Introduction
Urinary bladder cancer is the ninth most common
cancer worldwide (1), and occurs in a
ratio of ~5:1 with respect to non-muscle-invasive vs.
muscle-invasive phenotypes (2).
Non-muscle-invasive tumors may be treated by combined therapy
involving transurethral resection and intravesical chemotherapy
(3), whereas muscle-invasive bladder
cancer can be managed with radical cystectomy and pelvic
lymphadenectomy, according to the current gold standard method
(4). Critically, among all tumor
types, bladder cancer exhibits a significantly greater rate of
recurrence following treatment, and the per-patient cost of
treating this cancer is therefore considered to be the highest
(5). As current therapies continue to
produce unsatisfactory rates of morbidity and mortality, the
development of novel treatment strategies to manage bladder cancer
remains a challenge.
Rottlerin
[1-(6-[(3-acetyl-2,4,6-trihydroxy-5-methylphenyl)methyl]-5,7-dihydroxy-2,2-dimethyl-2H-1-benzopyran-8-yl)
−3-phenyl-2-propen-1-one; also known as mallotoxin] is a
traditional Indian medicine that is used against tapeworm, scabies
and herpetic ringworm. It has been used as a protein kinase C δ
(PKC-δ) inhibitor to verify the biological function of PKC-δ
(6). Recent scientific research has
confirmed that rottlerin has a range of molecular targets and
antitumor activities, including inhibition of cell proliferation,
suppression of cell growth, induction of apoptosis,
anti-angiogenesis and inhibition of reactive oxygen species
formation (7–10). However, the biological mechanism
underlying the antitumor effect of rottlerin remains unclear.
In eukaryotic cells, autophagy is important process
by which protein degradation and organelle turnover can occur, and
acts as a critical adaptive response during cell stress or
starvation to recycle energy and nutrients (11). The activation of autophagy is
associated with various stress conditions, whereas its dysfunction
is linked to numerous types of human diseases, including cancer
(12). Consequently, autophagy has
been widely considered to be a potential novel target for cancer
therapy (13). Autophagy and
apoptosis are intricately linked: Both are genetically regulated
and evolutionarily conserved processes that can determine cell
fate; however, while apoptosis invariably leads to cancer cell
death, autophagy may occur as a survival response to growth factors
or nutrient deprivation, but may also act as an important molecular
mechanism for tumor cell suicide (14). Numerous studies have indicated that if
cellular damage is extensive or if apoptosis is compromised,
autophagy may be used to kill cells (15,16).
In the present study, the antitumor activity of
rottlerin on EJ malignant bladder cancer cells was investigated and
its role in the induction of autophagy was examined. Based on the
results, we propose that rottlerin-induced autophagy may contribute
to the antitumor effect via apoptosis.
Materials and methods
Cell culture
EJ human bladder carcinoma cells were purchased from
ATCC (Manassas, VA, USA) and maintained in Invitrogen RPMI-1640
medium (Thermo Fisher Scientific, Inc., Carlsbad, CA, USA) with 10%
fetal bovine serum (Sigma-Aldrich; Merck Millipore, Billerica, MA,
USA), 1% non-essential amino acids and 1% penicillin/streptomycin
in a humidified incubator at 37°C supplied with 5%
CO2.
Reagents
Rottlerin (Sigma-Aldrich; Merck Millipore) was
dissolved in dimethyl sulfoxide (DMSO) as a stock solution of 20 mM
and stored at −20°C. Human microtubule-associated protein 1 light
chain 3 (LC3; cat. no. ABC432; working dilution, 1:1,000) antibody
was purchased from Sigma-Aldrich (Merck Millipore). Human caspase-3
(cat. no. ab13585; dilution, 1:1,000) and human poly (ADP-ribose)
polymerase (PARP; cat. no. ab75607; dilution, 1:400) antibodies
were purchased from Abcam (Cambridge, MA, USA). The human
anti-β-actin antibody (cat. no. sc-130065; dilution, 1:1,000) was
obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Horseradish peroxidase-conjugated donkey anti-rabbit (cat. no.
NA934; dilution, 1:1,000) and anti-mouse (cat. no. N1034; dilution,
1:10,000) IgG secondary antibodies were obtained from GE Healthcare
Life Sciences (Uppsala, Sweden).
MTT cell viability assay
Cell viability was measured by MTT assay. EJ cells
(5,000/well) were seeded into 96-well plates and cultured in a
CO2 incubator overnight. Rottlerin at various
concentrations was then added. Following incubation periods of 24,
48 and 72 h, 10 µl of MTT solution (Sigma-Aldrich; Merck
Millipore), dissolved in autoclaved phosphate-buffered saline (PBS)
at 5 mg/ml, was added. After 4 h, 150 µl of DMSO was added to
dissolve the formazan crystals, and the absorbance (optical
density; OD) of each well was measured using an automatic
microplate reader (Thermo Fisher Scientific, Inc.) at 490 nm. Each
experiment was repeated three times. Cell viability results were
calculated as a percentage, as follows: Cell viability % =
(ODtreatment-ODblank)/(ODcontrol-ODblank)×100.
Transmission electron microscopy
(TEM)
TEM was used to determine the appearance of
autophagosomes in treated and control EJ cells. Cells treated with
rottlerin (2 µM) or without for 48 h were harvested by
trypsinization, washed with ice-cold PBS and fixed in 2.5%
glutaraldehyde in 0.1 M phosphate buffer, then post-fixed in 1%
osmium tetroxide buffer. Following dehydration in a graded series
of ethanol, the cells were embedded in Spurr resin. Ultra-thin
sections (60 nm) were cut on an ultramicrotome and were
subsequently observed under a JEM-1230 transmission electron
microscope.
Western blot analysis
Cells were treated with rottlerin (2 µM) and washed
with ice-cold PBS at 48 h post-treatment, and cell lysate was
isolated using Protein Extraction Reagent (Beyotime Institute of
Biotechnology, Nantong, China). Equal amounts of cell lysate
protein (30 µg) were separated by gel electrophoresis on a 6–12%
gradient SDS-PAGE gel and transferred onto nitrocellulose
membranes. The membranes were blocked with 5% non-fat dried milk in
PBS for 1 h at room temperature prior to the application of the
specific primary antibodies for incubation overnight at 4°C.
