Introduction
The presence of circulating tumor cells (CTCs) in
peripheral blood indicates a systemic disease stage (1), and detection of CTCs in peripheral blood
is useful for estimating prognosis and monitoring disease
progression in breast (2), prostate
(3), skin (4) and colon (5) malignancies. The commonly used techniques
for enrichment and detection of CTCs are density gradient
separation (6,7), direct enrichment by filtration (8), immunomagnetic separation (9), flow cytometry (10), reverse transcription-polymerase chain
reaction (RT-PCR) (11,12) and microchip technology (13). The CELLSEARCH® System
(Veridex LLC, Raritan, NJ, USA) (14)
is a widely used automated method of immunomagnetic cell enrichment
(15–17) that detects CTCs by detection of
epithelial markers such as epithelial cell adhesion molecule
(EpCAM) and cytokeratin. The expression of these markers, however,
decreases during the epithelial-mesenchymal transition (EMT)
(18,19); thus, CTCs undergoing EMT may escape
detection.
Increased telomerase activity is a common
characteristic of malignant tumors, and telomerase activity
strongly correlates with carcinogenesis and disease progression
(20–22). As a novel means of detecting cells
with high telomerase activity in peripheral blood samples of cancer
patients, a telomerase-specific, replication-competent adenovirus
variant termed OBP-401 (TelomeScan®; Oncolys BioPharma,
Inc., Tokyo, Japan) was used (22).
In this variant, the human telomerase reverse transcriptase (TERT)
gene promoter drives the expression of the viral genes adenovirus
early region 1A (E1A) and E1B, while the cytomegalovirus promoter
drives the expression of the green fluorescent protein (GFP) gene,
which serves as a marker of viral replication (22).
In our previous study (23), we used OBP-401 to detect CTCs in
patients with gastric cancer. Gastric cancer is the fifth most
common cancer worldwide and the third most frequent cause of
cancer-associated mortality, affecting ~723,000 people in 2012
(24). In that study, blood samples
from 65 treatment-naïve gastric cancer patients were collected
prior to surgery (23). CTCs are
larger than normal blood cells (25,26), and
it was observed that the GFP+ cells in these samples with a
diameter ≥7.735 µm (termed L-GTP+ cells) expressed EpCAM and other
CTC markers (23). Additional results
indicated that the number of L-GFP+ cells was significantly
correlated with venous invasion (23)
and overall survival after a median follow-up period of 3 years
(27).
The number of CTCs following initial treatment
correlates significantly with treatment efficacy and prognosis in
patients with breast (2), prostate
(3) and colorectal cancer (5). However, whether this is the case for
patients with gastric cancer, is controversial. In the present
study, the number of CTCs (defined as L-GFP+ cells) were determined
prior and subsequent to treatment.
Materials and methods
Patients and healthy volunteers
The present report is an interim analysis of part of
our prospective study of CTCs as GFP+ cells in patients with
treatment-naïve gastric adenocarcinoma who underwent surgery at the
Digestive Disease Center of Showa University Northern Yokohama
Hospital (Tsuzuki-ku, Japan) between April 2010 and May 2011.
Peripheral blood samples (three samples per time point) were
obtained from 37 patients before surgery and at 4 and 24 weeks
after surgery. The inclusion criteria were as follows: i)
Histologically confirmed adenocarcinoma of the stomach by
endoscopic biopsy; ii) clinical solitary tumor; iii) no prior
endoscopic resection, chemotherapy or radiotherapy; iv) age, 20–80
years; v) Eastern Cooperative Oncology Group performance status
(28) of 0 or 1; vi) sufficient organ
function; and vii) written informed consent. The exclusion criteria
were as follows: i) Synchronous or metachronous malignancy; ii)
pregnancy or breast-feeding; iii) active or chronic viral
hepatitis; iv) active bacterial or fungal infection; v) diabetes
mellitus; vi) systemic administration of corticosteroids; and vii)
unstable hypertension. The pathological stage of the disease was
determined according to the 7th edition of the American Joint
Committee on Cancer/International Union Against Cancer
tumor-node-metastasis classification system (29). The depth of the tumor invasion in 4
patients without gastrectomy and the regional lymph node status of
7 patients without sufficient lymphadenectomy were surgically
diagnosed.
All patients were followed up at our hospital every
3 months after surgery. The patients underwent endoscopy and
computed tomography at least once per year depending on their
disease stage and course. Postoperative therapy was allowed if
required.
Peripheral blood samples were collected from healthy
volunteers to establish the cut-off size of GFP+ cells. All
volunteers were employees of Sysmex Corporation (Kobe, Japan), and
were interviewed individually prior to sample collection. The
volunteer group consisted of 7 men (mean age, 31.4 years; range,
24–39 years) and 3 women (mean age, 33.7 years; range, 26–48
years). All volunteers underwent medical check-ups upon employment
and annually thereafter. Check-ups included medical interviews,
auscultation, chest radiography, and blood and urine analyses. None
of the volunteers was receiving medical treatment, pregnant or
breast-feeding, or had donated blood within the month previous to
the initiation of the study.
