Introduction
There are two types of minimally invasive treatment
for liver cancer: i) Vascular treatment such as hepatic arterial
infusion chemotherapy, hepatic artery embolism and transcatheter
hepatic arterial chemoembolization; and ii) non-vascular treatment
such as percutaneous chemical ablation and percutaneous physical
ablation. Percutaneous physical ablation includes cryotherapy using
argon-helium superconductive operation system, radiofrequency
ablation, microwave coagulation, laser-induced interstitial
thermotherapy and high intensive focused ultrasound (1,2).
Argon-helium cryoablation is an extremely important
minimally invasive freezing technique that combines two
technologies: the ultralow temperature-programmed and
temperature-programmed methods. The method has produced better
effects compared to freeze and heat treatment alone (3); however, it is affected by many factors
such as the classification of liver function in patients,
superconducting knife diameter, tumor location, length of the
elimination times and the extent (4).
To decrease spontaneous rupture and hemorrhage, in the puncture
approach, the use of necessary needle hemostasis technique and the
decrease of the extent of freeze-thaw area should be considered
(5,6).
In the present study, we analyzed the effectiveness
of using 64-slice spiral computed tomography (CT) and perfusion
imaging to guide argon-helium cryoablation treatment of liver
cancer in 120 subjects.
Materials and methods
Data
From June 2014 to June 2015, we enrolled 60 patients
with advanced hepatocellular carcinoma before surgery. They were
placed in the observation group and were treated with argon-helium
cryoablation. In the observation group, there were 42 males and 18
females aged, 52–76 years (average, 65.6±14.2 years). According to
the Barcelona BCLC staging (7), there
were 45 cases of metaphase liver cancer and 15 cases of advanced
liver cancer. The maximum diameter of the tumor ranged from 2.5 to
4.6 cm (average, 3.4±1.1 cm) and the number of tumors ranged from 1
to 4 (average, 2.2±0.8). There were 52 cases of positive HBsAg, and
AFP ranged from 56 to 732 ng/ml (average, 348.3±72.6 ng/ml). A
retrospective summary of the 60 cases of metaphase and advanced
liver cancer were used as the control group. They had matching
gender, age, tumor characteristics, and HBsAg-positive rates with
AFP levels at 1:1.
Inclusion criteria for the study were: i) No liver
surgery, no radiotherapy and chemotherapy, no trauma, no particle
implantation; ii) tumor diameter was <5 cm, the number of lesion
≤3 and, the number of primary liver cancer was ≤5; iii) no severe
liver and renal insufficiency and stable vital signs; iv) liver
function Child-Pugh grade A or grade B; and v) no arteriovenous
fistula formation and no contrast agent allergy history with
security needle path. Exclusion criterias: i) Low quality computed
tomography (CT) image; ii) the convergence part of liver cancer
near the intrahepatic bile duct and other parts leading to ablation
difficulties; iii) incomplete or lost follow-up records; and iv)
patients who died during the follow-up period for any reason other
than liver cancer. This study obtained the informed consents and
approval from the Ethics Committee of the Affiliated Hospital of
Hebei University of Engineering (Hebei, China).
Study method
We used GE 64-slice spiral CT to enhance three
phases scanning preoperatively and determined the extent of lesions
and the blood supply situation to optimize the formulation of the
argon-helium knife treatment. We observed and controlled the
freezing-melting range by dynamic CT perfusion imaging to minimize
the complications. Postoperative curative effects and prognosis was
evaluated using 64-slice CT during the follow-up examinations. In
the control group we verified the clinical effects as well as
prognosis for routine X-ray. CT, magnetic resonance imaging (MRI)
or ultrasound.
Patients in the observation group were prepared in
routine time for plain scan and enhanced upper abdominal. We used
Stellent dual tube high pressure syringe in Medrad (Medrad,
Pittsburgh, PA, USA), and non-ionic contrast agent (350 mg I/ml).
