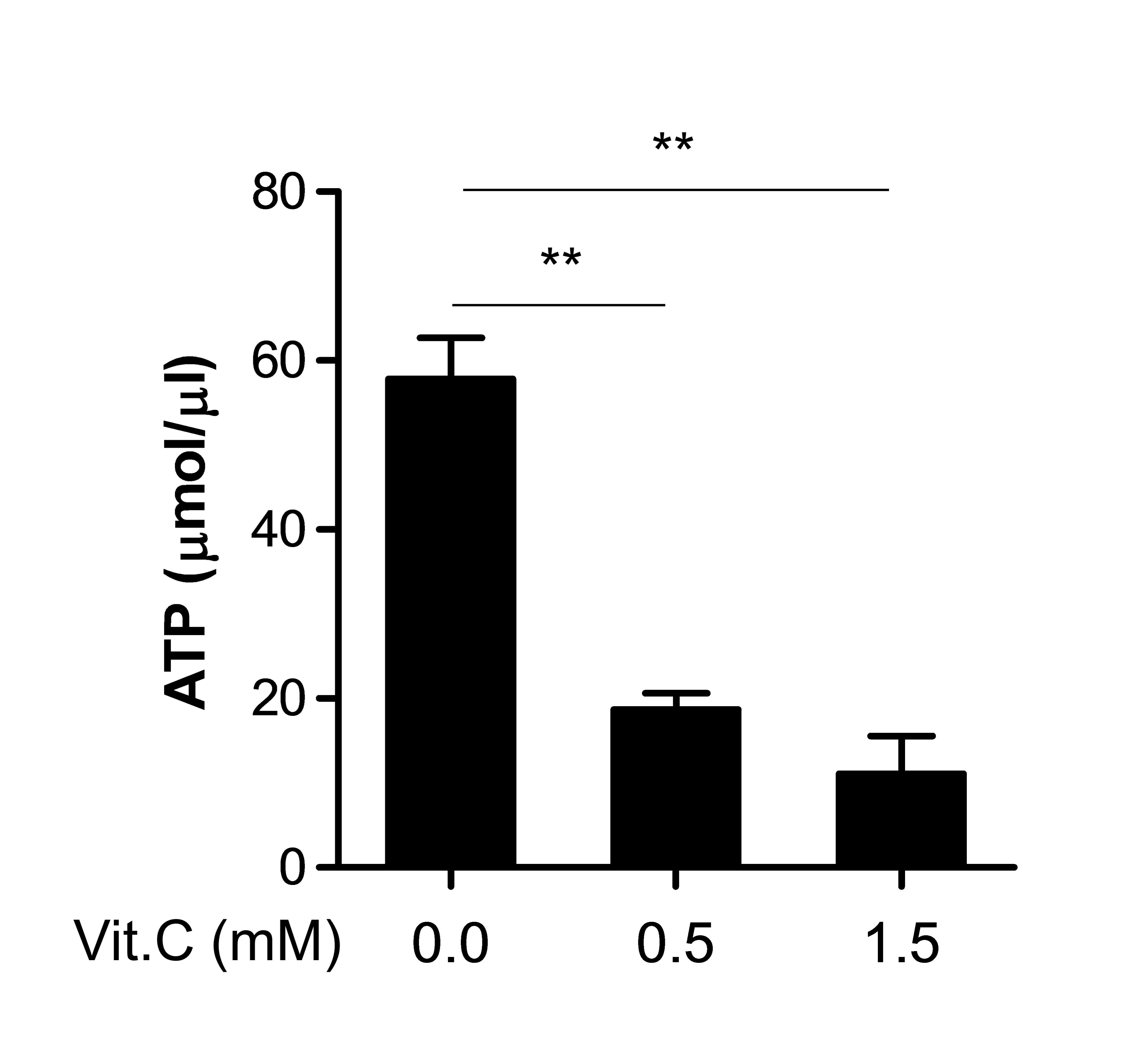
Figs. 3 and 4 demonstrate that vitamin C modulated
certain factors associated with the mitochondrial function,
including intracellular Ca2+ and ROS (18). Therefore, other mitochondrial
function, ATP production (18), was
additionally examined. ATP generation in the cells was markedly
decreased by vitamin C in a dose-dependent manner (Fig. 6), indicating that vitamin C may cause
overall mitochondrial dysfunction as well as apoptosis
induction.