The present study was approved by the Institutional
Review Board of Showa University Northern Yokohama Hospital
(Tsuzuki-ku, Japan; approval number 0903-03). The study protocol
was explained to patients and volunteers prior to obtention of
written informed consent. Our study was registered with the
University Hospital Medical Information Network in Japan (Tokyo,
Japan; registration number 000004026).
Viruses
OBP-401, a telomerase-specific,
replication-selective adenovirus variant in which the TERT promoter
drives the expression of the viral EIA and EIB genes, and into
which the GFP gene is integrated (22), was used. The sensitivity and
specificity of the OBP-401 assay have been reported by Kim et
al (30). Briefly, the assay was
evaluated five times using MDA-MB-468 breast carcinoma cells as
positive controls. The numbers of GFP+ cells in samples containing
1 MDA-MB-468 cell and 7.5 ml blood were 1, 1, 1, 2 and 3,
respectively, while the numbers of GFP+ cells in samples containing
20 MDA-MB-468 cells and 7.5 ml blood were 15, 17, 19, 22 and 24,
respectively. Viral samples were stored at −80°C.
Sample preparation and
immunostaining
Details of sample preparation and assay are
described in our previous report (23). A 7.5-ml peripheral vein blood sample
was obtained from each patient before surgery and at 4 and 24 weeks
after surgery. Blood was collected in tubes containing citric acid,
phosphoric acid and dextrose, and stored at 4°C. Samples were
assayed within 48 h of collection. Samples were centrifuged for 5
min at 540 × g, and the plasma phase was removed. Cell
pellets were washed four times with PBS (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) and twice with RPMI medium
(Sigma-Aldrich; Merck Millipore). Upon suspension in RPMI medium,
cells were infected with 4×108 plaque-forming units of
OBP-401 for 24 h at 37°C. Dead cells were stained with the red
fluorescent reactive dye L23102 (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Upon inactivation of OBP-401, cells were fixed
with 2% paraformaldehyde for 20 min at room temperature and treated
with a surface-active agent (Emalgen 2025 G; Kao Chemicals, Tokyo,
Japan) for 10 min at 40°C to degrade red blood cells. Cells were
incubated with phycoerythrin-labeled anti-human cluster of
differentiation (CD) 45 antibody (catalogue number 368510;
BioLegend, San Diego, CA, USA; 1:5 dilution) for 30 min at 25°C,
which was diluted into PBS containing 2% fetal bovine serum (FBS;
Sigma-Aldrich; Merck Millipore). Cells were washed with PBS
containing 2% FBS and mounted on glass slides for microscopic
analysis (two slides per sample).
Determination of GFP fluorescence
intensity threshold
The threshold for GFP fluorescence intensity was
determined as previously reported (23). Briefly, blood samples (7.5 ml) from
healthy volunteers were mixed with ~30,000 A549 (lung carcinoma),
HepG2 (hepatocellular carcinoma), HEC-1 (endometrial carcinoma),
KATO-III (gastric carcinoma), SBC-3 (small cell lung carcinoma),
LNCaP (prostate adenocarcinoma), MDA-MB-468 (breast carcinoma) or
OVCAR-3 (ovarian carcinoma) cells. The A549, HepG2, HEC-1,
KATO-III, SBC-3 and MDA-MB-468 cell lines were obtained from the
Health Science Research Resources Bank (Osaka, Japan), while the
LNCap and OVCAR-3 cell lines were obtained from the RIKEN Cell Bank
(Tokyo, Japan). Cell lines were cultured according to the vendor's
specifications. The samples were assayed using OBP-401, and GFP+
cells were visualized by fluorescence microscopy and counted. The
GFP signal intensity threshold was determined to be
2.85×107 molecules of equivalent fluorochrome on the
basis of the minimal GFP intensity level observed in the blood
samples mixed with the cells. There was no significant difference
in cell size prior and subsequent to OBP-401 infection.
Determination of cell size
threshold
In our previous study (23), the various sizes of GFP+ cells in each
sample made it difficult to determine which GFP+ cells best
represented CTCs. To establish a constant value, the optimum
threshold derived from a receiver operating characteristic curve
analysis based on cell size was used to define GFP+ CTCs; this
threshold was 7.735 µm. Thus, two groups of GFP+ cells were
analyzed: Small (S)-GFP+ cells, measuring <7.735 µM in diameter,
and large (L)-GFP+ cells, measuring ≥7.735 µm in diameter (27).
Cell counting and analysis. In a blind analysis,
GFP+ cells on slides were counted on a computer-controlled
fluorescence microscope (IX71; Olympus Corporation, Tokyo, Japan).
Cells with fluorescent emission counts of ≥2.85×107
molecules of equivalent fluorochrome were counted as GFP+ cells.
The criteria for a GFP+ cell did not include the presence of
epithelial markers such as EpCAM or cytokeratin, since tumor cells
undergoing EMT have been reported to lose epithelial markers
(31). CD45+ cells were excluded from
the analysis.
Statistical analysis
All statistical analyses were performed using JMP
Pro 11.1.1 software (SAS Institute, Cary, NC, USA). The
Kaplan-Meier analysis and the Wilcoxon test were used to calculate
relapse-free survival rates and for non-parametric comparisons of
the numbers of GFP+ cells. The relapse-free survival rate of the
patients who underwent non-curative surgery declined to 0 months.