Scanning parameters were as follows: Tube voltage was 80 kV, tube
current was 80 mAs, detector was set at 16 cm, layer thickness was
0.5 mm, layer spacing was 0.5 mm, matrix was 512×512 and scanning
speed was 0.5 sec/cycle. Perfusion scan process began scanning
after 8 sec when contrast agent was injected and scanned once from
8 to 28 sec every 2 and 3 sec from 34 to 52 sec, and every 5 sec
from 59 to 69 sec. This created a total of 21 dynamic volume data
and each volume data contained 320 images, thus 6,720 images were
obtained in total. The original images were handled in the
workstation after transmitted to Aquilion ONE and processed through
slope method. The correction of the respiratory motion position was
carried out under the body correction software in the body
perfusion software. Consequently the corrected data package was
introduced into the body perfusion software and the double input
mode was selected to carry out the analysis. The abdominal aorta
and the portal vein were selected as feeding artery and draining
vein. The region of interest was respectively arranged
corresponding to aorta abdominalis, portal vein, liver and spleen
to generate the TDC curve. The perfusion parameters of pseudo color
pictures (red for high perfusion, blue low perfusion and the
remaining colors between them) of TDC hepatic arterial perfusion
(HAP), portal vein perfusion (PVP) volume and hepatic arterial
perfusion index (HAPI) could be obtained in the lines of TDC curve.
Three axial, coronal and sagittal ROI perfusion parameters were
obtained randomly. Preoperative and postoperative ROI selection was
kept as consistent as possible. ROI selection was done by two
radiologists at deputy director level and each value was repeatedly
measured every 4 weeks and the average values were calculated. Four
to six weeks after operation, patients were examined using the same
method for post-processing imaging and analyses. After the first
examination, they were re-examined once every 3 months.
For preoperative CT imaging, we chose the correct
position, supine, prone position or lateral position. We then
determined the surface puncture, puncture path, measuring needles
angle and depth and the required number of cryotherapy probes to
develop the correct arrangement and sequences. Using CT images,
argon-helium superconductive operation system (Endocare, Irvine,
CA, USA) entered the tumor target with argon-helium knife special
puncture needle (the probe in 2 or 3 mm and the tip in 1.5 mm)
along the proposed puncture points. Subsequently, we went back from
the needle core and the expansion tube, leaving in the catheter
sheath, and accurately embedded argon-helium knife in the sheath
(sheath was withdrawn 3–5 cm). The ultra-low temperature operation
system initiated after CT imaging confirmed the correct position of
the argon-helium knife. The treatment mode was as follows: The
argon was frozen for 10–15 min then helium rewarmed for 3 min (this
was considered a cycle). Probe could be affected by the local
anatomical structure, in the cases of larger tumors, tumors that
are very close to adjacent tissue structure and tumors already
invading other tissues. During a single cycle we melted up to
70–90% of the tumor, however, it was difficult to melt the entire
tumor in one attempt. Residual tumor was treated during the second
cycle. During the cryoablation process, patients were monitored
closely and were asked to advise the investigators should there be
any discomfort during the process. CT scanning of the ablation zone
was performed periodically to better monitor the ice ball formation
and to avoid freezing injuries to adjacent vital structures. After
the operation, the patients were monitored closely to verify
whether there were any adverse reactions, such as fever and pain in
order to treat them properly.
Observation index
Differences in tumor treatment effects, AFP changes,
total frozen area, freezing time, complication, median survival
time and perfusion parameters were compared. The effects of tumor
treatment was in accordance with RECIST standards, which was
divided into complete remission (CR), partial remission (PR),
stable disease (SD) and disease progression (PD). The total
efficiency was calculated as: CR+PR+SD/total number of the cases ×
100%.
Statistical analysis
SPSS 19.0 statistical software (Chicago, IL, USA)
was used for data analysis. Quantitative data were expressed as
mean + standard deviation and qualitative data were expressed as
the number of cases or percentage (%). Comparison in the two groups
was tested using the independent samples t-test. The paired t-test
was used for same group comparisons and the comparison of two
groups was tested using the χ2 test. Median survival was
analyzed by Kaplan-Meier. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison between the effects of
tumor therapy and the occurrence of complications
The total effective rate for the observation group
was significantly higher than that in the control group and the
total incidence of complications in the observation group was
significantly lower than that of the control group. Differences
were statistically significant (P<0.05) (Table I).
 | Table I.Comparison of the effects of tumor
therapy and the occurrence of complications. |
Table I.
