Introduction
Cadherins belong to a family of transmembraneous
adhesion molecules that are important in maintaining cell polarity
and tissue integrity (1). They can
mediate calcium-dependent cell-to-cell adhesion by interacting with
the cytoplasmatic catenins, α, β, and γ (2,3). The
catenins link cadherins to the actin cytoskeleton, but also have
signaling functions of their own. Over the years, >20 types of
cadherins have been identified and characterized, including the
original E-, P- and N-cadherin (Type I), and cadherins 5 to 12
(Type II) (1–4). While the two subgroups share structural
similarities, they exhibit surprisingly little sequence homology.
Cadherins are involved in normal mammary gland development and
function, and they appear to influence breast cancer and its
clinical outcome (1,3–6). Berx and
van Roy (7) reviewed the role of
cadherins in malignant disease, and it has been demonstrated that
loss of E-cadherin expression was associated with increased
invasiveness and decreased differentiation. Interestingly,
re-induction of E-cadherin in invasive breast cancer cells did not
result in a less aggressive behavior in vitro, thereby
suggesting that E-cadherin is rather an indicator of a more
invasive phenotype than a causative factor (3,5,8).
Cadherin-11, also known as OB-cadherin was first
identified in mouse osteoblasts and is normally expressed in cells
with a mesenchymal phenotype, including the mesenchyme of the
kidney and brain during development (8,9).
Cadherin-11 is also expressed in cartilage synoviocytes and is an
important mediator of the synoviocyte reaction that characterizes
rheumatoid arthritis (10). In the
adult, cadherin-11 is strongly expressed in bone as well as certain
cancers that metastasize to bone (11). While the exact expression profile of
cadherin-11 in healthy mammary gland is not known, it has been
shown that it interacts with the fibroblast growth factor (FGF)
signaling pathway (2,8,12), and
thus modulates the response to growth factors. Cadherin-11 is
typically expressed in many types of condensing mesenchyme and when
expressed in epithelium, EMT is thought to have occurred (12–17). It
may also provide the cell with an ability to establish itself into
the bone environment (5,11). The majority of patients that succumb
to breast (or prostate) cancer have metastases to the skeleton. It
is possible that these cadherin-11-expressing tumor cells activate
either osteoclasts or osteoblasts, depending on the type of cancer
metastasis, leading to bone remodeling (11).
While the precise role of cadherins in cancer
remains unclear, they are important in the basic events and
processes in breast cancer tumorigenesis (4,12). Several
events in tumorigenesis are strongly connected to changes in
cadherin expression (14–18). One example is cadherin switching,
where cadherins change from those expressed in epithelial cells to
those predominant in mesenchymal cells (8). This event is part of a process that is
vital to malignant change, the epithelial-mesenchymal transition
(EMT). EMT is a key biologic process that was initially identified
as a developmental program that enables polarized epithelial cells
to acquire a motile mesenchymal phenotype (13–17,19,20).
This transition results in a more invasive and metastatic phenotype
(15–17,19).
Research suggests that cadherin switching is required for increased
motility but not for the morphological changes that accompany EMT
(13,19). The reverse process of EMT, the
mesenchymal-epithelial transition (MET) involves the conversion of
mesenchymal cells to their epithelial derivatives (14). In carcinoma progression, reactivation
of the EMT program promotes tumor metastasis by driving tumor cell
invasion and enhancing tumor cell survival (16,17). These
changes are highly dynamic and many intermediate phenotypes exist.
Dubois-Marshall et al (16)
described two distinct possible mechanisms of EMT arising in breast
cancer, one of which is uncoupled from cadherin switching. Some
difficulty lies in the fact that it is not yet fully understood how
cadherins expression profiles change in EMT. This emanates from the
fact that there are multiple ways to regulate cadherin expression,
and many, but not all of these overlap (2). Recently, cadherins were demonstrated to
regulate stem cell maintenance and differentiation (20). The use of mesenchymal stem cells for
tissue repair requires the migration and homing to the site of
damaged tissue and it has been shown that both the migratory and
proliferation potential of these cells are affected by cadherin-2
and cadherin-11 (20).