Subsequently the membranes were washed three times with PBS
containing Tween-20, and incubated with secondary antibodies at
room temperature for 1 h. The blots were detected with an ECL kit
(Beyotime Institute of Biotechnology) using a Storm 840
PhosphorImager system (Molecular Dynamics, Inc., Sunnyvale, CA,
USA). The images were further analyzed using Image Quant TL 8.1
software (GE Healthcare Life Sciences).
Flow cytometric analysis
Apoptosis was analyzed by flow cytometry using
propidium iodide (PI) and an annexin V-fluorescein isothiocyanate
FITC staining kit (BD Biosciences, Franklin Lakes, NJ, USA). Cells
were detached by trypsinization at 37°C for 5 min. Detached cells
were rinsed twice with RPMI-1640 medium and cold PBS, then
separated by centrifugation at 168 × g for 5 min at room
temperature. Cells were resuspended in 1X binding buffer at a
concentration of 1×106 cells/ml. Cell/buffer solution
(100 µl; 1×105 cells) was transferred to a round-bottom
tube and incubated with 5 µl of PI and annexin V-FITC for 15 min at
room temperature in darkness. Finally, 400 µl of 1X binding buffer
was added to each sample tube and evaluated by flow cytometry
(FACSCalibur; BD Biosciences) according to the manufacturer's
instructions.
Cell cycle analysis
Cells were trypsinized, washed twice with cold PBS,
fixed in ice-cold 70% ethanol and stored at 4°C. Prior to analysis,
cells were washed again with PBS, suspended in 50 g/ml PI and 0.25
g/ml RNase A, and further incubated for 30 min in the dark.
Cytometric analyses were performed using flow cytometer and
CellQuest v1.0.2 software (BD Biosciences). For each determination,
~10,000 cells were counted.
Clonogenic (colony formation)
assay
The clonogenic assay was performed to evaluate in
vitro cell survival following treatment with rottlerin. Cells
were seeded in 6-well plates (2×105/well) and treated
with rottlerin (2 µM) at 24 h after seeding. Untreated cells were
used as a negative control for this analysis. After a further 24 h
incubation, cells were trypsinized and washed once with PBS, then
counted and seeded in 6-well plates (200 cells/well). Each
experiment was repeated three times. All experimental samples were
incubated in an environment of 5% CO2 at 37°C for 14
days to form appropriately large clones consisting of ≥50 cells.
Cells were subsequently stained with crystal violet staining
solution (Beyotime Institute of Biotechnology). The visible
colonies (≥50 cells) were counted and typical images were captured
using a Nikon camera.
Statistical analysis
All statistical analyses were performed with SPSS
software version 17.0 (SPSS, Inc., Chicago, IL, USA). Results are
presented as the mean ± standard error of the mean. Different test
conditions were compared using a one-way analysis of variance or
Student's t-test, with P<0.05 considered to indicate a
statistically significant difference.
Results
Rottlerin inhibits the growth of human
malignant bladder cancer EJ cells
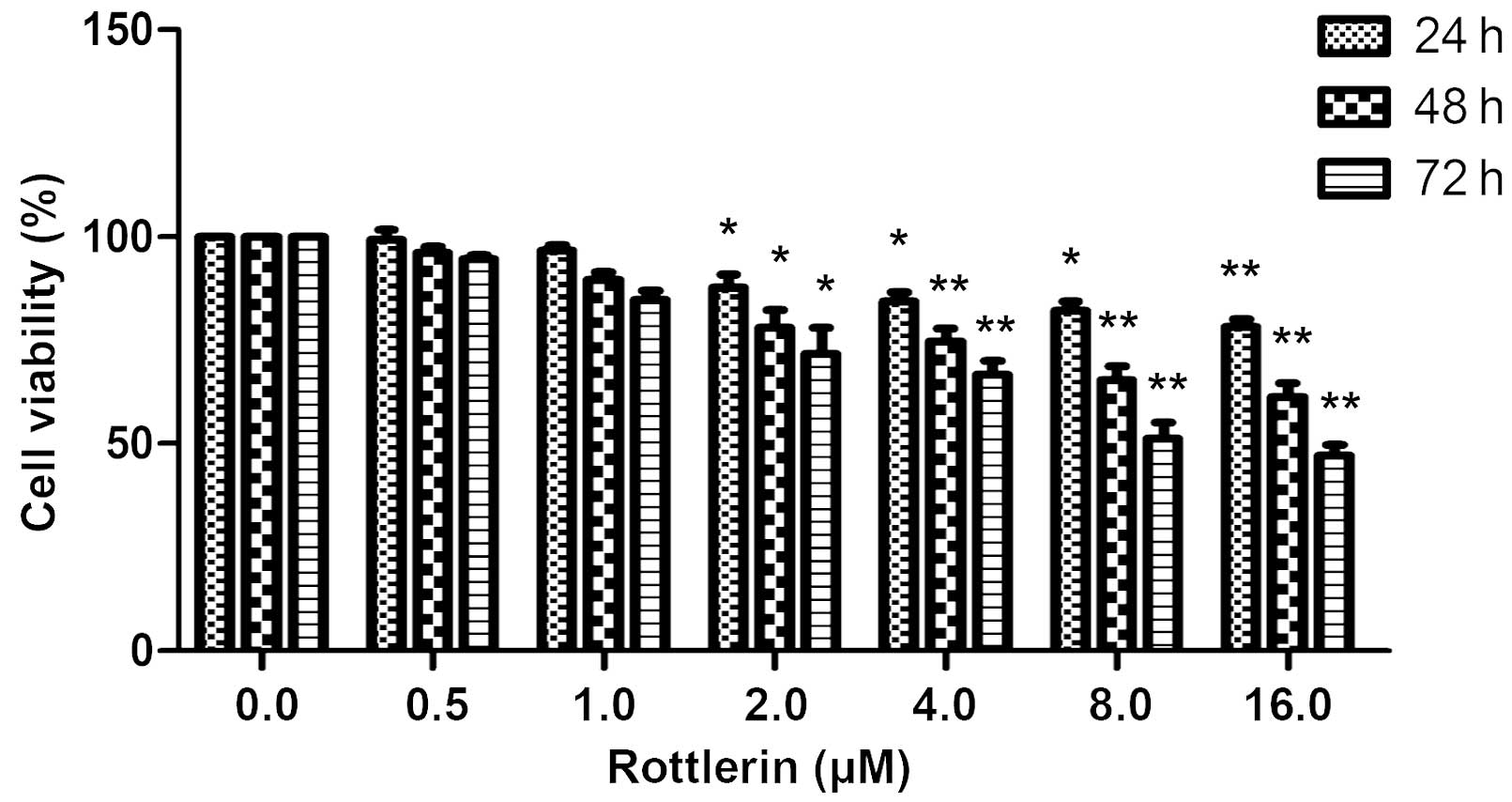
Using an MTT assay, the effects of rottlerin on EJ
bladder cancer cells were determined. The cells were treated with
0–16 µΜ rottlerin for 24, 48 or 72 h prior to the assessment of
cell viability. As shown in Fig. 1,
rottlerin had a growth inhibitory effect on EJ cells in a
dose-dependent and time-dependent manner, indicating that rottlerin
has antitumor effects in this cell type; however, concentrations of
0, 0.5 and 1 µΜ rottlerin had non-significant effects on the cells,
and 2 µΜ was therefore selected for further experiments.
