Introduction
Glioblastoma multiforme (GBM) is an epithelial tumor
of the central nervous system, which most commonly presents as a
solitary lesion: The occurrence of multiple lesions is rare
(1). GBM is most commonly occurs in
individuals aged between 45 and 70 years old, with a worldwide
incidence of 1–10% (1–3). The most common clinical symptom of GBM
is epilepsy (1). Magnetic resonance
imaging (MRI) is the primary diagnostic tool for GBM (4). Tumors involving the corpus callosum,
which grow bilaterally into occipital and temporal lobes, result in
a butterfly pattern on MRI, termed ‘butterfly glioma’ (5). Definitive diagnosis is based on
histopathological examination of intraoperatively removed
tumors/tumor sections. Morphological diagnosis is based on criteria
defined by the World Health Organization (6,7). Surgical
resection is recommended if feasible, followed by chemotherapy and
radiotherapy, which is the standard treatment for GBM (8). GBM exhibits a highly unfavorable
prognosis; The majority of patients only survive for 12–15 months
following disease onset (9). Previous
in vivo studies of GBM have focused on the etiology,
pathology, clinical symptoms, imaging features, treatment and
prognosis of the disease (1–3). In vitro studies of GBM cell
proliferation have also been performed (10,11),
however, the mechanism of disease progression and its corresponding
MRI features remain unclear. In this study, a case of rapidly
progressing GBM is presented, and the findings of MRI, surgical and
pathological examinations are discussed. Written informed consent
was obtained from the patient.
Case report
In April 2010, a 60-year-old male patient with
mental confusion was admitted to The First Affiliated Hospital
(Hangzhou, China) after experiencing convulsions for 1 day.
Physical examination revealed that the pupil size of both eyes was
equal; however, the pupillary light reflex was slow. In addition,
high muscular tension of the limbs was observed and positive
bilateral Babinski signs were identified. Blood examinations
revealed a leukocyte count of 12.0×109/l and a
neutrophil granulocyte level of 92.6%. Kidney function and blood
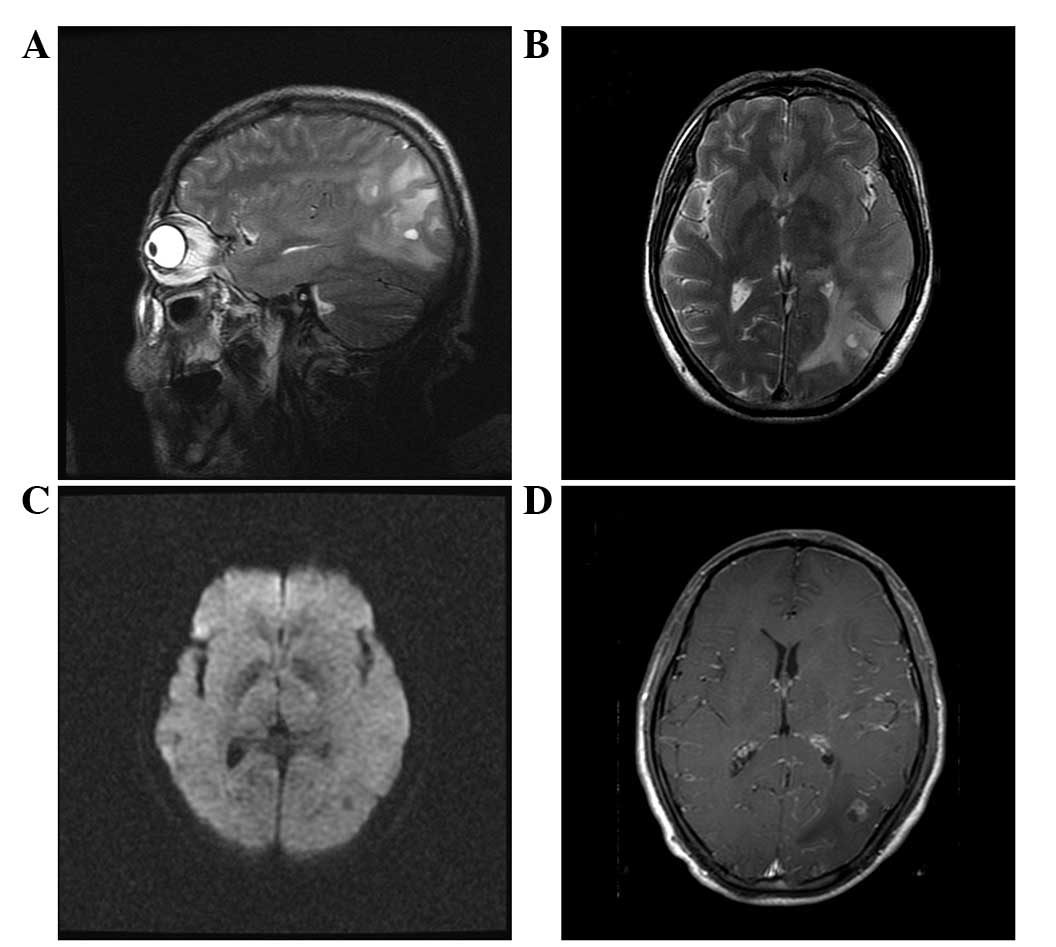
gas composition were normal. MRI examination revealed abnormal
signals from the temporal-occipital-parietal lobes and thus
metastatic tumors were initially suspected and the possibility of a
brain abscess was excluded (Fig. 1).