Discussion
The clinical efficacy of vitamin C treatment in
patients with cancer is controversial (4). However, several studies have reported
that plasma concentrations of vitamin C in humans following
high-dose intravenous injection are 5–15 mM, while those achieved
following oral administration are limited to 0.15–0.20 mM (4,19,20). In the majority of cancer cell lines,
concentrations of vitamin C <5 mM cause 50% cell death, however
concentrations >20 mM are nontoxic in normal cells (4). Notably, in the present study, the
EC50 of vitamin C in AGC cells was 1.0–1.3 mM. These
values have important clinical implications, as the concentrations
are in the range achieved following intravenous injection of
high-dose vitamin C (4). In addition,
it was demonstrated that intravenous injection of vitamin C has
remarkably few side effects in a study of >20,000 patients over
2 years (20). Therefore, the use of
high-dose vitamin C treatment in cancer therapy should be
re-evaluated.
The ER and mitochondria are important intracellular
organelles in the TNFR-independent apoptosis pathway (14). Excess calcium that has escaped from
the ER may enhance the production of ROS from mitochondria, and the
increased mitochondrial generation of Ca2+ ions and ROS
synergistically may lead to the alteration of the mitochondrial
permeability transition pore, resulting in cell death (13,14). In
addition, previous studies have reported that a high dose of
vitamin C increases the susceptibility of cancer cells to apoptosis
by activating mitochondrial 14-3-3σ and 14-3-3β (21,22). In
line with these reports, the present study demonstrated that a high
dose of vitamin C increases the levels of intracellular
Ca2+ and ROS, and decreases the production of
intracellular ATP, which is mainly produced by mitochondria. This
indicates that mitochondrial dysfunction may be an important
process in the vitamin C-induced cell death of AGS cells.
Furthermore, the results from the current study
indicated that the expression of the autophagy indicator protein
LC3 II was markedly increased following treatment with high-dose
vitamin C. It has been demonstrated that autophagy is involved in
the turnover of unnecessary proteins and whole organelles, and is
therefore predominantly a cytoprotective process to help maintain
the healthy condition of normal cells (6). However, excessive activation of
autophagy under certain circumstances is linked to mechanisms of
cell death (6). Thus, the vitamin
C-induced autophagy in the present study may be linked to
apoptosis, but not to necrosis or cytoprotective processes. Future
studies are required to examine the differences between autophagy
induction in healthy cells and in those treated with vitamin C.
In conclusion, the present study demonstrated that
vitamin C is able to induce apoptosis in human gastric cancer cells
in a caspase-dependent manner. This process involves mitochondrial
dysregulation via intracellular Ca2+ efflux from ER, ROS
generation from mitochondria and decreased levels of ATP.
Additionally, the effective dose of vitamin C for inducing
apoptotic cell death in humans is achievable by intravenous
injection of high-dose vitamin C. Therefore, high-dose vitamin C
treatment may be developed in the future as a novel effective
therapeutic strategy for patients with gastric cancer.
Acknowledgements
The present study was supported by a grant from the
National Research and Development Program for Cancer Control,
Ministry of Health and Welfare, Republic of Korea (Seoul, Korea;
grant no. 0820050).
References
|
1
|
Shin A, Kim J and Park S: Gastric cancer
epidemiology in Korea. J Gastric Cancer. 11:135–140. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lordick F, Allum W, Carneiro F, Mitry E,
Tabernero J, Tan P, Van Cutsem E, van de Velde C and Cervantes A:
Unmet needs and challenges in gastric cancer: The way forward.
Cancer Treat Rev. 40:692–700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Levine M, Dhariwal KR, Welch RW, Wang Y
and Park JB: Determination of optimal vitamin C requirements in
humans. Am J Clin Nutr. 62:(6 Suppl). 1347S–1356S. 1995.PubMed/NCBI
|
|
4
|
Ohno S, Ohno Y, Suzuki N, Soma G and Inoue
M: High-dose vitamin C (ascorbic acid) therapy in the treatment of
patients with advanced cancer. Anticancer Res. 29:809–815.