Rottlerin activates autophagy in EJ
cells
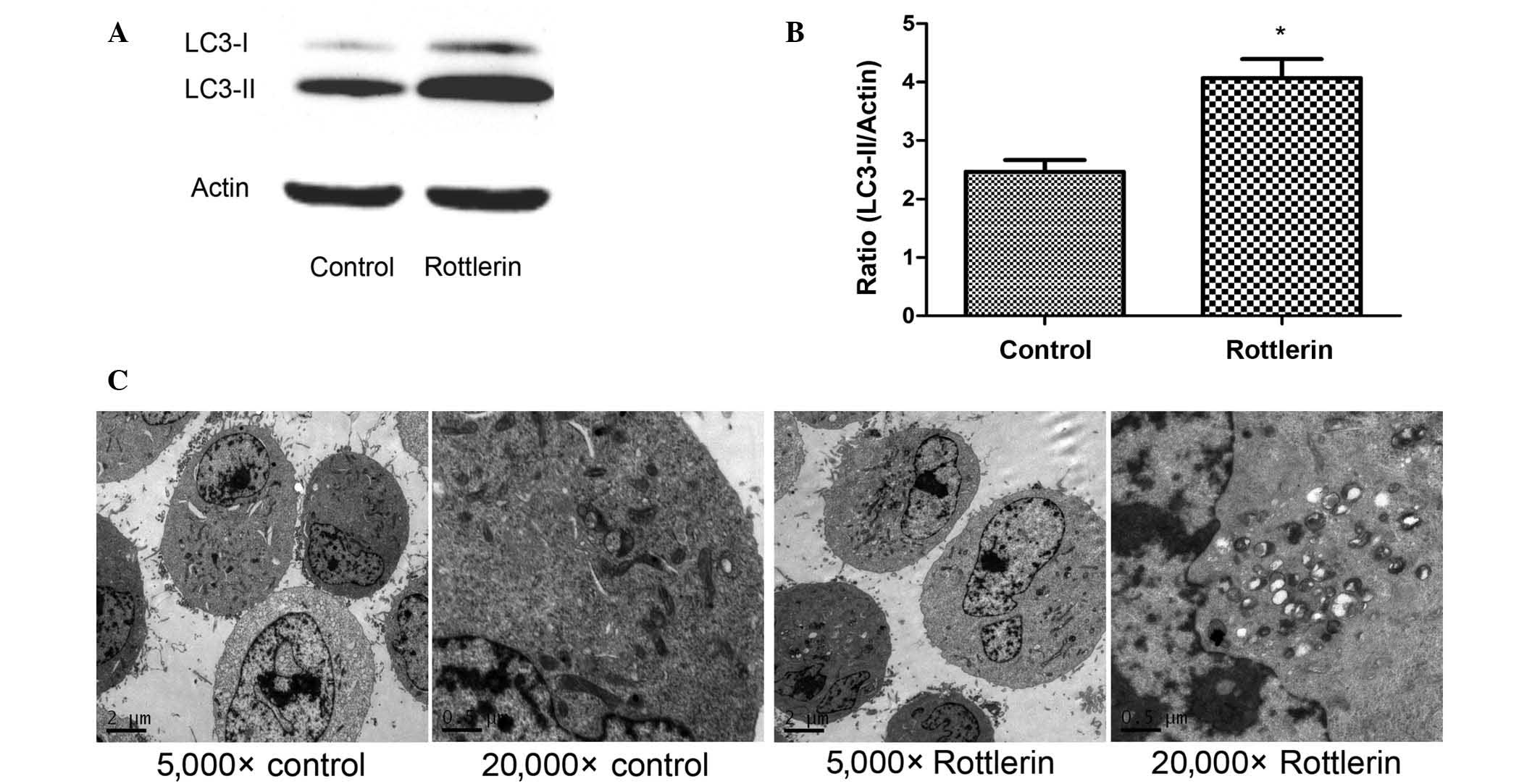
To evaluate the activation of autophagy by
rottlerin, the expression levels of the autophagy-related protein
LC3 were assessed by western blotting analysis and autophagosome
formation was observed by TEM. During autophagosome formation, the
microtubule-associated LC3-I is converted to the membrane-bound
form LC3-II (17). In EJ cells, the
expression level of LC3 increased following treatment with
rottlerin (Fig. 2A) as compared with
the control cells, and quantification of the protein expression
levels demonstrated that this difference was significant (P=0.014)
(Fig. 2B). These results indicate
that autophagy is activated by rottlerin. To further confirm this
finding, TEM studies were performed, revealing a greater amount of
autophagosomes in rottlerin-treated EJ cells, whereas no
autophagosomes were observed in the untreated control group
(Fig. 2C). Collectively these data
suggest that autophagy is induced by rottlerin in EJ cells.