P≤0.05 was considered to indicate a statistically significant
difference.
Results
Participant characteristics
The clinicopathological characteristics of 37
patients (26 men and 11 women; mean age, 60.7 years; range, 33–76
years) are summarized in Table I. The
median follow-up period of the surviving patients was 39 months. A
total of 33 patients underwent pathological curative surgery, and 5
of these patients experienced disease recurrence. In total, 6
patients succumbed to disease; 16 patients underwent distal
gastrectomy; 18 patients underwent total gastrectomy; and 3
underwent exploratory laparotomy. Of the 37 patients, 17 received
chemotherapy post-surgery; 13 patients received oral chemotherapy
(S-1); and 4 received oral chemotherapy combined with infusion
(S-1/cisplatin and S-1/docetaxel).
 | Table I.Patient characteristics and
pathological findings. |
Table I.
Patient characteristics and
pathological findings.
| Variable | Number of
patients |
|---|
| Gender |
|
| Male | 26 |
|
Female | 11 |
| Age, years (mean;
range) | 60.7; 33–76 |
| Gastrectomy |
|
|
Distal | 16 |
|
Total | 18 |
|
None | 3 |
| Curability |
|
| R0 | 33 |
| R1 | 0 |
| R2 | 4 |
| TNM stage |
|
| I | 22 |
| II | 5 |
|
III | 6 |
| IV | 4 |
| Depth of tumor
invasion |
|
| T1 | 20 |
| T2 | 5 |
| T3 | 5 |
| T4 | 7 |
| Lymph node
metastasis |
|
| N0 | 22 |
| N1 | 4 |
| N2 | 3 |
| N3 | 8 |
| Distant
metastasis |
|
| M0 | 33 |
| M1 | 4 |
| Main histological
typea |
|
|
Differentiated | 13 |
|
Undifferentiated | 24 |
| Lymphatic
invasion |
|
| L0 | 18 |
| L1 | 16 |
| LX | 3 |
| Venous
invasion |
|
| V0 | 19 |
|
V1–2 | 15 |
| VX | 3 |
| Postoperative
chemotherapy |
|
| Yes
(oral) | 13 |
| Yes
(oral and infusion) | 4 |
| No | 20 |
| Outcome |
|
| Relapse
free | 28 |
|
Relapse | 5 |
|
Non-curative surgery | 4 |
Association between the number of
L-GFP+ cells and patient outcome
The association between the number of L-GFP+ cells
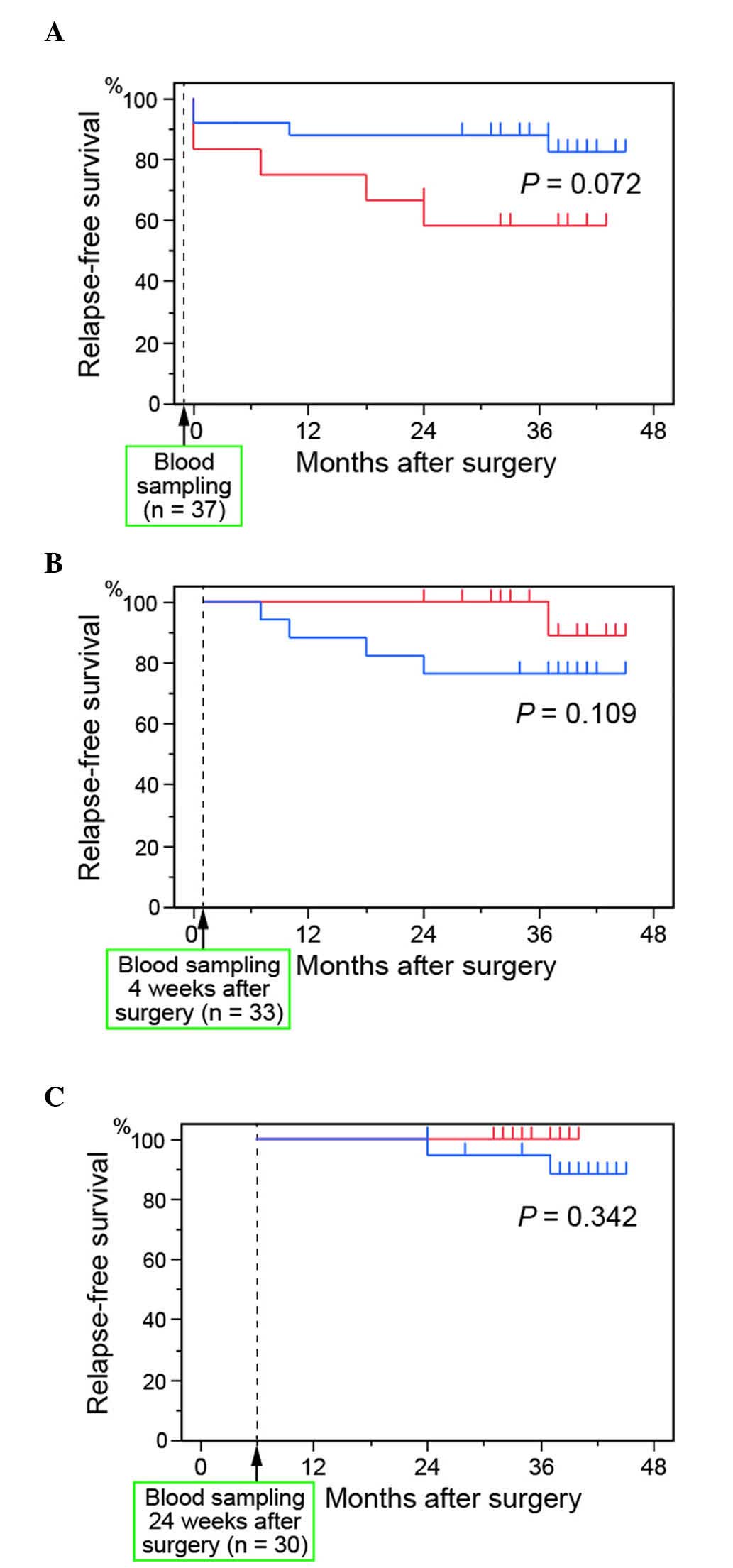
and the relapse-free survival rate is shown in Fig. 1. Of the 37 patients enrolled in our
study, 33 and 30 were relapse free at 4 and 24 weeks, respectively,
after surgery. There were no significant differences in the
relapse-free survival rates of patients with ≥6 L-GFP+ cells and