The patient reported no history of disease, however, he had
experienced head trauma with basal fracture 10 years previously.
Serum tumor markers, including carcinoembryonic antigen,
α-fetoprotein, carbohydrate antigen 199 and 125, total prostate
specific antigen and ferritin were normal. Furthermore, erythrocyte
sedimentation rate was normal and serum was negative for
antinuclear (cat. no. FA 1510-1003-1; 1:10; EUROIMMUN AG, Luebeck,
germany) and antineutrophil cytoplasmic (cat. no. FA 1200-1003;
1:10; EUROIMMUN AG) antibodies.
Cerebrospinal fluid (CSF) pressure was 180
mmH2O (normal range, 80–180 mmH2O) with
transparency and no blood cells. Biochemical analysis of the CSF
revealed a protein concentration of 0.49 g/l (normal range,
0.15–0.45 g/l) and carcinoembryonic antigen levels of <0.50
ng/ml (normal range, <0.573 ng/ml). Glucose and chloride levels
were within the normal ranges. Computed tomography (CT) images of
the lung were normal. Electroencephalogram demonstrated a slow
spike wave in the left hemisphere in addition to an epileptic
lesion in the left central parietal lobe.
Subsequently, the patient was administered mannitol
(125 ml, every 12 h for 26 days) to lower intracranial pressure,
diazepam (10 mg, daily for 3 days), Tegretol (0.2 g, twice daily
for 26 days) to treat the symptoms of epilepsy and ceftriaxone (2.0
g, daily for 9 days) for infection control. After treatment, the
patient recovered and regained full consciousness with a normal
body temperature and was able to eat and walk without the
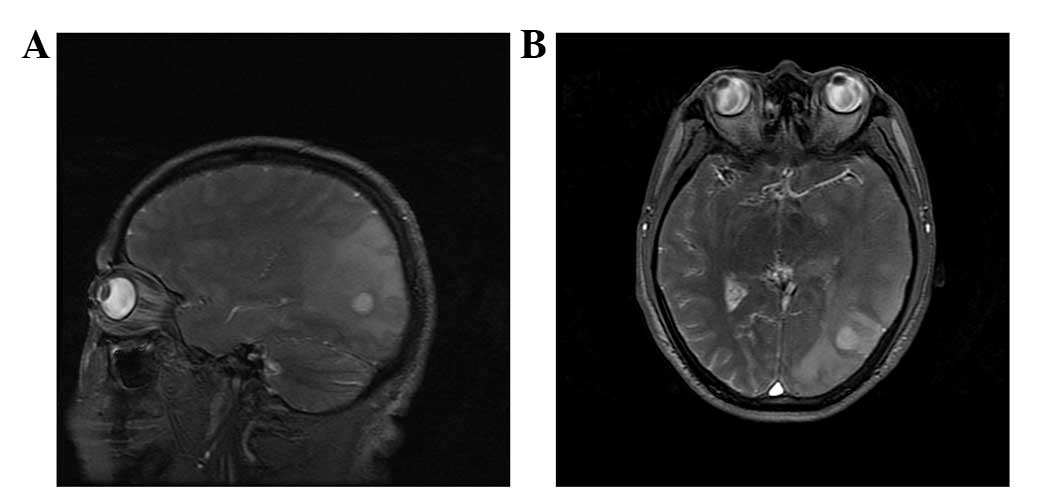
occurrence of seizures. MRI examination performed 12 days after
hospitalization revealed multiple lesions between the left temporal
and left occipital lobes, which had increased in size since the
initial MRI examination, and thus parasitic infection could not be
ruled out (Fig. 2). The spirometra