Rottlerin promotes apoptosis and cell
cycle arrest in EJ cells
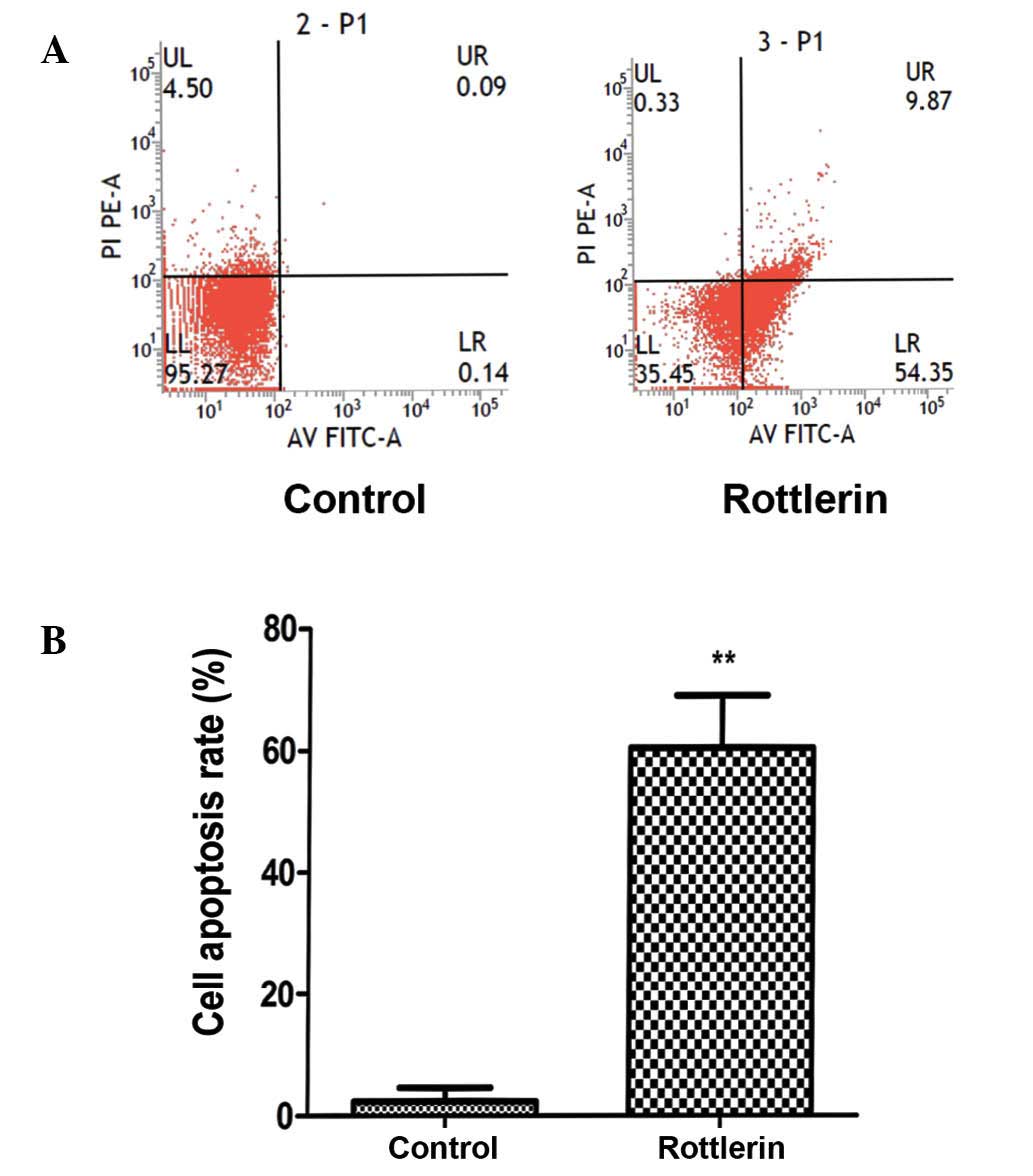
To assess apoptosis, a PI and annexin V-FITC assay
was performed. The results revealed that a marked increase in
apoptosis occurred following a 48-h treatment (Fig. 3A). Quantification of the apoptosis
rates demonstrated a significant increase in apoptosis in cells
treated with rottlerin (P<0.01) (Fig.
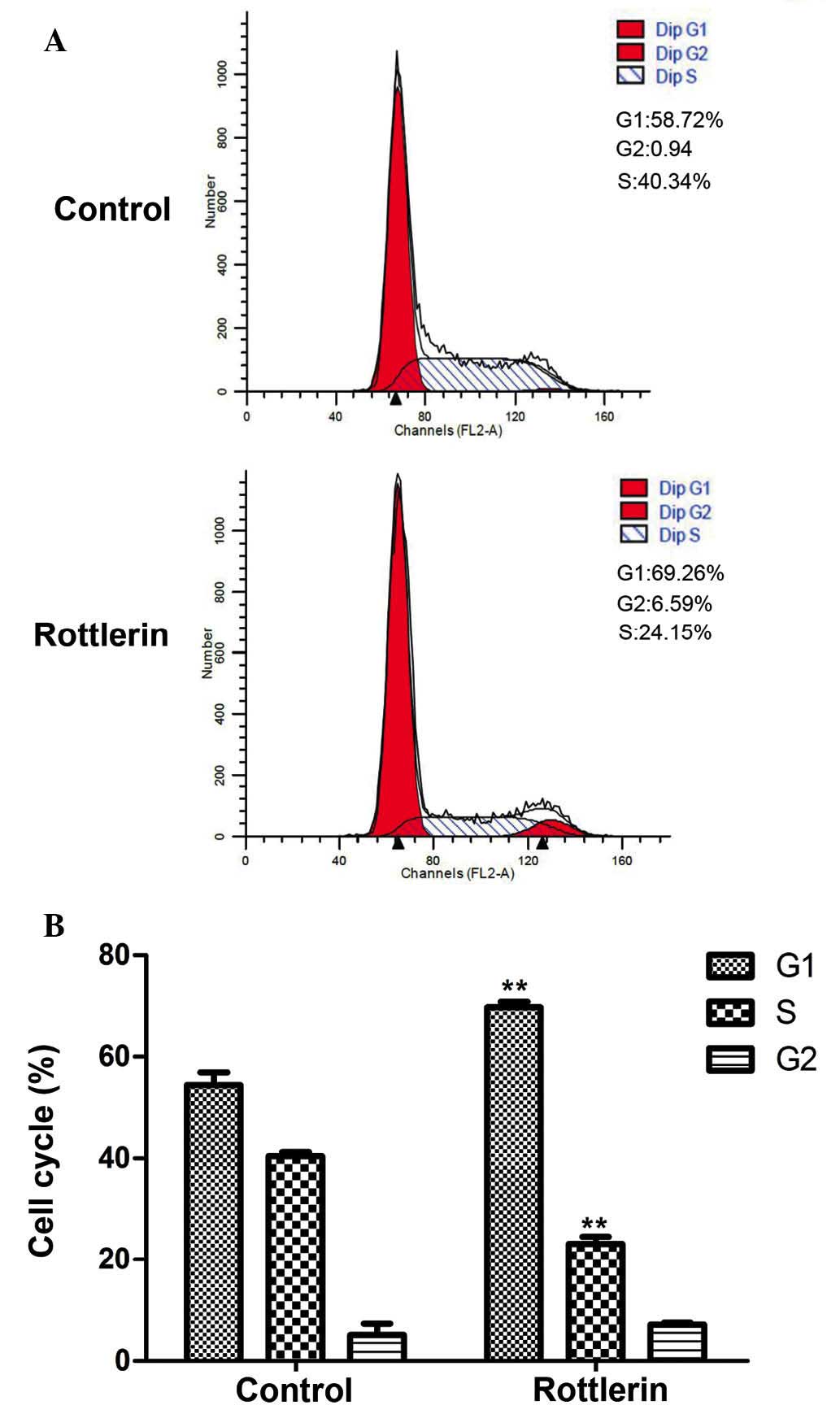
3B). Cell cycle distribution was also analyzed by flow
cytometry (Fig. 4A and B). EJ cells
were predominantly blocked in G1 phase, indicating that the cell
cycle distribution of EJ cells was affected by rottlerin. These
results indicate that, after 48 h treatment, the percentage of