Pishvaian et al (6) have demonstrated that cadherin-11 mRNA
and protein, as well as a cadherin-11 variant mRNA are expressed in
invasive and poorly differentiated breast cancer cell lines. In
these cells, cadherin-11 is localized to the cell membrane in a
detergent-soluble complex, where it associates with α and
β-catenin, and may facilitate tumor cell invasion and metastasis.
Assefnia et al and Dakshanamurthy et al (21,22)
demonstrated that cadherin-11 is increased in early stages of human
breast cancer and in other malignancies. When compared to healthy
breast tissue, cadherin-11 was markedly elevated in DCIS and also
in the stroma of invasive breast cancers compared to normal stroma.
While this seems counter-intuitive at first, it illustrates that
the functional diversity of cadherins in physiological cell is also
reflected in processes connected to malignant disease.
There is paucity of studies evaluating cadherin-11
expression in human invasive breast cancer. The aim of the present
study was to investigate cadherin-11 expression in malignant breast
tissue samples and benign and/or healthy breast tissue samples. The
expression was then correlated with several clinicopathological
parameters.
Materials and methods
Patient samples
Human breast tissue microarray (TMA) slides were
obtained from US Biomax Inc. (Rockville, MD, USA). These TMAs
consists of malignant and benign breast tumors, and healthy breast
tissue adjacent to a malignant tumor or from women undergoing
reduction mammoplasty. Clinicopathological information was
obtained, including age, tumor grade, tumor size and histology.
Hormone receptor status, e.g., estrogen, progesterone and HER2, as
well as cadherin-11 expression were analyzed using
immunohistochemistry (IHC).
Immunohistochemistry for
cadherin-11
Tissue sections of paraffin-embedded formalin-fixed
tissue blocks were deparaffinized with xylene for 5 min each,
followed by two washes with 100% ethanol for 10 min. The slides
were then incubated in 95% ethanol for another 10 min and washed
with dH2O twice for 5 min. Antigen retrieval was
performed by placing slides in 10 mmol/l citrate buffer (pH 6.0)
and microwave treatment for 15 min. Tissue sections were cool down
to room temperature (RT), washed in phosphate-buffered saline (PBS)
and distilled water. Afterwards, sections were blocked with Ultra V
Block (Lab Vision, Westinghouse Drive, Fremont, CA, USA) for 4 min.
After a consecutive PBS wash, slides were incubated with the
monoclonal Mouse anti cadherin-11 IgG2B Clone # 283416 Catalog
Number: MAB1790 (R&D Systems). Negative controls were performed
on all tissue sections by replacing primary antibodies with diluted
isotype immunoglobulin (ImmunoCruz™ Staining system, Santa Cruz
Biotechnology). Then the slides were incubated with goat
anti-polyvalent and streptavidin-HRP (both Lab Vision) for 60 min,
followed by an incubation with 3-amino-9-ethylcarbazole (AEC).
Finally, slides were washed in PBS, counterstained with hematoxylin
for 5 sec and cover-slipped.
Semi-quantitative reverse
transcription polymerase chain reaction (RT-PCR) for
Cadherin-11
A total of 0.5–1 µg of total RNA was extracted from
each of the breast cancer samples, subsequently subjected to DNase
(RNase-Free DNase Set; Qiagen, Hilden, Germany) treatment and then
incubated with 0.5 µg/µl random hexamers (Promega Corp., Madison,
WI, USA). The final volume was adjusted to 5 µl with diethyl
pyrocarbonate-treated double distilled water (DEPC-treated
ddH2O), before being heat-denatured at 70°C for 5 min
and chilled on ice. The samples were then added to a reaction mix
consisting of 4 µl of 5X RT-buffer (250 mM Tris-HCl, pH 8.3, 375 mM
KCl, 15 mM MgCl2), 2 µl dNTP mix stock solution (10 mM
each Pharmacia Biotech, Uppsala, Sweden), 1 µl RNase inhibitor
(Applied Biosystems, Vienna, Austria), 1 µl dithiothreitol (DTT),
and 1 µl MMLV (Moloney murine leukemia virus)-RT (200 U/µl,
Amersham Bioscience Ltd.). The reaction mix was vortexed and
centrifuged briefly before being incubated at 37°C for 1 h. The
reaction was stopped by heating to 80°C for 10 min. The tubes were
chilled briefly on ice before they were centrifuged and stored at
−20°C.
