Introduction
Cancer is the second leading cause of mortality
after heart disease (1). Cancer
comprises a group of diseases characterized by the unregulated
division and proliferation of cells. Lung cancer is a leading
causes of mortality in men and women (2). The main reason behind the discouraging
survival statistics is that the majority of lung cancer subjects
present with late-stage disease and are not curable using current
therapies (3).
Chemoprevention is one of the promising areas in
anticancer strategies in which extensive research is being
performed globally. Use of the combination approach is an upcoming
area of research (4). To the best of
our knowledge, there is currently a paucity of information with
regard to the combined use of curcumin and quercetin in modulating
ultrastructural changes during lung cancer.
Thus, the focus of the present study was to evaluate
the efficacy of the combined chemoprevention approach using
curcumin and quercetin in modulating ultrastructural changes during
lung carcinogenesis.
Materials and methods
Chemicals
Benzo(a)pyrene (BP), NADPH, GSH, NBT, DTNB,
Curcumin and Quercetin was procured from Sigma-Aldrich (St. Louis,
MO, USA).
Animals
A total of 24 male laka Mice, weighing 18–20 g, were
procured from the Xuzhou Medical College (Jiangsu, China). The rats
were housed in polypropylene cages in a temperature-controlled room
(21±2°C) on a 12-h light/dark cycle (lights on at 06:00), and had
free access to food and water. Prior to initiating the experiments,
the animals were adapted to the laboratory conditions for a week.
The study was approved by the Ethics Committee of Xuzhou Medical
College.
Experimental design
The mice were divided into five treatment groups.
Animals in group I served as normal controls and were given water
and diet ad libitum. Mice in this group were also
administered corn oil intraperitoneally, which was used as the
vehicle for treatment in BP-treated animals. Animals in group II
were given a single injection (P) of BP at a dose level of 100
mg/kg of body weight (b.wt.) dissolved in corn oil, for a total
duration of 10 weeks (5). Group III
animals were given curcumin at a dose level of 60 mg/kg of b.wt.
three times a week orally in water. Animals in group IV were given
quercetin at a dose level of 40 mg/kg of b.wt. in water three times
a week orally. Animals in group V animals were given a combined
treatment of curcumin and quercetin in a similar manner as was
given to group III and IV animals, respectively. The abovementioned
treatment with phytochemicals was started 10 days prior to BP
injection.
Ultrastructural studies
A small section of lung (1×1 cm; 4 µm thick) was
removed, immersed in PBS and fixed with formaldehyde and
glutaraldehyde, prior to incubation in 0.2 M sodium cacodylate
buffer (pH 7.2) for 10–12 h at 4°C. The specimens were then
thoroughly washed 3 times in 0.1 M cacodylate buffer (Biosharp,
Hefei, China) and post-fixed for 60 min in 1% osmium tetraoxide
(Biosharp), created in the cacodylate buffer. The tissues were
thoroughly washed in buffer to remove any extraneous traces of
osmium tetraoxide and then dehydrated in ascending series of
acetone, allowing 20 min per change, in each concentration of
acetone. Specimens were then filtered and subsequently embedded in
resin. Specimen blocks were formed by polymerization of the pure
embedding resin at 60°C for 48–72 h. Ultrathin sections of various
specimen blocks were made using ultramicrotome (Jiancheng Biotech,
Nanjing, China). Initially, semithin sections (1 µm) were cut using
sharp glass knives to locate the area of interest in the different
treatment groups. These semithin sections were stained with 0.5%
toludine blue produced in 1% borax solution. Ultrathin sections of
interference colors from golden to silver were cut and loaded on
fine copper grids. The sections were then double-stained with
uranyl acetate and lead citrate. The ultrathin sections were
subsequently reviewed under a transmission electron microscope
(Olympus, Tokyo, Japan).