cells arrested in G1 phase was increased from 54.4±2.5 to 69.7±1.1%
(P=0.005).
Rottlerin induces apoptotic cell death
through a caspase-independent pathway
Rottlerin induces apoptosis in certain cancer cell
lines (18), and the activation of a
caspase cascade is important in apoptotic induction and execution
(19). To confirm the involvement of
caspases in rottlerin-induced apoptosis, the expression levels of
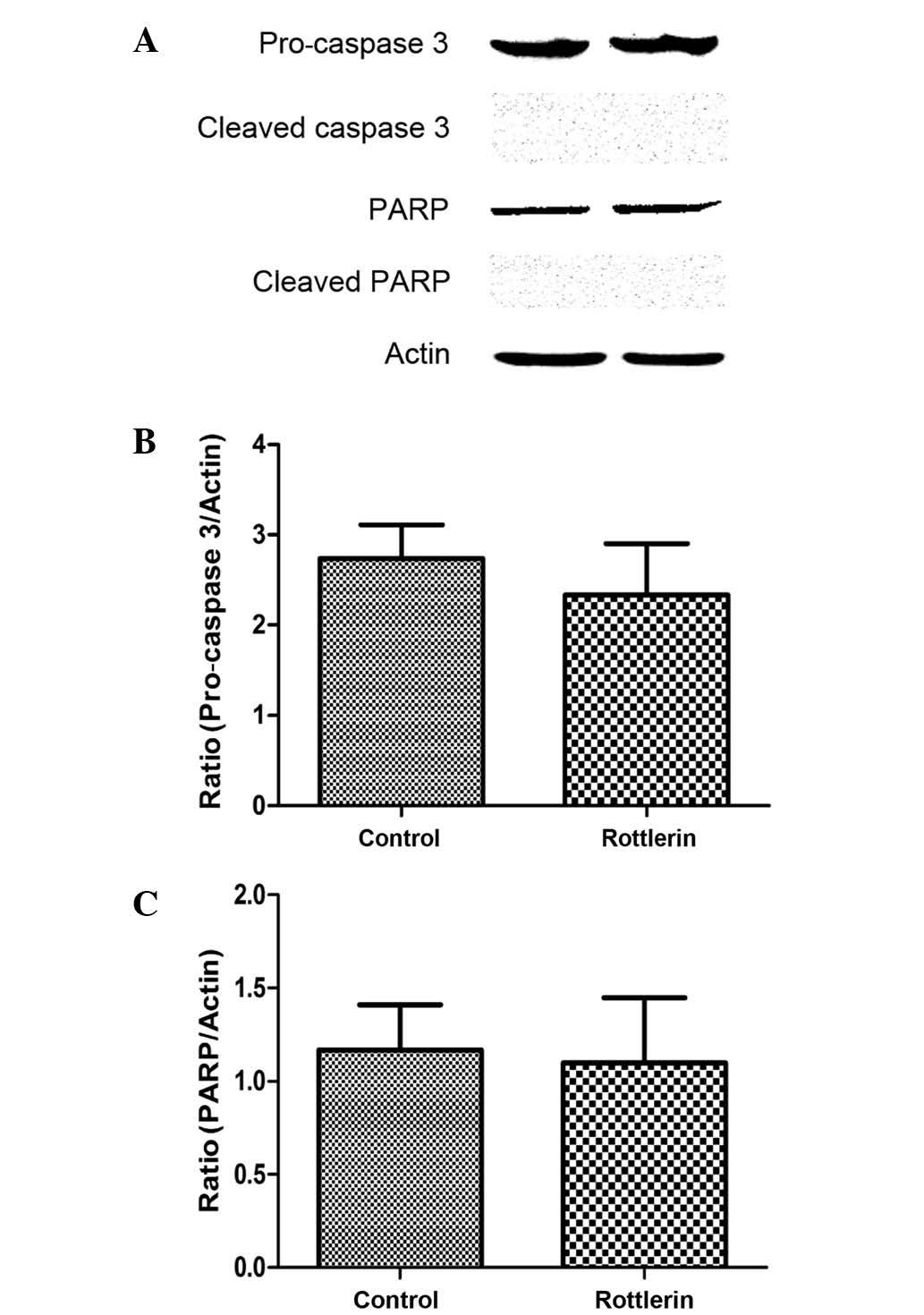
pro-caspase-3 and PARP were measured by western blot. The results
indicated that pro-caspase-3 and PARP levels were not increased in
rottlerin-treated cells (Fig. 5A-C),
and the cleaved forms of caspase-3 and PARP were also not detected
(Fig. 5A). These findings indicate
that caspases are not involved in rottlerin-induced apoptosis in EJ
cells.