mansoni test was positive. Therefore, the patient remained
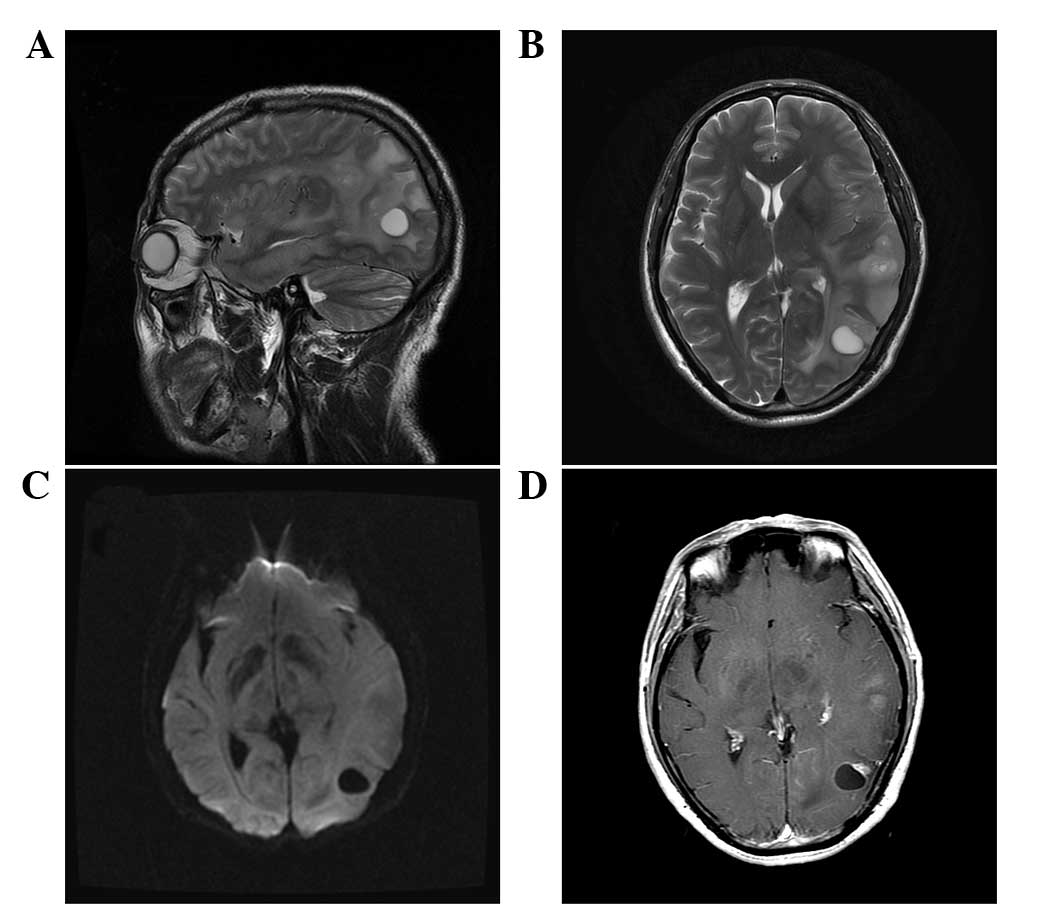
hospitalized to treat the infection. MRI examination 23 days after
hospitalization revealed multiple lesions, which had markedly
increased in size and number when compared with the previous MRI
findings. Subsequently, GBM was suspected (Fig. 3) and resection of tumor in the left
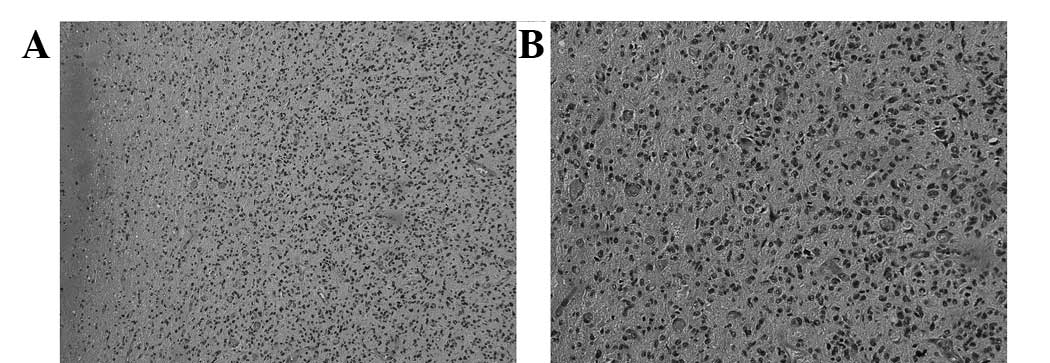
temporal occipital lobe was performed. Histopathological
examination identified GBM [World Health Organization grade IV
(12)] in the left temporal and
parietal lobes (Fig. 4). No further
treatment was administered. Seven months after surgery, the patient
experienced a sudden headache and dysfunction of the extremities.
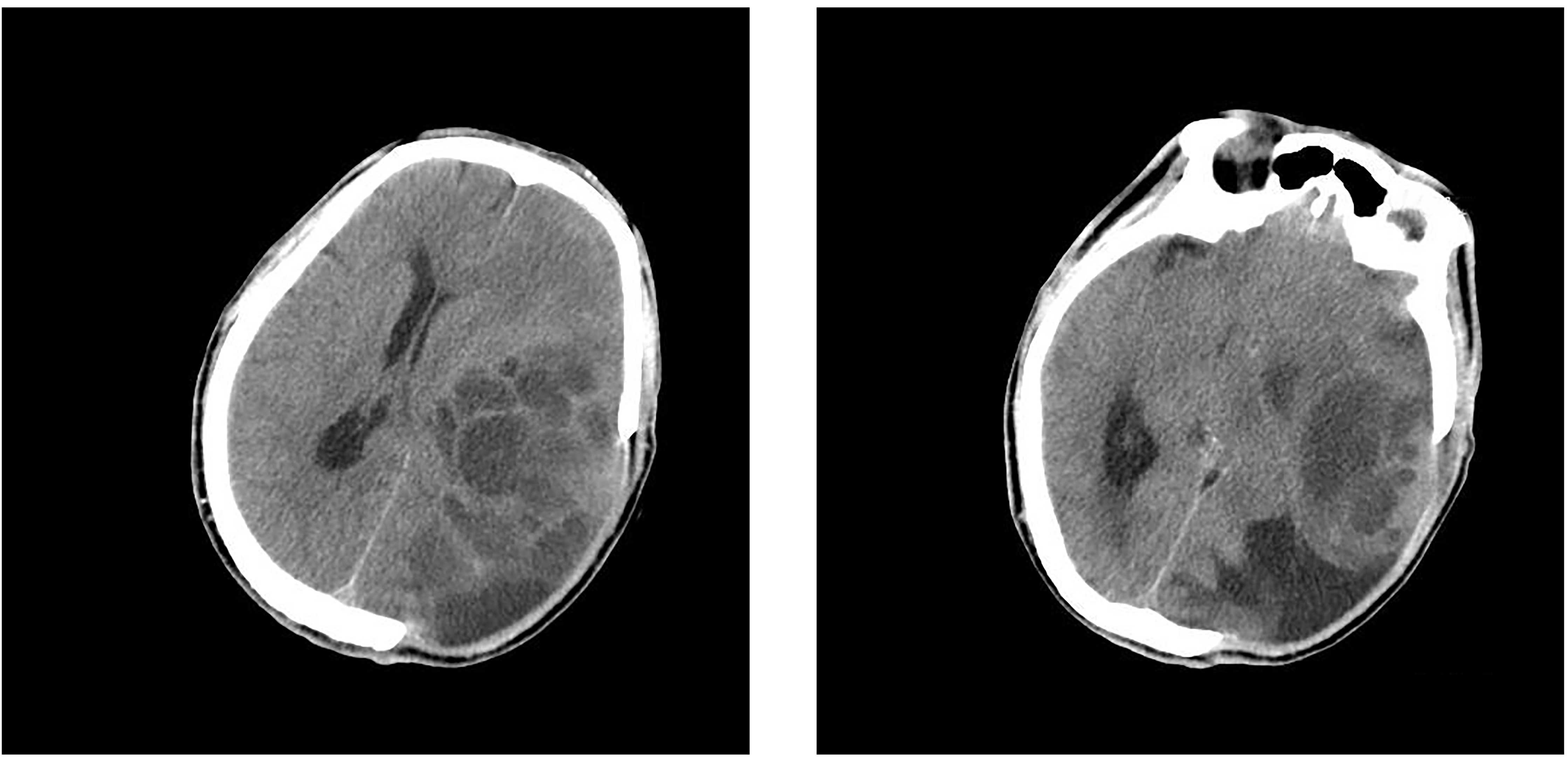
CT of the head revealed GBM recurrence (Fig. 5). The patient was subsequently
administered mannitol treatment, however he succumbed to the
disease in January 2011.