Comparison of the effects of tumor
therapy and the occurrence of complications.
| Group types | Case | CR | PR | SD | PD | Total effective
rate | Fever | Pain | Skin frostbite | Nausea and
vomiting | Abdominal
bleeding | Bile duct fistula and
hepatic abscess | Others | Total incidence
rate |
|---|
| Observation | 60 | 12 | 16 | 20 | 12 | 48 (80.0) | 4 | 3 | 1 | 3 | 2 | 3 | 2 | 18 (30.0) |
| Control | 60 | 8 | 14 | 15 | 23 | 37 (61.7) | 5 | 4 | 2 | 4 | 5 | 6 | 3 | 29 (48.3) |
| χ2 |
|
|
|
|
| 4.881 |
|
|
|
|
|
|
| 4.232 |
| P-value |
|
|
|
|
| 0.027 |
|
|
|
|
|
|
| 0.040 |
Comparison of AFP changes, total
freezing area and freezing time
Following treatment, AFP was significantly lower in
the observation group. Total freezing area and freezing time were
significantly lower than those in the control group. Differences
were statistically significant (P<0.05) (Table II).
 | Table II.Comparison of AFP changes, total
freezing area and freezing time. |
Table II.
Comparison of AFP changes, total
freezing area and freezing time.
| Groups | AFP (ng/ml) | Total freezing area
(cm2) | Freezing time
(min) |
|---|
| Observation | 124.5±42.6 | 7.2±1.5 | 20.7±4.2 |
| Control | 206.3±51.7 | 9.6±1.7 | 34.9±4.8 |
| t-test | 6.325 | 10.624 | 8.452 |
| P-value | <0.001 | <0.001 | <0.001 |
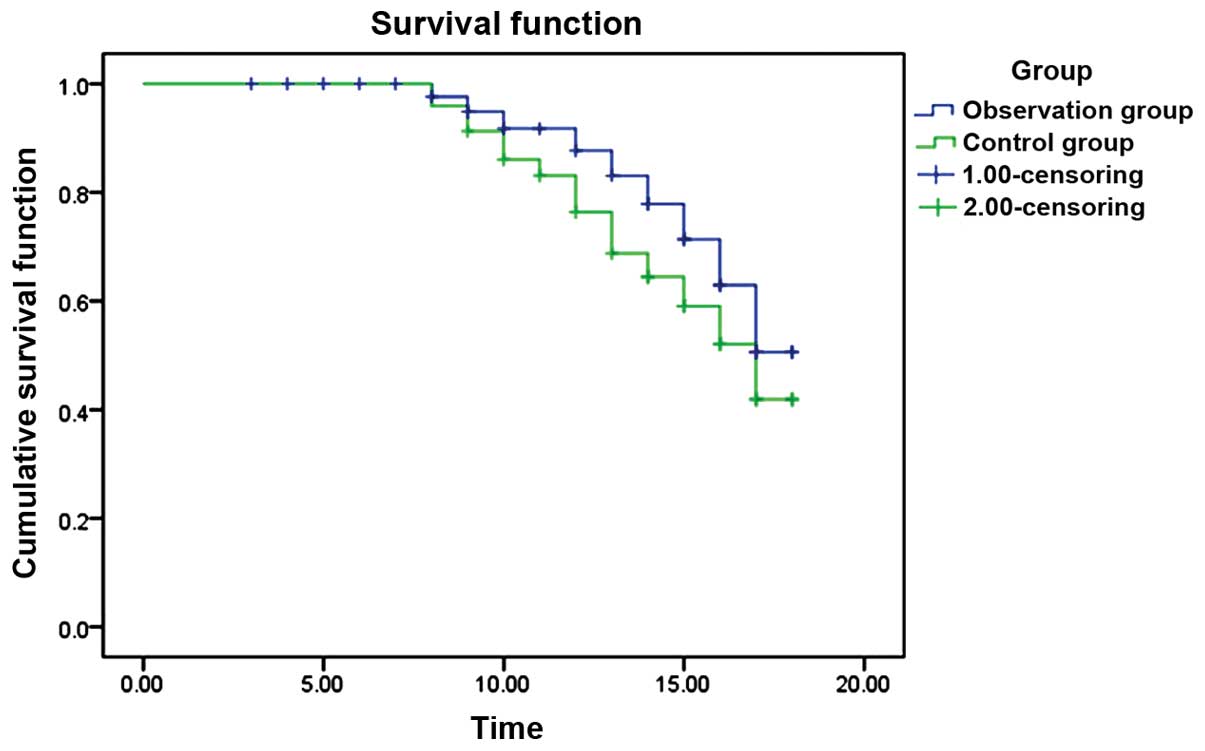
Comparison of median survival
time
The two groups were followed up for an average of 14
months. In the observation group, 18 cases (30.0%) succumbed while
in the control group the number of deaths was 29 (48.3%). The
mortality rate in the observation group decreased significantly.