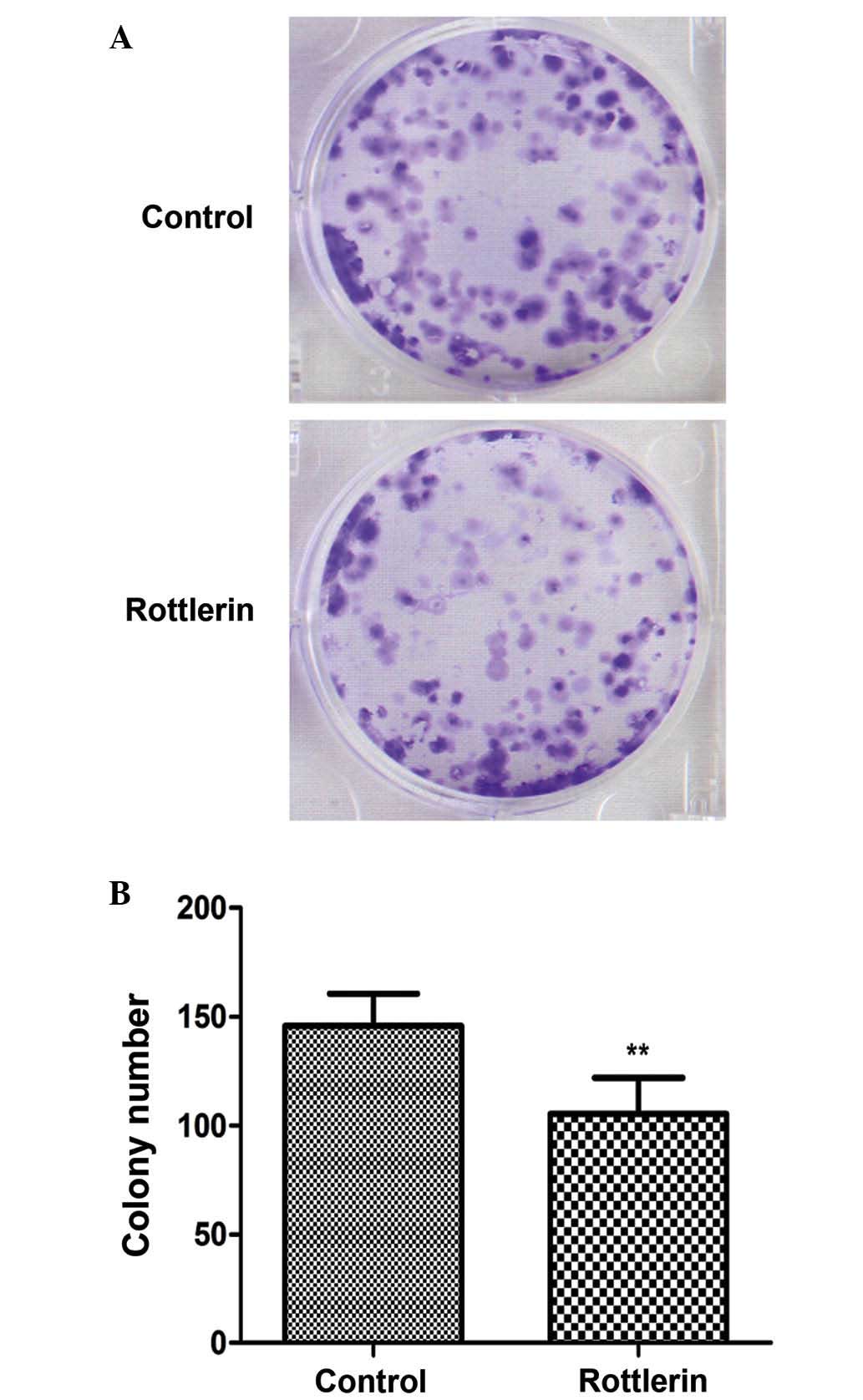
Rottlerin reduces the clonogenic
capacity of EJ cells
As shown in Fig. 6, EJ
cells exposed to rottlerin 48 h treatment exhibited a prominent
reduction in the clonogenic capacity compared with untreated cells
(P=0.003).
Discussion
The present study provides evidence that induction
of autophagy potentiates the rottlerin-induced apoptotic cell death
in human bladder cancer cells in vitro.
Rottlerin is a natural polyphenolic ketone that may
be isolated from the pericarps of Mallotus philippinensis
(20). Herbs and their derived
products have been the mainstay of traditional medicines all over
the world (21). The phytochemicals
presenting in these plants and their food products are generally
non-toxic and have the capacity to prevent chronic diseases
(22). Generally the plant products
encompass high concentrations of flavonoids and phenolic content
(23). Flavonoids serve a vital role
in protection against human diseases, including lipid peroxidation
involved in atherogenesis, thrombosis, carcinogenesis,
hepatotoxicity and a variety of other disease conditions (24,25).
Rottlerin as a plant extract has numerous different functions.