PCR was performed by adding 20 µl reaction mix to
2.5 µl 10X PCR-buffer, 2 µl dNTP mix (10 mM each; New England
Biolabs, Hertfordshire, UK), 0.25 µl primer (100 µM), 5 µl Taq
polymerase (5 U/ml) to the cadherin-11 primers (primer sequences:
Forward, 5′-ACCAGATGTCTGTGTCAGA-3′ and reverse,
3′-GTCATCCTTGTCATCTGCA-5′. The gene GAPDH (primer sequences:
Forward, 5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse,
3′-GAAGATGGTGATGGGATTTC-5′) was used as a reference for
normalization. A total volume of 45 µl was reached by adding
DEPC-treated ddH2O. Cycling conditions were as follows:
Depending on the primers, 25–35 cycles were carried out of 94°C for
1 min, 68°C for 2 min, 72°C for 2 min, with an extension of 5 sec,
with each subsequent cycle. ddH2O was used instead of
total RNA for negative controls. Agarose gel electrophoresis was
performed by adding 20 µl of each of the PCR products and
subjecting them to 1.2% NuSieve® (Lonza Ltd., Basel,
Switzerland) 3:1 agarose gel electrophoresis in 1X TBE buffer,
separated by applying a constant voltage at 80 V for 1–2 h. DNA
bands were then visualized by ethidium bromide, using UV
transilluminator (Syngene®, Cambridge, UK). Band size
was determined by a co-loaded DNA size marker.
Evaluation of immunohistochemical
staining and statistical analysis
Immunostained slides were scored under a microscope
(Olympus BX51; Olympus, Tokyo, Japan). The staining intensity of
hormone receptors was scored according to Remmele et al
(23). The HER2 receptor status has
been evaluated according to standardized assessment (24). Only slides with a IHC 3+ status of
HER2 receptors were categorized as positive. Chi-square and
Student's t-test were used to compare cadherin-11 protein
expression and age. Associations between cadherin-11 and
clinical-pathological parameters were analyzed using Pearson's rho
correlation test (2-sided). For all analyses, P<0.05 was
considered to indicate a statistically significant difference. Data
were analyzed using SAS version 8.1 (SAS Institute Inc., Cary, NC,
USA).
Results
A total of 82 malignant tumor samples and 70 healthy
breast tissue and benign breast lesions were analyzed by IHC and
semi-quantitative RT-PCR. The patient tumor characteristics are
shown in Table I. The median age of
the patients with malignant tumors was 51 years, and median age of
patients with benign or healthy tissue was 48 years. The difference
in median age was not statistically significant. Of the malignant
tumors, 75% (n=62) were infiltrating ductal carcinomas, and the
remaining histological types included infiltrating lobular
carcinomas (n=3) and otherwise specified (n=17). Regarding tumor
size, 56% of malignant tumors were stage 2 (n=45), 24.4% were stage
1 (n=20), 19.5% were stage T3 (n=16), and 1% were stage 4 (n=1).
The majority of tumors were grade 2 (53.7%, n=44), followed by
grade 1 (18.3%, n=15) and Grade 3 (17%, n=14). In 9 cases (11%),
the tumor grades were unknown. The estrogen receptor status was
positive in 71% of the samples (n=58), negative in 19.5% of cases
(n=16), with 9.8% (n=8) unknown. The progesterone receptor status
was positive in 42 (51.2%) cases, negative in 26.8% (n=22) cases
and 22% were unknown. The HER2 receptor status was positive in 22
cases (26.8%) were HER2 receptors evaluated as positive and
negative in 60 cases (73.2%).
 | Table I.Patients characteristics. |
Table I.