Discussion
GBM [World Health Organization stage IV (12)] is one of the most malignant tumors
worldwide. The first case of GBM was reported by Bradley in 1880
(13). At present, the incidence rate
of GBM is 1–10% (1,13). Due to the high grade of malignancy,
GBM exhibits a highly unfavorable prognosis; some patients
succumbed within 3 months of disease onset (14). GBM exhibits no specific clinical,
imaging or histological characteristics. Depending on the
localization and progression of GBM, the clinical symptoms may
include headache, ataxia, dizziness, visual disturbances and
frequent syncope (15,16). The occurrence of seizures in patients
who have not been previously diagnosed with epilepsy may also be an
indication for neuroimaging due to suspected GBM (2). Due to the lack of specific symptoms, GBM
is often misdiagnosed as an infection, inflammatory process or
circulatory and immunological diseases (15). The patient in the present case was
hospitalized due to the occurrence of seizures.
GBM tumors develop as a result of infiltrative
growth via nerve fiber pathways or metastatic spread via CSF,
therefore satellite lesions form in adjacent areas of the brain
(1,13). Multiple lesions were identified in the
present case and we hypothesize that the lesions located near the
sulci may have been caused by local spread. GBM develops as a
result of infiltrative growth, which increases intracranial
pressure and subsequently may lead to hydrocephaly (3). The inferior horn of the lateral
ventricles of the left hemisphere was evidently compressed in the
present case. The aggravation of clinical symptoms may be
associated with the rapid growth of glioblastoma, which damages
local tissue structure and increases the position effect causing
impaired brain function.
MRI is the primary diagnostic tool for GBM. Simpson
et al (8) evaluated the MRI
findings of 645 GBM patients and reported that in 38, 56 and 6% of
patients, the tumor diameter at diagnosis was <5, 5–10 and
>10 cm, respectively. The high proliferation rate of GBM causes
rapid tumor growth, and a previous study reported that the tumor
cell doubling time for GBM ranges between 2 days and several weeks
(10). It is hypothesized that tumor
cell doubling time may predict survival time (10,14). The
median survival time following diagnosis for patients with GBM is
9.8 days (10). To the best of our
knowledge, no in vivo studies have investigated the imaging
features that are associated with GBM progression. In the present
case, the initial MRI examination indicated that the maximum
diameter of the lesion on the left temporal-occipital lobe was ~7
mm with cystic and enhanced solid sections. MRI performed 12 days
after hospitalization revealed that the lesion had increased to 13
mm in diameter. The lesion exhibited revealed homogenous low
signals on diffusion-weighted images, however, the peri-tumor edema
range remained unchanged. MRI performed 23 days after
hospitalization revealed that the diameter of the lesion had
increased to 17 mm in the cystic sections and the solid sections
exhibited enhancement. The rapid increase in lesion number and size
demonstrated by MRI imaging in the present case was in accordance
with a previous in vitro study of GBM cell culture (10). Thus, the rapid morphological
progression of cystic lesion sections may indicate GBM. In the
meanwhile, the enlarged GBM is mainly from the cystic parts rather
than solid parts might attribute to the necrotic features of GBM
(17). In the present study,
diagnosis of GBM was uncertain in the early stages due to the
positive results obtained from the spirometra mansoni test.