The present results demonstrate that rottlerin has a
growth inhibitory effect on EJ cells in a dose-dependent and
time-dependent manner, and also reduces the clonogenic capacity of
these cells; thus, in vitro cell survival capacity is
reduced following treatment with rottlerin. Recently, increasing
evidence has demonstrated that autophagy and apoptosis often occur
in the same cells, initiated in response to the same stimuli
(26). In this circumstance,
autophagy either promotes or inhibits apoptosis. The majority of
studies have indicated that autophagy inhibition sensitizes tumor
cells to a wide spectrum of cancer therapies, while others have
shown that treatment-induced tumor cell death requires intact
autophagic machinery (27–32). For instance, atorvastatin is
considered by some to cause autophagy to promote apoptosis in human
prostate cancer cells (33), whilst
others have a contrasting views, and consider that inhibition of
autophagy potentiates atorvastatin-induced apoptotic cell death
(34). Although there is known
crosstalk between autophagy and apoptosis, since they share certain
signaling pathways and proteins, the mechanism where this bridging
occurs has not been fully defined (35). In the present study autophagy was
induced by rottlerin, as indicated by a number of observations.
Firstly, the morphological appearances of autophagy were
identified, including increased amounts of autophagosomes on TEM,
which is the gold standard. In addition, quantification of LC3-II
protein expression levels revealed apparent changes in treated
cells. Finally, biochemical changes that are characteristic of
apoptotic cell death were observed, including an increased sub-G1
population.
As expected, treatments with rottlerin induced
autophagy and also produced a higher rate of apoptotic cell death
compared with controls in human bladder cancer cells in
vitro. Apoptosis is a kind of programmed cell death that is
important in maintaining adult tissue homeostasis and supporting
the embryonic tissue remodeling (36). There are three main apoptotic
pathways: i) The mitochondrial (or intrinsic) pathway, mediated by
the Bcl-2 superfamily members which interact with the mitochondrial
membrane; ii) the death receptor (extrinsic) pathway, governed by
specific death receptors that bind specific ligands, including
tumor necrosis factor (TNF), TNF-related apoptosis-inducing ligand
(which binds to the DR4 and DR5 death receptors), and FasL (a
ligand that binds to the Fas receptor); and iii) the endoplasmic
reticulum stress pathway, which is predominantly regulated by
inositol-requiring enzyme 1 and CHOP-mediated proapoptotic
signaling (37–40).
Caspases, a family of cysteine-dependent
aspartate-directed proteases, play a key role during the process of
apoptosis. Most of the apoptotic signaling pathways converge on the
activation of intracellular caspases and generate a complex
biochemical cascade that propagates death signaling (41). Rottlerin-induced apoptotic cell death
is considered to be mediated through a decrease of mitochondrial
membrane potential and translocation of apoptosis-inducing factor
(AIF) into the nucleus; results suggest that rottlerin-induced
apoptosis is mediated through mitochondrial membrane depolarization
and AIF translocation into the nucleus, via a caspase-independent
pathway (42). However, other
researchers have suggested that rottlerin-induced apoptosis is
mediated through the caspase pathway (43). The results of the present study reveal
that pro-caspase-3, cleaved caspase-3, total PARP and cleaved-PARP
protein expression levels were not apparently altered in cells
treated with rottlerin. From our results, it may be concluded that
rottlerin induces apoptosis through a caspase-independent pathway.
Further investigation should focus on the regulation of apoptosis
mechanisms to increase the antitumor effect of rottlerin
treatment.
Acknowledgements
The present study was supported by the National
Natural Science Foundation Project of China (grant no. 81560416)
and Natural Science Foundation Project of Gansu province(grant nos.
145RJYA256 and 1606RJZA044).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vishnu P, Mathew J and Tan WW: Current
therapeutic strategies for invasive and metastatic bladder cancer.
Onco Targets Ther. 4:97–113. 2011.PubMed/NCBI
|
|
3
|
Shariat SF, Karam JA, Lotan Y and
Karakiewizc PI: Critical evaluation of urinary markers for bladder
cancer detection and monitoring. Rev Urol. 10:120–135.
2008.PubMed/NCBI
|
|
4
|
Bellmunt J, Orsola A, Wiegel T, Guix M, De
Santis M and Kataja V: ESMO Guidelines Working Group: Bladder
cancer: ESMO clinical practice guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 22:(Suppl 6). Svi45–Svi49. 2011.
View Article : Google Scholar
|
|
5
|
Tanaka T, Miyazawa K, Tsukamoto T, Kuno T
and Suzuki K: Pathobiology and chemoprevention of bladder cancer. J
Oncol. 2011:5283532011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gschwendt M, Müller HJ, Kielbassa K, Zang
R, Kittstein W, Rincke G and Marks F: Rottlerin, a novel protein
kinase inhibitor. Biochem Biophys Res Commun. 199:93–98. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liao YF, Hung YC, Chang WH, Tsay GJ, Hour
TC, Hung HC and Liu GY: The PKC delta inhibitor, rottlerin, induces
apoptosis of haematopoietic cell lines through mitochondrial
membrane depolarization and caspases' cascade. Life Sci.
77:707–719. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ni H, Ergin M, Tibudan SS, Denning MF,
Izban KF and Alkan S: Protein kinase C-delta is commonly expressed
in multiple myeloma cells and its downregulation by rottlerin
causes apoptosis. Br J Haematol. 121:849–856. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valacchi G, Pecorelli A, Sticozzi C,
Torricelli C, Muscettola M, Aldinucci C and Maioli E: Rottlerin
exhibits antiangiogenic effects in vitro. Chem Biol Drug
Des. 77:460–470. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maioli E, Greci L, Soucek K, Hyzdalova M,
Pecorelli A, Fortino V and Valacchi G: Rottlerin inhibits ROS
formation and prevents NF kappaB activation in MCF-7 and HT-29
cells. J Biomed Biotechnol. 2009:7429362009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mizushima N and Klionsky DJ: Protein
turnover via autophagy: Implications for metabolism. Annu Rev Nutr.
27:19–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Doria A, Gatto M and Punzi L: Autophagy in
human health and disease. N Engl J Med. 368:18452013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hippert MM, O'Toole PS and Thorburn A:
Autophagy in cancer: Good, bad, or both? Cancer Res. 66:9349–9351.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hannigan AM and Gorski SM: Macroautophagy:
The key ingredient to a healthy diet? Autophagy. 5:140–151. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nishino I: Autophagic vacuolar myopathy.