Patients characteristics.
| Characteristic | Malignant tumor
(n=82) | Benign tumors and/or
normal tissue (n=70) | P-value |
|---|
| Median age (range),
years | 51 (42–62) | 48 (41–55) |
|
|
Cadherin-11a, n (%) |
|
| <0.0001 |
| 0 | 2 (2.4) | 37 (52.9) |
|
| + | 21 (25.6) | 13 (18.6) |
|
| ++ | 34 (41.5) | 12 (17.1) |
|
| +++ | 25 (30.5) | 8 (11.4) |
|
| Histology, n (%) |
|
| N/A |
| Invasive
ductal | 62 (75.6) | N/A |
|
| Invasive
lobular | 3 (3.7) |
|
|
|
Other | 17 (20.7) |
|
|
| Tumor grade, n
(%) |
|
| N/A |
| G1 | 15 (18.3) |
|
|
| G2 | 44 (53.7) |
|
|
| G3 | 14 (17.0) |
|
|
|
Unknown | 9 (11.0) | N/A |
|
| Tumor size, n
(%) |
|
| N/A |
| T1 | 20 (24.4) |
|
|
| T2 | 45 (56.1) |
|
|
| T3 | 16 (19.5) | N/A |
|
| T4 | 1 (1.2) |
|
|
| ER, n (%) |
|
| N/A |
| 0 | 16 (19.5) |
|
|
| 5% | 10 (12.2) |
|
|
|
5–10% | 8 (9.8) |
|
|
|
>10% | 40 (48.8) |
|
|
|
Unknown | 8 (9.8) | N/A |
|
| PR, n (%) |
|
| N/A |
| 0 | 22 (26.8) |
|
|
| 5% | 12 (14.6) |
|
|
|
5–10% | 16 (19.5) |
|
|
|
>10% | 14 (17.1) |
|
|
|
Unknown | 18 (22.0) | N/A |
|
| HER2, n (%) |
|
| N/A |
|
Positiveb | 22 (26.8) |
|
|
|
Negative | 60 (73.2) | N/A |
|
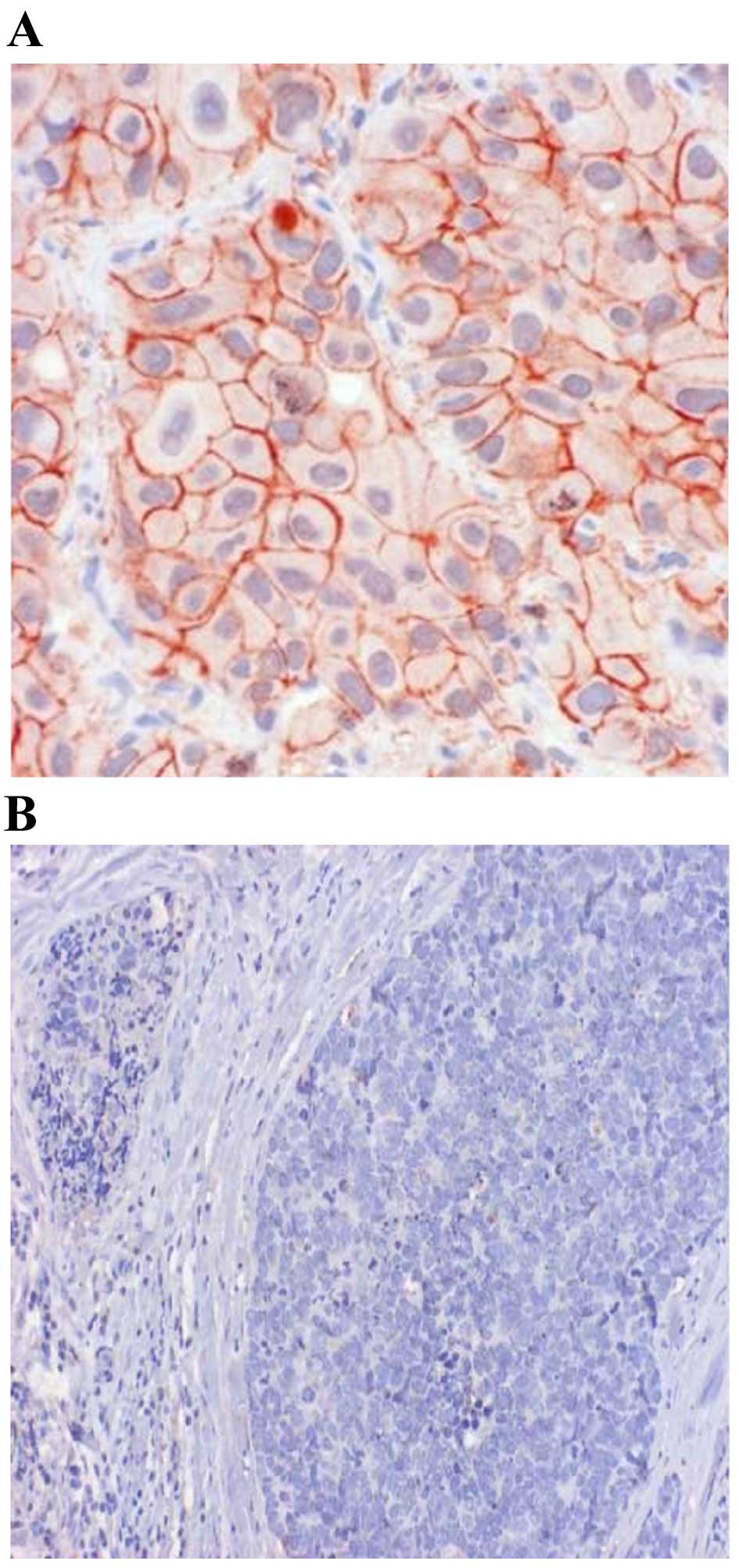
Fig. 1 shows the IHC
results for cadherin-11. In malignant tissue samples, 25 cases
(30.5%) exhibited strong positivity for cadherin-11, 34 cases
(41.5%) had moderate positivity, and weak positivity in another 21
cases (25.6%). Of 82 cases, only two (2.4%) were negative for
cadherin-11. As for benign/healthy samples, only 8 cases (11.4%)