2009.PubMed/NCBI
|
|
5
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nikoletopoulou V, Markaki M, Palikaras K
and Tavernarakis N: Crosstalk between apoptosis, necrosis and
autophagy. Biochim Biophys Acta. 1833:3448–3459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ha YM, Park MK, Kim HJ, Seo HG, Lee JH and
Chang KC: High concentrations of ascorbic acid induces apoptosis of
human gastric cancer cell by p38-MAP kinase-dependent up-regulation
of transferrin receptor. Cancer Lett. 277:48–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Simon F, Varela D, Riveros A, Eguiguren AL
and Stutzin A: Non-selective cation channels and oxidative
stress-induced cell swelling. Biol Res. 35:215–222. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee GW, Park HS, Kim EJ, Cho YW, Kim GT,
Mun YJ, Choi EJ, Lee JS, Han J and Kang D: Reduction of breast
cancer cell migration via up-regulation of TASK-3 two-pore domain
K+ channel. Acta Physiol (Oxf). 204:513–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakamura TY, Yamamoto I, Nishitani H,
Matozaki T, Suzuki T, Wakabayashi S, Shigekawa M and Goshima K:
Detachment of cultured cells from the substratum induced by the
neutrophil-derived oxidant NH2Cl: Synergistic role of
phosphotyrosine and intracellular Ca2+ concentration. J
Cell Biol. 131:509–524. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Northington FJ, Chavez-Valdez R, Graham
EM, Razdan S, Gauda EB and Martin LJ: Necrostatin decreases
oxidative damage, inflammation, and injury after neonatal HI. J
Cereb Blood Flow Metab. 31:178–189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pinton P, Giorgi C, Siviero R, Zecchini E
and Rizzuto R: Calcium and apoptosis: ER-mitochondria
Ca2+ transfer in the control of apoptosis. Oncogene.
27:6407–6418. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lemasters JJ, Theruvath TP, Zhong Z and
Nieminen AL: Mitochondrial calcium and the permeability transition
in cell death. Biochim Biophys Acta. 1787:1395–1401. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bravo-Sagua R, Rodriguez AE, Kuzmicic J,
Gutierrez T, Lopez-Crisosto C, Quiroga C, Díaz-Elizondo J, Chiong
M, Gillette TG, Rothermel BA and Lavandero S: Cell death and
survival through the endoplasmic reticulum- mitochondrial axis.
Curr Mol Med. 13:317–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carr A and Frei B: Does vitamin C act as a
pro-oxidant under physiological conditions? FASEB J. 13:1007–1024.
1999.PubMed/NCBI
|
|
16
|
Padayatty SJ, Katz A, Wang Y, Eck P, Kwon
O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK and Levine M: Vitamin
C as an antioxidant: Evaluation of its role in disease prevention.
J Am Coll Nutr. 22:18–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mizushima N and Yoshimori T: How to
interpret LC3 immunoblotting. Autophagy. 3:542–545. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Csordás G and Hajnóczky G:
SR/ER-mitochondrial local communication: Calcium and ROS. Biochim
Biophys Acta. 1787:1352–1362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levine M, Conry-Cantilena C, Wang Y, Welch
RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King
J and Cantilena LR: Vitamin C pharmacokinetics in healthy
volunteers: Evidence for a recommended dietary allowance. Proc Natl
Acad Sci USA. 93:3704–3709. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Padayatty SJ, Sun AY, Chen Q, Espey MG,
Drisko J and Levine M: Vitamin C: Intravenous use by complementary
and alternative medicine practitioners and adverse effects. PloS
One. 5:e114142010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagappan A, Park KI, Park HS, Kim JA, Hong
GE, Kang SR, Lee DH, Kim EH, Lee WS, Won CK and Kim GS: Vitamin C
induces apoptosis in AGS cells by down-regulation of 14-3-3σ via a
mitochondrial dependent pathway. Food Chem. 135:1920–1928. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim JE, Kang JS and Lee WJ: Vitamin C
induces apoptosis in human colon cancer cell line, HCT-8 via the
modulation of calcium influx in endoplasmic reticulum and the
dissociation of bad from 14-3-3beta. Immune Netw. 12:189–195. 2012.
View Article : Google Scholar : PubMed/NCBI
|