Differences were statistically significant (χ2 2=4.232,
P=0.040). In the observation group, the median survival time was
>17 months and median survival time in the control group was 17
months. Differences were statistically significant (log-rank test
χ2=10.744, P=0.001) (Fig.
1).
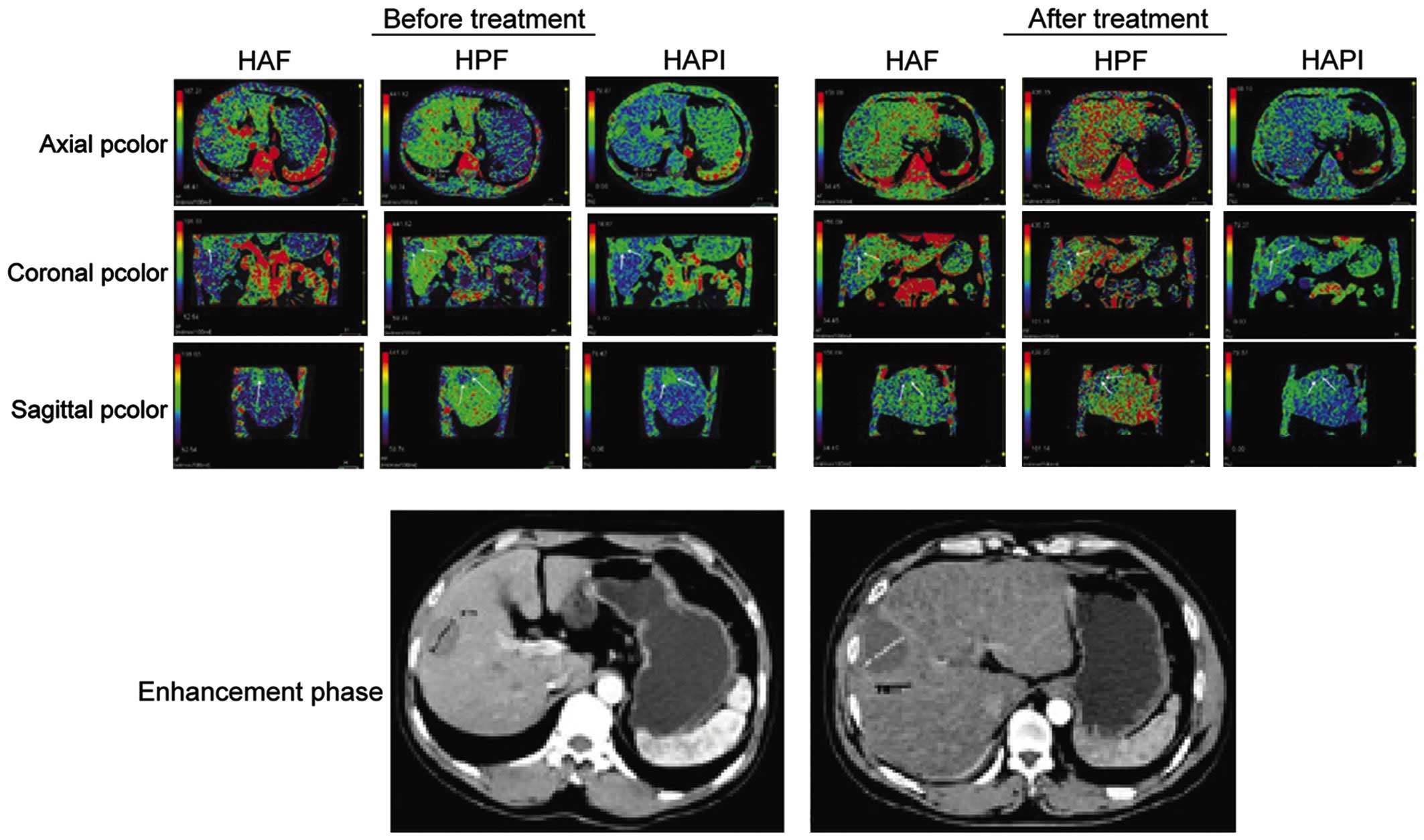
Comparison of perfusion parameters in
the observation group
Following treatment, HAP, PVP and HAPI levels were
significantly lower than those before treatment. Differences were
statistically significant (P<0.05) (Table III and Fig. 2).
 | Table III.Comparison of perfusion parameters in
the observation group before and after treatment. |
Table III.