Results
Ultrastructure of lung of normal
control mice
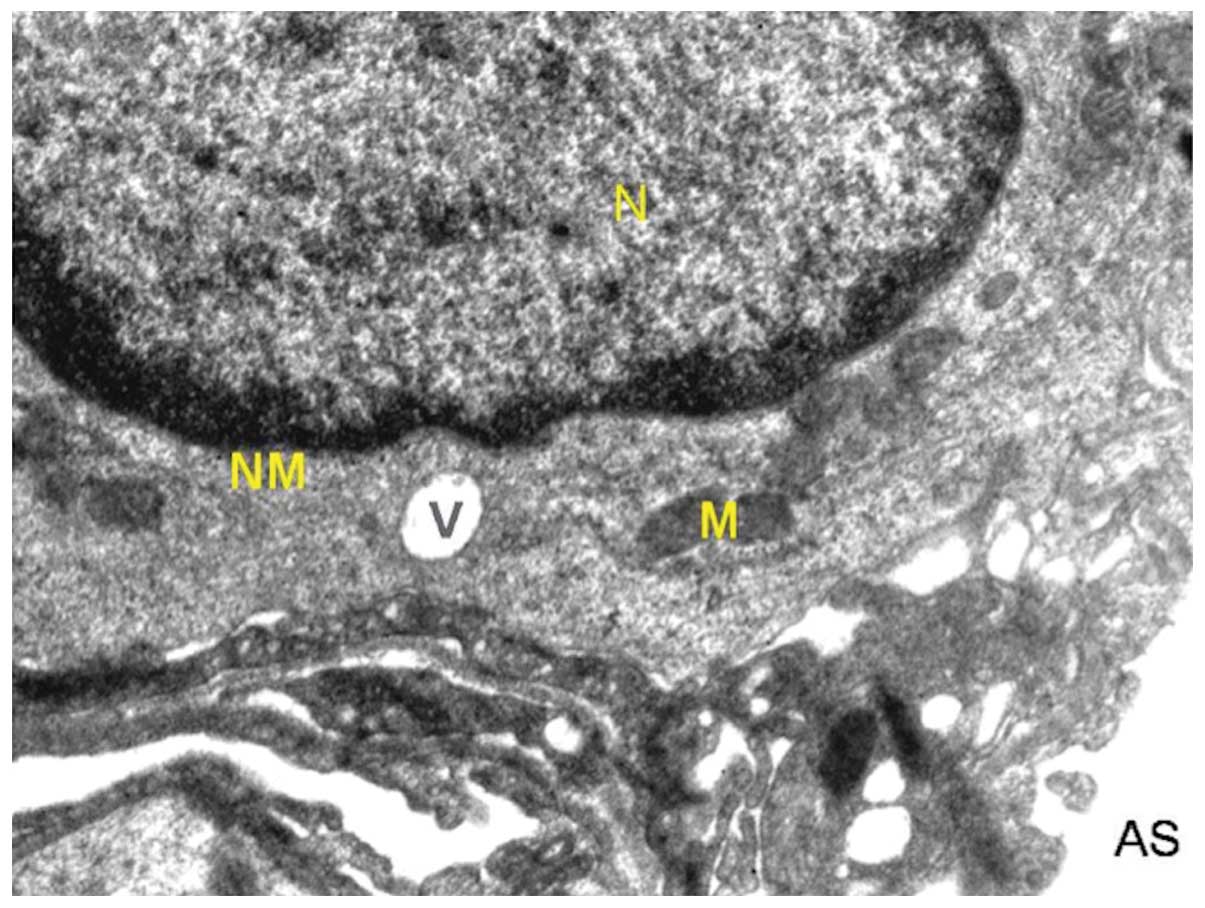
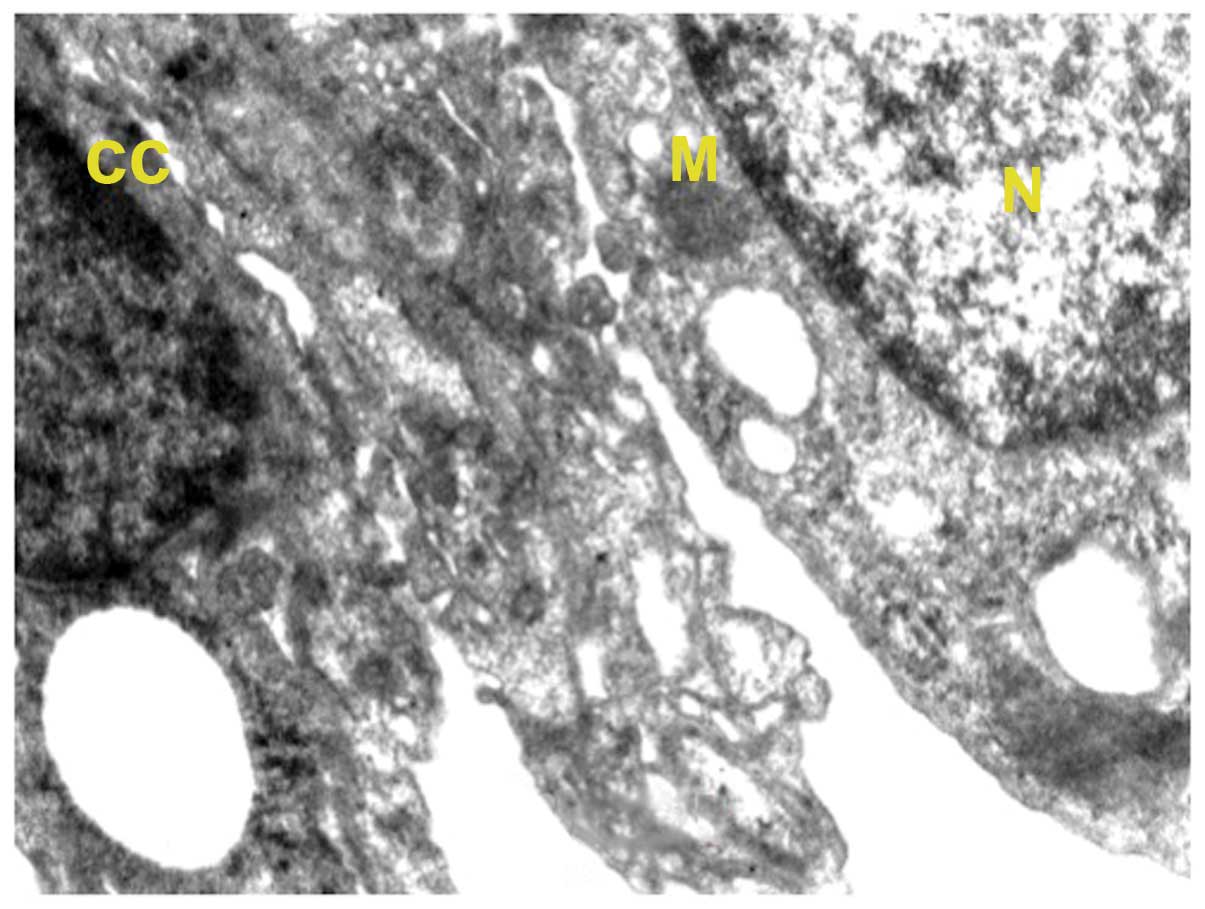
In the normal control group, the alveolar cells were
normal in appearance with a normal nucleus with intact nuclear
membrane (Fig. 1). Two types of
alveolar cells are recognized, types I and II. The alveolar
epithelium (type I) consisted of cells forming the thin layer of
lining, with the alveolar air spaces with nucleus occasionally
bulging into the alveolus lumen. Type II cells exhibited microvilli
present on the surface of the alveolar epithelium of the mouse lung
and seemed to be integral with neat arrangement. Mitochondria were
many and they appeared to be regular in shape with approximate
roundness and ellipse. The inner matrix of mitochondria was of
medium density and the cell-to-cell contact was clearly distinct.
Inclusion bodies were present in the type II cells. The type II
cells contained remnants of surfactant-rich lamellar bodies.
Surfactant released from these cells covered the alveolar surface
to reduce surface tension in order that alveolar surfaces did not
collapse onto one another on expiration. Multi-lobular nuclei were
also observed. Additionally, vacuolization was observed once or
twice in the normal control cells. The basement membrane was
visible, forming an interface between alveolar and capillary
endothelia. Red blood cells were also visible along with the
nucleus of capillary cells.