patients with <6 L-GFP+ cells, either before (P=0.072; Fig. 1A) or at 4 (P=0.109; Fig. 1B) or 24 (P=0.342; Fig. 1C) weeks after surgery. At 4 weeks
after surgery, the relapse-free survival rate was, however,
relatively higher in patients with ≥6 GFP+ cells than in patients
with <6 GFP+ cells.
Association between patient outcome
and changes in the number of GFP+ cells
The numbers of L-GFP+ cells in peripheral blood
samples from patients without relapse following curative surgery
(R-), patients with relapse following curative surgery (R+) and
patients who underwent non-curative surgery (Non-C) were compared
at three time points. The mean numbers of L-GFP+ cells at 0, 4 and
24 weeks after surgery were as follows: R-, 5.6, 8.3 and 6.1,
respectively; R+, 6.0, 4.0 and 7.2, respectively; and Non-C, 6.8,
0.8 and 4.0, respectively (Fig. 2A).
There was a significant difference between the number of L-GFP+
cells in samples from the R- patients and the Non-C patients at 4
weeks after surgery (P=0.034).
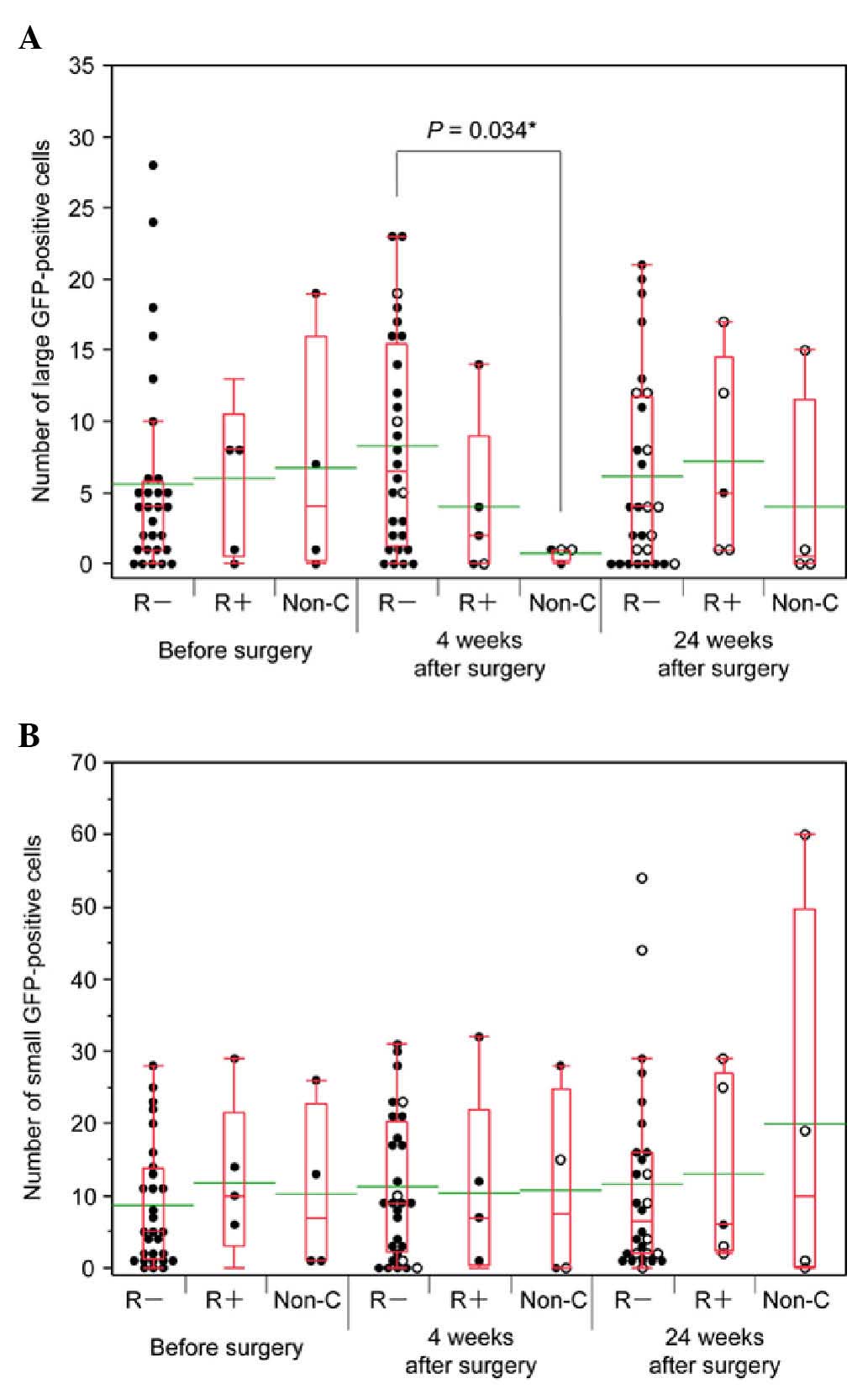 | Figure 2.Association of GFP+ cell number with
patient outcome. (A and B) The black dots indicate the number of
GFP+ cells in 7.5-ml blood samples from chemotherapy-negative
patients. The white dots indicate the number of GFP+ cells in
samples from patients receiving chemotherapy. The bottom and top of
the box represent the lower and upper quartiles, respectively,
while the band across the box represents the median. The lower and
upper bars at the ends of the whiskers indicate the lowest datum
within 1.5 interquartile ranges of the lower quartile and the
highest datum within 1.5 interquartile ranges of the upper
quartile, respectively. The green bars indicate the mean value. (A)
Numbers of L-GFP+ cells (≥7.735 µm in diameter). The mean numbers
of L-GFP+ cells before surgery, 4 weeks post-surgery and 24 weeks
post-surgery were as follows: R-, 5.6, 8.3 and 6.1; R+, 6.0, 4.0
and 7.2; and Non-C, 6.8, 0.8 and 4.0, respectively. There was a
significant difference in the number of L-GFP+ cells in samples
from R- patients and Non-C patients (P=0.034). (B) Numbers of
S-GFP+ cells (<7.735 µm in diameter). The mean numbers of S-GFP+