exhibited strong positivity for cadherin-11, 12 (17.1%) were
moderate positive, and 13 (18.6%) were weak positive. However, more
benign/normal tissues tested negative for cadherin-11 than
malignant tumors (52.9 vs. 2.4%, respectively). This difference was
statistically significant (P<0.0001).
Correlations between cadherin-11 protein expression
and other clinical-pathological parameters are shown in Table II. The expression of cadherin-11
protein was not correlated with patient age, tumor size, grading,
or hormone receptors status.
 | Table II.Correlation between cadherin-11
protein expression and clinicopathological parameters. |
Table II.
Correlation between cadherin-11
protein expression and clinicopathological parameters.
| Parameter | Age | Grading | Tumor size | ER | PR | HER2 |
|---|
| Cadherin-11 |
|
|
Correlation coeff. | −0.109 | −0.034 | 0.340 | 0.034 | 0.029 | 0.128 |
| Sig.
(2-tailed) | 0.331 | 0.762 | 0.701 | 0.760 | 0.795 | 0.252 |
| Age |
|
|
Correlation coeff. |
| 0.107 | 0.071 | −0.125 | −0.167 | −0.017 |
| Sig.
(2-tailed) |
| 0.340 | 0.525 | 0.263 | 0.133 | 0.882 |
| Grading |
|
|
Correlation coeff. |
|
| −0.185 | 0.043 | 0.049 | 0.081 |
| Sig.
(2-tailed) |
|
| 0.097 | 0.703 | 0.664 | 0.470 |
| Tumor size |
|
|
Correlation coeff. |
|
|
| 0.125 | 0.199 | 0.057 |
| Sig.
(2-tailed) |
|
|
| 0.173 | 0.074 | 0.610 |
| ER |
|
|
Correlation coeff. |
|
|
|
| −0.169 | 0.036 |
| Sig.
(2-tailed) |
|
|
|
| 0.129 | 0.748 |
| PR |
|
|
Correlation coeff. |
|
|
|
|
| 0.138 |
|
| Sig.