Semin Pediatr Neurol. 13:90–95. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang YX, Xu SQ, Chen XH, Liu RS and Liang
ZQ: Autophagy involvement in olanzapine-mediated cytotoxic effects
in human glioma cells. Asian Pac J Cancer Prev. 15:8107–8113. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deretic V, Delgado M, Vergne I, Master S,
De Haro S, Ponpuak M and Singh S: Autophagy in Immunity against
mycobacterium tuberculosis: A model system to dissect immunological
roles of autophagy. Curr Top Microbiol Immunol. 335:169–188.
2009.PubMed/NCBI
|
|
18
|
Singh BN, Kumar D, Shankar S and
Srivastava RK: Rottlerin induces autophagy which leads to apoptotic
cell death through inhibition of PI3K/Akt/mTOR pathway in human
pancreatic cancer stem cells. Biochem Pharmacol. 84:1154–1163.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhivotovsky B, Samali A, Gahm A and
Orrenius S: Caspases: Their intracellular localization and
translocation during apoptosis. Cell Death Differ. 6:644–651. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu QY, Zhang L, Lugea A, Moro A,
Edderkaoui M, Eibl G, Pandol SJ and Go VL: Determination of
rottlerin, a natural protein kinases C inhibitor, in pancreatic
cancer cells and mouse xenografts by RP-HPLC method. J Chromatogr
Sep Tech. 4(pii): 1000622013.PubMed/NCBI
|
|
21
|
Gangwar M, Goel RK and Nath G: Mallotus
philippinensis Muell. Arg (Euphorbiaceae): Ethnopharmacology and
phytochemistry review. Biomed Res Int. 2014:2139732014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kumar VP, Chauhan NS, Padh H and Rajani M:
Search for antibacterial and antifungal agents from selected Indian
medicinal plants. J Ethnopharmacol. 107:182–188. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arfan M, Hazrat K, Magdalena K, Kosińka A,
Wiczkowski W and Amarowicz R: Antioxidant activity of phenolic
fractions of Mallotus philippinensis bark extract. J Food Sci.
27:109–117. 2009.
|
|
24
|
Tiwari AK: Imbalance in antioxidant
defence and human diseases: Multiple approach of natural
antioxidants therapy. Current Science. 81:1179–1187. 2001.
|
|
25
|
Gangwar M, Gautam MK, Sharma AK, Tripathi
YB, Goel RK and Nath G: Antioxidant capacity and radical scavenging
effect of polyphenol rich Mallotus philippenensis fruit extract on
human erythrocytes: An in vitro study. Scientific World
Journal. 2014:2794512014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li X, Li X, Wang J, Ye Z and Li JC:
Oridonin up-regulates expression of P21 and induces autophagy and
apoptosis in human prostate cancer cells. Int J Biol Sci.
8:901–912. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liang XH, Jackson S, Seaman M, Brown K,
Kempkes B, Hibshoosh H and Levine B: Induction of autophagy and
inhibition of tumorigenesis by beclin 1. Nature. 402:672–676. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen YS, Song HX, Lu Y, Li X, Chen T,
Zhang Y, Xue JX, Liu H, Kan B, Yang G and Fu T: Autophagy
inhibition contributes to radiation sensitization of esophageal
squamous carcinoma cells. Dis Esophagus. 24:437–443. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu DL, Gao M, Yang Y, Qi Y, Wu K and Zhao
S: Inhibition of autophagy promotes cell apoptosis induced by the
proteasome inhibitor MG-132 in human esophageal squamous cell
carcinoma EC9706 cells. Oncol Lett. 9:2278–2282. 2015.PubMed/NCBI
|
|
30
|
Choi S, Lim MH, Kim KM, Jeon BH, Song WO
and Kim TW: Cordycepin-induced apoptosis and autophagy in breast
cancer cells are independent of the estrogen receptor. Toxicol Appl
Pharmacol. 257:165–173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Li R, Zhu S, Zhou R, Wang L, Du J,
Wang Y, Zhou B and Mai L: Cordycepin induces apoptosis and
autophagy in human neuroblastoma SK-N-SH and BE(2)-M17 cells. Oncol
Lett. 9:2541–2547. 2015.PubMed/NCBI
|
|
32
|
He Z, Mangala LS, Theriot CA, Rohde LH and
Wu and Zhang Y: Cell killing and radiosensitizing effects of
atorvastatin in PC3 prostate cancer cells. J Radiat Res.
53:225–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He Z, Yuan J, Qi P, Zhang L and Wang Z:
Atorvastatin induces autophagic cell death in prostate cancer cells
in vitro. Mol Med Rep. 11:4403–4408. 2015.PubMed/NCBI
|
|
34
|
Kang M, Jeong CW, Ku JH, Kwak C and Kim
HH: Inhibition of autophagy potentiates atorvastatin-induced
apoptotic cell death in human bladder cancer cells in vitro.
Int J Mol Sci. 15:8106–8121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ringer L, Sirajuddin P, Tricoli L, Waye S,
Choudhry MU, Parasido E, Sivakumar A, Heckler M, Naeem A,
Abdelgawad I, et al: The induction of the p53 tumor suppressor
protein bridges the apoptotic and autophagic signaling pathways to
regulate cell death in prostate cancer cells. Oncotarget.
5:10678–10691. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Orelio C, Harvey KN, Miles C, Oostendorp
RA, van der Horn K and Dzierzak E: The role of apoptosis in the
development of AGM hematopoietic stem cells revealed by Bcl-2
overexpression. Blood. 103:4084–4092. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ashkenazi A and Dixit VM: Death receptors:
Signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bleackley RC and Heibein JA: Enzymatic
control of apoptosis. Nat Prod Rep. 18:431–440. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Song KS, Kim JS, Yun EJ, Kim YR, Seo KS,
Park JH, Jung YJ, Park JI, Kweon GR, Yoon WH, et al: Rottlerin
induces autophagy and apoptotic cell death through a
PKC-delta-independent pathway in HT1080 human fibrosarcoma cells:
The protective role of autophagy in apoptosis. Autophagy.
4:650–658. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kumar D, Shankar S and Srivastava RK:
Rottlerin-induced autophagy leads to the apoptosis in breast cancer
stem cells: Molecular mechanisms. Mol Cancer. 12:1712013.
View Article : Google Scholar : PubMed/NCBI
|