cells before surgery, 4 weeks post-surgery and 24 weeks
post-surgery were as follows: R-, 8.7, 11.3 and 11.6; R+, 11.8,
10.4 and 13.0; and Non-C, 10.3, 10.8 and 20.0, respectively.
Although there were no significant differences, the number of
S-GFP+ cells in samples from Non-C patients 24 weeks after surgery
was relatively higher than the numbers of S-GFP+ cells in the
samples from R- (P=0.932) and R+ patients (P=0.713). R-, patients
without relapse; R+, patients with relapse; Non-C, patients who
underwent non-curative surgery; GFP, green fluorescent protein; L,
large; S, small. |
The mean numbers of S-GFP+ cells at 0, 4 and 24
weeks after surgery were as follows: R-, 8.7, 11.3 and 11.6,
respectively; R+, 11.8, 10.4 and 13.0, respectively; and Non-C,
10.3, 10.8 and 20.0, respectively (Fig.
2B). There were no significant differences between the three
groups either prior or subsequent to surgery. The number of S-GFP+
cells in samples from Non-C patients at 24 weeks after surgery was,
however, relatively high.
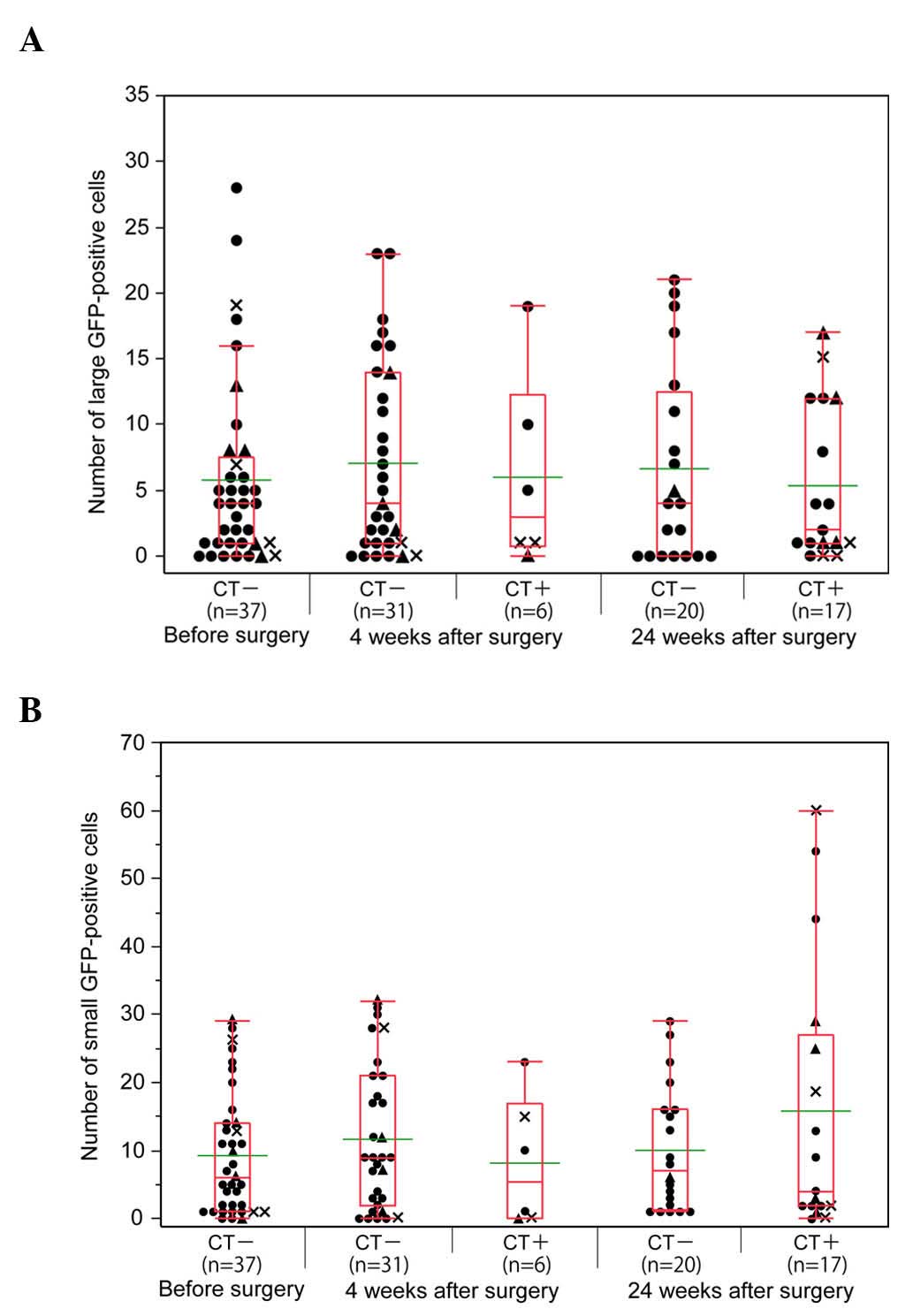
The associations between the number of GFP+ cells,
postoperative chemotherapy and outcome are represented in Fig. 3. Of the 37 patients, 6 and 17 were
receiving postoperative chemotherapy 4 and 24 weeks after surgery.
The number of L-GFP+ cells was similar prior and subsequent to
surgery, regardless of chemotherapy (Fig.
3A). Although there was no significant difference, the number
of S-GFP+ cells was relatively high in the samples from the
patients who were receiving postoperative chemotherapy 24 weeks
after surgery (Fig. 3B).
Discussion
In the present study, the association between the
number of CTCs (L-GFP+ cells) and treatment outcome was assessed in
patients with gastric cancer, which was the third leading cause of
cancer-associated mortality worldwide in 2012 (24). The usefulness of CTCs for diagnosis
and estimation of prognosis has been reported for breast (15,31),
prostate (32), lung (33), esophagus (11,12), colon
(34) and gastric cancer (23,27). The
results of the present study indicate that the number of CTCs
during treatment is unrelated to prognosis in gastric cancer.
A major finding of our study is that there is no