(2-tailed) |
|
|
|
|
| 0.215 |
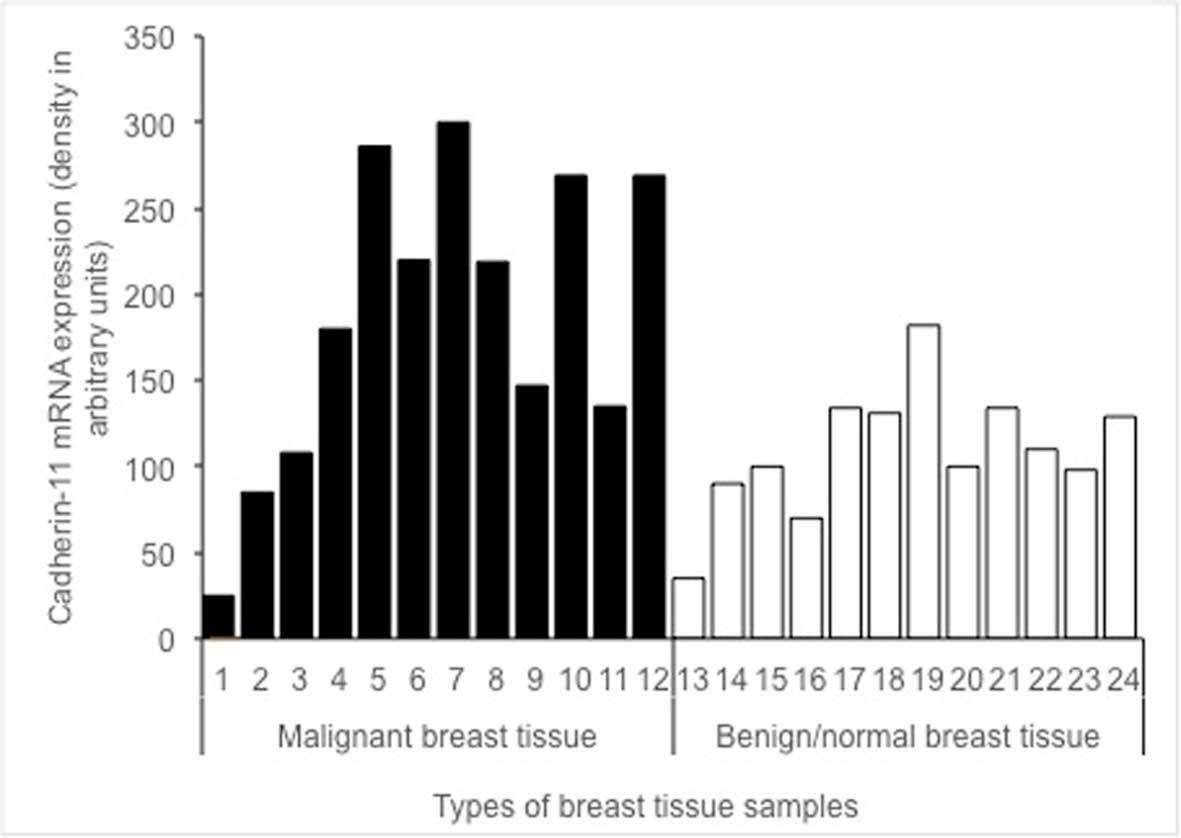
The expression of cadherin-11 mRNA in malignant
tissues (black lanes 1–12) vs. benign/healthy tissues (white lanes
13–24) is shown in Fig. 2. The
difference in cadherin-11 mRNA levels between malignant, and benign
and/or healthy tissue samples was statistically significant
(P=0.040).
Discussion
The present study demonstrates a significant
difference in both mRNA transcription and protein expression of
cadherin-11 in malignant breast tissue, when compared to benign
and/or healthy tissue. These findings are consistent with past
research and further emphasize the role of cadherins in the
fundamental mechanics of the disease (3,5,6,13,19). Furthermore, this also points to the
suspected role of cadherin-11 in EMT (12–17,19).