However, histopathological examination following surgery confirmed
the diagnosis of GBM. In cases where a tumor is suspected and
resection procedures are limited, fine needle biopsy is recommended
to minimize trauma (18). The present
case revealed that GBM may progress rapidly with a doubling time of
10 days and multiple cystic alterations. If diagnosis of GBM is
unclear, early biopsy is recommended.
References
|
1
|
Salvati M, Caroli E, Orlando ER, Frati A,
Artizzu S and Ferrante L: Multicentric glioma: Our experience in 25
patients and critical review of the literature. Neurosurg Rev.
26:275–279. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sanli AM, Turkoglu E, Dolgun H and Sekerci
Z: Unusual manifestations of primary Glioblastoma Multiforme: A
report of three cases. Surg Neurol Int. 1:872010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Castro-Costa CM, de Araújo RW, de
Arruda MA, de Araújo PM and de Figueiredo EG: Increased
intracranial pressure in a case of spinal cervical glioblastoma
multiforme. Analysis of these two rare conditions. Arq
Neuropsiquiatr. 52:64–68. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ulutin C, Fayda M, Aksu G, Cetinayak O,
Kuzhan O, Ors F and Beyzadeoglu M: Primary glioblastoma multiforme
in younger patients: A single-instruction experience. Tumori.
92:407–411. 2006.PubMed/NCBI
|
|
5
|
Agrawal A: Butterfly glioma of the corpus
callosum. J Cancer Res Ther. 5:43–45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Louis DN, Ohgaki H, Wiestler OD and
Cavenee WK: WHO Classification of Tumours of the Central Nervous
System. 1. 4th. IARC Press; Lyon: 2007
|
|
7
|
Zülch KJ: Histological Typing of Tumours
of the Central Nervous System. World Health Organization; Geneva:
1979
|
|
8
|
Simpson JR, Horton J, Scott C, Curran WJ,
Rubin P, Fischbach J, Isaacson S, Rotman M, Asbell SO, Nelson JS,
et al: Influence of location and extent of surgical resection on
survival of patients with glioblastoma multiforme: Results of three
consecutive Radiation Therapy Oncology Group (RTOG) clinical
trials. Int J Radiat Oncol Biol Phys. 26:239–244. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Furneaux CE, Marshall ES, Yeoh K, Monteith
SJ, Mews PJ, Sansur CA, Oskouian RJ, Sharples KJ and Baguley BC:
Cell cycle times of short-term cultures of brain cancers as
predictors of survival. Br J Cancer. 99:1678–1683. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu J, Rizak JD, Fan Y, Guo X, Li J, Huma T
and Ma Y: Establishment and partial characterization of a human
tumor cell line, GBM-HSF, from a glioblastoma multiforme. Human
Cell. 27:129–136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fuller GN and Scheithauer BW: The 2007
Revised World Health Organization (WHO) Classification of Tumours
of the Central Nervous System: newly codified entities. Brain
Pathol. 17:304–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bradley WL: Case of gliosarcomatous tumors
of the brain. Proc Con Med Soc. 2:39–41. 1880.
|
|
14
|
Cvetkoivč-Dožič D, Skender-Gazibara M and
Dožič S: Morphological and molecular features of diffuse
infiltrating astrocytoma. Arch Oncol. 12:38–39. 2004.
|
|
15
|
Lakhan SE and Harle L: Difficult diagnosis
of brainstem glioblastoma multiforme in a woman: A case report and
review of the literature. J Med Case Rep. 3:872009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Levine SA, McKeever PE and Greenberg HS:
Primary cerebellar glioblastoma multiforme. J Neurooncol.
5:231–236. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kleihues P, Burger PC, Collins VP and
Cavenee WK: World Health Organization Classification of
TumoursPathology and Genetics of Tumours of the Nervous System.
IARC Press; Lyon: 2000
|
|
18
|
Katsetos CD, Dráberová E, Legido A and
Dráber P: Tubulin targets in the pathobiology and therapy of
glioblastoma multiforme. II. gamma-Tubulin. J Cell Physiol.
221:514–520. 2009. View Article : Google Scholar : PubMed/NCBI
|