significant correlation between patient prognosis and the number of
L-GFP+ cells in samples from gastric cancer patients following
surgery. This result is consistent with that of our previous study,
which used PCR to detect CTCs in patients with esophageal cancer
(11). Significant associations
between the number of CTCs during treatment and prognosis have been
reported for patients with breast (2), prostate (3) and colorectal (5) cancer. The difference in the results of
these studies and the present study may reflect the type of cancer
examined. The CTC number in our study may have been affected by the
postoperative chemotherapy regimens, which were not the same for
all patients. In addition, the median follow-up period of 3 years
in our study may be too short to accurately evaluate the
association between CTCs and prognosis. It is contrary to our
expectation that the number of L-GFP+ cells in samples from the
Non-C patients was significantly lower than R- patients 4 weeks
after surgery. Since the number of Non-C patients in the present
study is small, this significant difference should be confirmed by
a large-scale prospective study.
The second major finding of our study is that the
number of S-GFP+ cells increases following chemotherapy, which
suggests that chemotherapy reduces the size of CTCs. The present
authors suggest that the ratio of drug-resistant CTCs (e.g., cancer
stem-like cells) to drug-sensitive CTCs increases following
chemotherapy. Therefore, drug-resistant CTCs may be smaller than
drug-sensitive CTCs. It is also possible that OBP-401 infection
increases telomerase activity in non-cancer cells, which are
smaller than CTCs.
A limitation of our study is that the metastatic
potential of the GFP+ cells was not determined. Additional studies
in a larger population of patients with different cancer types are
required to clarify the clinical applicability of CTC detection.
Future studies should analyze the functions of viable CTCs upon
cell sorting and identify CTCs with metastatic potential using
additional tools such as DNA ploidy analysis (35,36). Gene
expression profiling of CTCs and primary tumors will also provide
important insights into the mechanisms of cancer metastasis.