Our results are in agreement with the data of
Pishvaian et al (6), who
examined the expression of cadherin-11 in breast cancer cell lines
and demonstrated that cadherin-11 mRNA and protein were expressed
in the most invasive cell lines, but not in any of the noninvasive
cell lines. Based on these results, it is anticipated that
cadherin-11 expression may be well correlated with the invasive
phenotype in cancer cells and could serve as a molecular marker for
the more aggressive, invasive subset of breast tumors. Pishvaian
et al (6) reported that
cadherin-11 expression was significantly upregulated in malignant
tissue samples and that it was localized on the cell membrane of
the malignant cells, which is also in line with the results
presented in Fig. 1. Similarly, the
difference in mRNA expression in malignant and benign tissue
samples was statistically significant (P=0.040) in the present
study (Fig. 2). Cadherin-11 was
preferentially expressed in basal-like breast cancer (13). The differences between expression of
cadherin-11 protein and grading or hormone receptors status were
not statistically significant in our study. There was also no
correlation between estrogen and progesterone receptors in
malignant breast tissue samples (Table
II). The lack of correlation may be due to the median age of
the sample group (51 years), since older, post-menopausal women are
more likely to develop estrogen receptor positive breast cancer.
Previous studies have demonstrated that there is no relationship
between age and progesterone receptor positivity (25–27).
The present study was limited in several ways.
Firstly, the control group consisted of tissue samples with
undefined benign pathologies, which may influence the expression
profile of cadherins. Secondly, the protein expression of
cadherin-11 was performed using immunohistochemistry, which is
subjective, and proper evaluation of the score is lacking. We also
lacked clinical data on bone metastasis, which could have proven
relevant in this study.
The current study succeeded in demonstrating that
cadherin-11 expression is upregulated in invasive human breast
cancer. We hypothesize that the expression confers a more
mesenchymal cellular phenotype, which promotes invasion and
metastasis in invasive tumors.
Cadherin-11 is a major therapeutic target in
rheumatoid arthritis (10,21). Using a new proteochemometric
computational drug repurposing method, it was identified that the
drug celecoxib, a United States Food and Drug Administration
approved drug, and 2,5-dimethyl-celecoxib, a celecoxib analogue
without cyclooxygenase 2 inhibitory activity, had the structural
potential to bind cadherin-11 (22).
As cadherin-11 may be an important target in cancer progression
(21,28), this finding could potentially
translate into clinical application in cancer therapy. In
conclusion, our results indicate that cadherin-11 expression is
upregulated in malignant human breast cancer. Based on the fact
that cadherin-11 is typically expressed in cells of mesenchymal
origin, this suggests that EMT took place. These data suggest that
cadherin-11 is important for malignant progression and is a
potential therapeutic target in breast cancer.
References
|
1
|
Knudsen KA and Wheelock MJ: Cadherins and
the mammary gland. J Cell Biochem. 95:488–496. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andrews JL, Kim AC and Hens JR: The role
ad function of cadherins in the mammary gland. Breast Cancer Res.
14:2032012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Farina AK, Bong YS, Feltes CM and Byers
SW: Post-transcriptional regulation of cadherin-11 expression by
GSK-3 and beta-catenin in prostate and breast cancer cells. PLoS
One. 4:e47972009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Albergaria A, Ribeiro AS, Vieira AF, Sousa
B, Nobre AR, Seruca R, Schmitt FC and Paredes J: P-cadherin role in
normal breast development and cancer. Int J Dev Biol. 55:811–822.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tamura D, Hiraga T, Myoui A, Yoshikawa H
and Yoneda T: Cadherin-11-mediated interactions with bone marrow
stromal/osteoblastic cells support selective colonization of breast
cancer cells in bone. Int J Oncol. 33:17–24. 2008.PubMed/NCBI
|
|
6
|
Pishvaian MJ, Feltes CM, Thompson P,
Bussemakers MJ, Schalken JA and Byers SW: Cadherin-11 is expressed
in invasive breast cancer cell lines. Cancer Res. 59:947–952.
1999.PubMed/NCBI
|
|
7
|
Berx G and van Roy F: Involvement of
members of the cadherin superfamily in cancer. Cold Spring Harb
Perspect Biol. 1:a0031292009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maeda M, Johnson KR and Wheelock MJ:
Cadherin switching: Essential for behavioral but not morphological
changes during epithelium-to-mesenchyme transition. J Cell Sci.
118:873–887. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee DM, Kiener HP, Agarwal SK, Noss EH,
Watts GF, Chisaka O, Takeichi M and Brenner MB: Cadherin-11 in
synovial lining formation and pathology in arthritis. Science.