Comparison of perfusion parameters in
the observation group before and after treatment.
| Item | HAP (ml/min ·100
ml) | PVP (ml/min ·100
ml) | HAPI (%) |
|---|
| Before treatment | 124.6±33.2 | 82.7±24.3 | 69.4±18.5 |
| After treatment | 45.5±10.6 | 63.2±16.9 | 30.5±8.2 |
| t-test | 15.624 | 9.328 | 7.826 |
| P-value | <0.001 | <0.001 | <0.001 |
Discussion
The principle of argon-helium cryoablation is as
follows: i) High pressure normal temperature argon gas (cold
medium) and high pressure normal temperature helium (heat medium)
are introduced sequentially; ii) argon expands rapidly at the point
of the knife and the temperature in lesion tissue reaches −140°C
within 60 sec; and iii) helium expands rapidly at the point of the
knife and thaws the ice ball rapidly by elevating the temperature
up to 40–45°C. Thermocouple installed at the point of the knife
constantly monitors the temperature. Basic studies on cryoablation
revealed that intracellular ice had the tendency to induce cell
death (8). The sudden formation of
ice crystals in cells rapidly destroys the fluidity and osmotic
pressure balance of the cell membrane. By increasing the freezing
speed, the number of dead cells were markedly elevated. Owing to
the recrystallization of ice crystals, keeping cells frozen for an
extended period can further increase cell damage. On termination of
the freezing period, the thawing period is initiated. Ice crystals
expand when helium rapidly increases the temperature, which induces
the explosion of ice balls formed during the freezing period and
this explosion destroys the tumor. After the heating period there
is another round of freezing to attack any tumor cells that escaped
the initial freezing round. After 2 cycles of freezing and thawing,
lesion tissues can be destroyed completely (9).
Argon-helium cryoablation treatment can cause
hepatic failure, myoglobinuria, cold shock and other complications.
The extent of freeze thaw area is an important factor (10) and the total acreage of freeze can be
an independent predictor of whether postoperative complications
would increase. Results showed that the total treatment efficiency
in the observing group significantly increased and the total
incidence of complications was significantly reduced. Values of
HAP, PVP and HAPI were significantly lower compared with before
treatment. We believe that the application of 64-slice CT helped
the planning process of percutaneous cryoablation. Intraoperative
argon-helium knife in combination with 64-slice CT to control the
freezing and thawing acreage, can expand the scope of the damage,
reduce recurrence and reduce complications (11).
In the present study, for the first time we used a
1.64-slice spiral CT to plan treatment using an argon-helium knife.
The application of the CT perfusion analysis software package in
preoperative blood supply and staging of liver cancer and tumor
range evaluation made the argon-helium targeting cryoablation more
accurate and reliable (12). The
application of 64-slice spiral CT with the z-axis coverage of up to
4 cm, was useful in gaining a more comprehensive analysis of the
hemodynamic characteristics of liver cancer and we applied the
perfusion pattern to evaluate the flow pattern of the liver cancer
(13). In order to guide the surgical
approach and expand or reduce the scope of freezing and thawing,
the 64-slice CT 3D reconstruction technique was used in this study
(14). The study had a few defects:
i) Success of the scan was dependent on the patients respiratory
training; ii) time scan range of breath holding scan was different,
thus the choice of time period needed to be repeated; iii) there
was no uniform standard in result assessment and time of freezing
for argon-helium cryoablation.
We concluded that the use of 64-slice spiral CT
perfusion imaging in the treatment of liver cancer can
significantly improve the treatment efficiency of argon-helium
cryoablation. This method can extend the survival time and reduce
the complications.
Acknowledgements
The present study was supported by the Medical
Science Research Key Project of Hebei Province (no. 20110157).