In summary, the results of the present study
indicate that CTCs may be useful as predictors of disease
progression in treatment-naïve gastric cancer patients; however,
CTCs do not constitute a prognostic factor in patients during
treatment.
There was no significant association between the
change in the number of CTCs, treatment or prognosis in gastric
cancer patients who underwent curative surgery. However, the
present study used a short follow-up period, and only a small
number of participants were included. In addition, whether all GFP+
cells have true metastatic potential was unclear. Therefore,
further studies are warranted to confirm the findings of the
present study.
Acknowledgements
The present study was supported in part by a
Grant-in-Aid for Challenging Exploratory Research (grant number
23659308) from Japan Society for the Promotion of Science (Tokyo,
Japan). The authors are grateful to all the patients and volunteers
who donated blood for the study. The authors would like to thank
Professor Toshiyoshi Fujiwara (Okayama University Graduate School
of Medicine, Okayama, Japan) for helpful comments and suggestions;
Mr. Yasuo Urata (Oncolys BioPharma, Tokyo, Japan) for supplying
OBP-401; Dr Yukio Tsujino, Dr Toshiyuki Ozawa and Dr Akinori Masago
(Sysmex Corporation, Kobe, Japan) for their valuable support; and
the clinical staff of Showa University Northern Yokohama Hospital
(Yokohama, Japan).
References
|
1
|
Liotta LA, Kleinerman J and Saidel GM:
Quantitative relationships of intravascular tumor cells, tumor
vessels, and pulmonary metastases following tumor implantation.
Cancer Res. 34:997–1004. 1974.PubMed/NCBI
|
|
2
|
Camara O, Rengsberger M, Egbe A, Koch A,
Gajda M, Hammer U, Jörke C, Rabenstein C, Untch M and Pachmann K:
The relevance of circulating epithelial tumor cells (CETC) for
therapy monitoring during neoadjuvant (primary systemic)
chemotherapy in breast cancer. Ann Oncol. 18:1484–1492. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scher HI, Jia X, de Bono JS, Fleisher M,
Pienta KJ, Raghavan D and Heller G: Circulating tumour cells as
prognostic markers in progressive, castration-resistant prostate
cancer: A reanalysis of IMMC38 trial data. Lancet Oncol.
10:233–239. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
A L Reid, Millward M, Pearce R, Lee M,
Frank MH, Ireland A, Monshizadeh L, Rai T, Heenan P, Medic S, et
al: Markers of circulating tumour cells in the peripheral blood of
patients with melanoma correlate with disease recurrence and
progression. Br J Dermatol. 168:85–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu CY, Tsai HL, Uen YH, Hu HM, Chen CW,
Cheng TL, Lin SR and Wang JY: Circulating tumor cells as a
surrogate marker for determining clinical outcome to mFOLFOX
chemotherapy in patients with stage III colon cancer. Br J Cancer.
108:791–797. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arai Y, Kanamaru H, Yoshimura K, Okubo K,
Kamoto T and Yoshida O: Incidence of lymph node metastasis and its
impact on long-term prognosis in clinically localized prostate
cancer. Int J Urol. 5:459–465. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vona G, Sabile A, Louha M, Sitruk V,
Romana S, Schütze K, Capron F, Franco D, Pazzagli M, Vekemans M, et
al: Isolation by size of epithelial tumor cells: A new method for
the immunomorphological and molecular characterization of
circulating tumor cells. Am J Pathol. 156:57–63. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Talasaz AH, Powell AA, Huber DE, Berbee
JG, Roh KH, Yu W, Xiao W, Davis MM, Pease RF, Mindrinos MN, et al:
Isolating highly enriched populations of circulating epithelial
cells and other rare cells from blood using a magnetic sweeper
device. Proc Natl Acad Sci USA. 106:3970–3975. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He W, Wang H, Hartmann LC, Cheng JX and
Low PS: In vivo quantitation of rare circulating tumor cells by
multiphoton intravital flow cytometry. Proc Natl Acad Sci USA.
104:11760–11765. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ito H, Kanda T, Nishimaki T, Sato H,
Nakagawa S and Hatakeyama K: Detection and quantification of
circulating tumor cells in patients with esophageal cancer by
real-time polymerase chain reaction. J Exp Clin Cancer Res.
23:455–464. 2004.PubMed/NCBI
|
|
12
|
Honma H, Kanda T, Ito H, Wakai T, Nakagawa
S, Ohashi M, Koyama Y, Valera VA, Akazawa K and Hatakeyama K:
Squamous cell carcinoma-antigen messenger RNA level in peripheral
blood predicts recurrence after resection in patients with
esophageal squamous cell carcinoma. Surgery. 139:678–685. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagrath S, Sequist LV, Maheswaran S, Bell
DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky
A, et al: Isolation of rare circulating tumour cells in cancer
patients by microchip technology. Nature. 450:1235–1239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cohen SJ, Punt CJ, Iannotti N, Saidman BH,
Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et
al: Relationship of circulating tumor cells to tumor response,
progression-free survival, and overall survival in patients with
metastatic colorectal cancer. J Clin Oncol. 26:3213–3221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Riethdorf S, Fritsche H, Müller V, Rau T,
Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Jänicke F, et al:
Detection of circulating tumor cells in peripheral blood of
patients with metastatic breast cancer: A validation study of the
cell search system. Clin Cancer Res. 13:920–928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davis JW, Nakanishi H, Kumar VS,
Bhadkamkar VA, McCormack R, Fritsche HA, Handy B, Gornet T and
Babaian RJ: Circulating tumor cells in peripheral blood samples
from patients with increased serum prostate specific antigen:
Initial results in early prostate cancer. J Urol. 179:2187–2191;
discussion 2191. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hou JM, Greystoke A, Lancashire L,
Cummings J, Ward T, Board R, Amir E, Hughes S, Krebs M, Hughes A,
et al: Evaluation of circulating tumor cells and serological cell
death biomarkers in small cell lung cancer patients undergoing
chemotherapy. Am J Pathol. 175:808–816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Książkiewicz M, Markiewicz A and Zaczek
AJ: Epithelial-mesenchymal transition: A hallmark in metastasis
formation linking circulating tumor cells and cancer stem cells.