315:1006–1010. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang CF, Lira C, Chu K, Bilen MA, Lee YC,
Ye X, Kim SM, Ortiz A, Wu FL, Logothetis CJ, et al: Cadherin-11
increases migration and invasion of prostate cancer cells and
enhances their interaction with osteoblasts. Cancer Res.
70:4580–4589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nieto MA: Epithelial-mesenchymal
transitions in development and disease: Old views and new
perspectives. Int J Dev Biol. 53:1541–1547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sarrió D, Rodriguez-Pinilla SM, Hardisson
D, Cano A, Moreno-Bueno G and Palacios J: Epithelial-mesenchymal
transition in breast cancer relates to the basal-like phenotype.
Cancer Res. 68:989–997. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chao YL, Shepard CR and Wells A: Breast
carcinoma cells re-express E-cadherin during mesenchymal to
epithelial reverting transition. Mol Cancer. 9:1792010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clevers H and Nusse R: Wnt/ß-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dubois-Marshall S, Thomas JS, Faratian D,
Harrison DJ and Katz E: Two possible mechanisms of epithelial to
mesenchymal transition in invasive ductal breast cancer. Clin Exp
Metastasis. 28:811–818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aceto N, Toner M, Maheswaran S and Haber
D: En route to metastasis: Circulating tumor cell clusters and
epithelial-to-mesenchymal transition. Trends in Cancer. 1:44–52.
2015. View Article : Google Scholar
|
|
18
|
Satcher RL, Pan T, Cheng CJ, Lee YC, Lin
SC, Yu G, Li X, Hoang AG, Tamboli P, Jonasch E, et al: Cadherin-11
in renal cell carcinoma bone metastasis. PLoS One. 9:e898802014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Chao F, Huang B, Liu D, Kim J and
Huang S: HOXC8 promotes breast tumorigenesis by transcriptionally
facilitating cadherin-11 expression. Oncotarget. 5:2596–2607. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alimperti S and Andreadis ST: CDH2 and
CDH11 act as regulators of stem cell fate decisions. Stem Cell Res.
14:270–282. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Assefnia S, Dakshanamurthy S, Auvil JM
Guidry, Hampel C, Anastasiadis PZ, Kallakury B, Uren A, Foley DW,
Brown ML, Shapiro L, et al: Cadherin-11 in poor prognosis
malignancies and rheumatoid arthritis: Common target, common
therapies. Oncotarget. 5:1458–1474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dakshanamurthy S, Issa NT, Assefnia S,
Seshasayee A, Peters OJ, Madhavan S, Uren A, Brown ML and Byers SW:
Predicting new indications for approved drugs using a
proteochemometric method. J Med Chem. 55:6832–6848. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Remmele W and Schicketanz KH:
Immunohistochemical determination of estrogen and progesterone
receptor content in human breast cancer. Computer-assisted image
analysis (QIC score) vs. subjective grading (IRS). Pathol Res
Pract. 189:862–866. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hicks DG and Schiffhauer L: Standardized
assessment of the HER2 status in breast cancer by
immunohistochemistry. Lab Med. 42:459–467. 2011. View Article : Google Scholar
|
|
25
|
Savci-Heijink CD, Halfwerk H, Hooijer GK,
Horlings HM, Wesseling J and van de Vijver MJ: Retrospective
analysis of metastatic behaviour of breast cancer subtypes. Breast
Cancer Res Treat. 150:547–557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Salmen J, Neugebauer J, Fasching PA,
Haeberle L, Huober J, Wöckel A, Rauh C, Schuetz F, Weissenbacher T,
Kost B, et al: Pooled analysis of the prognostic relevance of
progesterone receptor status in five German cohort studies. Breast
Cancer Res Treat. 148:143–151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun JY, Wu SG, Li FY, Lin HX and He ZY:
Progesterone receptor loss identifies hormone receptor-positive and
HER2-negative breast cancer subgroups at higher risk of relapse: A
retrospective cohort study. Onco Targets Ther. 9:1707–1713.
2016.PubMed/NCBI
|
|
28
|
van Roy F: Beyond E-cadherin: Roles of
other cadherin superfamily members in cancer. Nat Rev Cancer.
14:121–134. 2014. View
Article : Google Scholar : PubMed/NCBI
|