References
|
1
|
Vitali GC, Laurent A, Terraz S, Majno P,
Buchs NC, Rubbia- Brandt L, Luciani A, Calderaro J, Morel P,
Azoulay D, et al: Minimally invasive surgery versus percutaneous
radio frequency ablation for the treatment of single small (≤3 cm)
hepatocellular carcinoma: a case-control study. Surg Endosc. 3:3–5.
2015.
|
|
2
|
Hao XJ, Li JP, Jiang HJ, Li DQ, Ling ZS,
Xue LM and Feng GL: CT assessment of liver hemodynamics in patients
with hepatocellular carcinoma after argon-helium cryoablation.
Hepatobiliary Pancreat Dis Int. 12:617–621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang C, Lu Y, Chen Y, Feng Y, An L, Wang
X, Su S, Bai W, Zhou L, Yang Y, et al: Prognostic factors and
recurrence of hepatitis B-related hepatocellular carcinoma after
argon-helium cryoablation: a prospective study. Clin Exp
Metastasis. 26:839–848. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang CP, Lu YY, Wang XZ, An LJ, Su SH,
Zhou L, Chen Y, Jia HJ, Feng YY and Yang YP: Percutaneous
argon-helium cryoablation for primary hepatocellular carcinoma:
report of 300 cases. Med J Chin PLA. 133:1413–1417. 2008.
|
|
5
|
Ma GL, Bai RJ, Jiang HJ, Hao XJ, Dong XP,
Li DQ, Liu XD and Wei L: Early changes of hepatic hemodynamics
measured by functional CT perfusion in a rabbit model of liver
tumor. Hepatobiliary Pancreat Dis Int. 11:407–411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li JP, Zhao DL, Jiang HJ, Huang YH, Li DQ,
Wan Y, Liu XD and Wang JE: Assessment of tumor vascularization with
functional computed tomography perfusion imaging in patients with
cirrhotic liver disease. Hepatobiliary Pancreat Dis Int. 10:43–49.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei S, Hao X, Zhan D, Xiong M, Li K, Chen
X and Huang Z: Are surgical indications of Barcelona Clinic Liver
Cancer staging classification justified? J Huazhong Univ Sci
Technolog Med Sci. 31:637–641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu HB, Ge CL, Huang ZH, Wang H, Liu ZY and
Zhang JR: Effect of targeted argon-helium cryoablation on the
portal region in canine livers. Nan Fang Yi Ke Da Xue Xue Bao.
29:538–540. 2009.(In Chinese). PubMed/NCBI
|
|
9
|
Wu S, Hou J, Ding Y, Wu F, Hu Y, Jiang Q,
Mao P and Yang Y: Cryoablation versus radiofrequency ablation for
hepatic malignancies: a systematic review and literature-based
analysis. Medicine (Baltimore). 94:e22522015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang YZ, Zhou SC, Zhou H and Tong M:
Radiofrequency ablation versus cryosurgery ablation for
hepatocellular carcinoma: a meta-analysis. Hepatogastroenterology.
60:1131–1135. 2013.PubMed/NCBI
|
|
11
|
Jain R, Narang J, Schultz L, Scarpace L,
Saksena S, Brown S, Rock JP, Rosenblum M, Gutierrez J and Mikkelsen
T: Permeability estimates in histopathology-proved
treatment-induced necrosis using perfusion CT: can these add to
other perfusion parameters in differentiating from
recurrent/progressive tumors? AJNR Am J Neuroradiol. 32:658–663.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sabir A, Schor-Bardach R, Wilcox CJ,
Rahmanuddin S, Atkins MB, Kruskal JB, Signoretti S, Raptopoulos VD
and Goldberg SN: Perfusion MDCT enables early detection of
therapeutic response to antiangiogenic therapy. AJR Am J
Roentgenol. 191:133–139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haider MA, Farhadi FA and Milot L: Hepatic
perfusion imaging: concepts and application. Magn Reson Imaging
Clin N Am. 18465–475. (x)2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsushima Y, Funabasama S, Aoki J, Sanada S
and Endo K: Quantitative perfusion map of malignant liver tumors,
created from dynamic computed tomography data. Acad Radiol.
11:215–223. 2004. View Article : Google Scholar : PubMed/NCBI
|