Pathobiology. 79:195–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gorges TM, Tinhofer I, Drosch M, Röse L,
Zollner TM, Krahn T and von Ahsen O: Circulating tumour cells
escape from EpCAM-based detection due to epithelial-to-mesenchymal
transition. BMC Cancer. 12:1782012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim NW, Piatyszek MA, Prowse KR, Harley
CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL and Shay
JW: Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blackburn EH: Telomere states and cell
fates. Nature. 408:53–56. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujiwara T, Kagawa S, Kishimoto H, Endo Y,
Hioki M, Ikeda Y, Sakai R, Urata Y, Tanaka N and Fujiwara T:
Enhanced antitumor efficacy of telomerase-selective oncolytic
adenoviral agent OBP-401 with docetaxel: Preclinical evaluation of
chemovirotherapy. Int J Cancer. 119:432–440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ito H, Inoue H, Sando N, Kimura S, Gohda
K, Sato J, Murakami K, Ito S, Odaka N, Satodate H and Kudo SE:
Prognostic impact of detecting viable circulating tumour cells in
gastric cancer patients using a telomerase-specific viral agent: A
prospective study. BMC Cancer. 12:3462012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
World Health Organization, . International
Agency for Research on Cancer. GLOBOCAN 2012. Estimated Cancer
Incidence, Mortality and Prevalence World Wide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspxAccessed
June 9, 2014.
|
|
25
|
Lin HK, Zheng S, Williams AJ, Balic M,
Groshen S, Scher HI, Fleisher M, Stadler W, Datar RH, Tai YC and
Cote RJ: Portable filter-based microdevice for detection and
characterization of circulating tumor cells. Clin Cancer Res.
16:5011–5018. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng S, Lin HK, Lu B, Williams A, Datar
R, Cote RJ and Tai YC: 3D microfilter device for viable circulating
tumor cell (CTC) enrichment from blood. Biomed Microdevices.
13:203–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ito H, Inoue H, Kimura S, Ohmori T,
Ishikawa F, Gohda K and Sato J: Prognostic impact of the number of
viable circulating cells with high telomerase activity in gastric
cancer patients: A prospective study. Int J Oncol. 45:227–234.
2014.PubMed/NCBI
|
|
28
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. 7th. Blackwell
Publishing Ltd.; Oxford: 2010
|
|
30
|
Kim SJ, Masago A, Tamaki Y, Akazawa K,
Tsukamoto F, Sato J, Ozawa T, Tsujino Y and Noguchi S: A novel
approach using telomerase-specific replication-selective adenovirus
for detection of circulating tumor cells in breast cancer patients.
Breast Cancer Res Treat. 128:765–773. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LW and Hayes DF: Circulating tumor cells, disease
progression, and survival in metastatic breast cancer. N Engl J
Med. 351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moreno JG, Miller MC, Gross S, Allard WJ,
Gomella LG and Terstappen LW: Circulating tumor cells predict
survival in patients with metastatic prostate cancer. Urology.
65:713–718. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Krebs MG, Sloane R, Priest L, Lancashire
L, Hou JM, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G,
et al: Evaluation and prognostic significance of circulating tumor
cells in patients with non-small-cell lung cancer. J Clin Oncol.
29:1556–1563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Katsumata K, Sumi T, Mori Y, Hisada M,
Tsuchida A and Aoki T: Detection and evaluation of epithelial cells
in the blood of colon cancer patients using RT-PCR. Int J Clin
Oncol. 11:385–389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bonsing BA, Beerman H, Kuipers-Dijkshoorn
N, Fleuren GJ and Cornelisse CJ: High levels of DNA index
heterogeneity in advanced breast carcinomas. Evidence for DNA
ploidy differences between lymphatic and hematogenous metastases.
Cancer. 71:382–391. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Klijanienko J, el-Naggar AK, de Braud F,
Rodriguez-Peralto JL, Rodriguez R, Itzhaki M, Russo A, Janot F,
Luboinski B and Cvitkovic E: Tumor vascularization, mitotic index,
histopathologic grade, and DNA ploidy in the assessment of 114 head
and neck squamous cell carcinomas. Cancer. 75:1649–1656. 1995.
View Article : Google Scholar : PubMed/NCBI
|