Ultrastructure of lung of
benzo[a]pyrene-treated mice
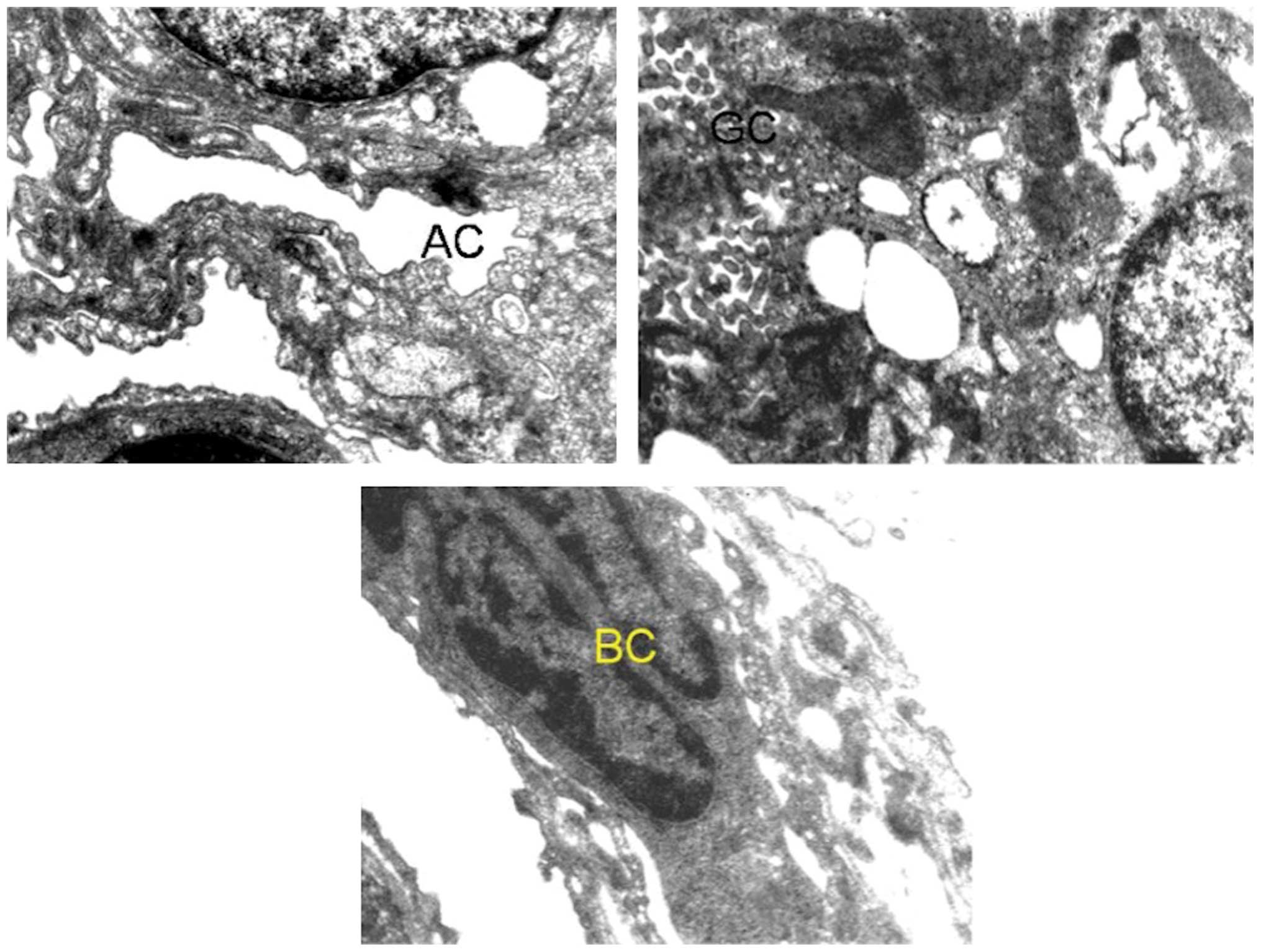
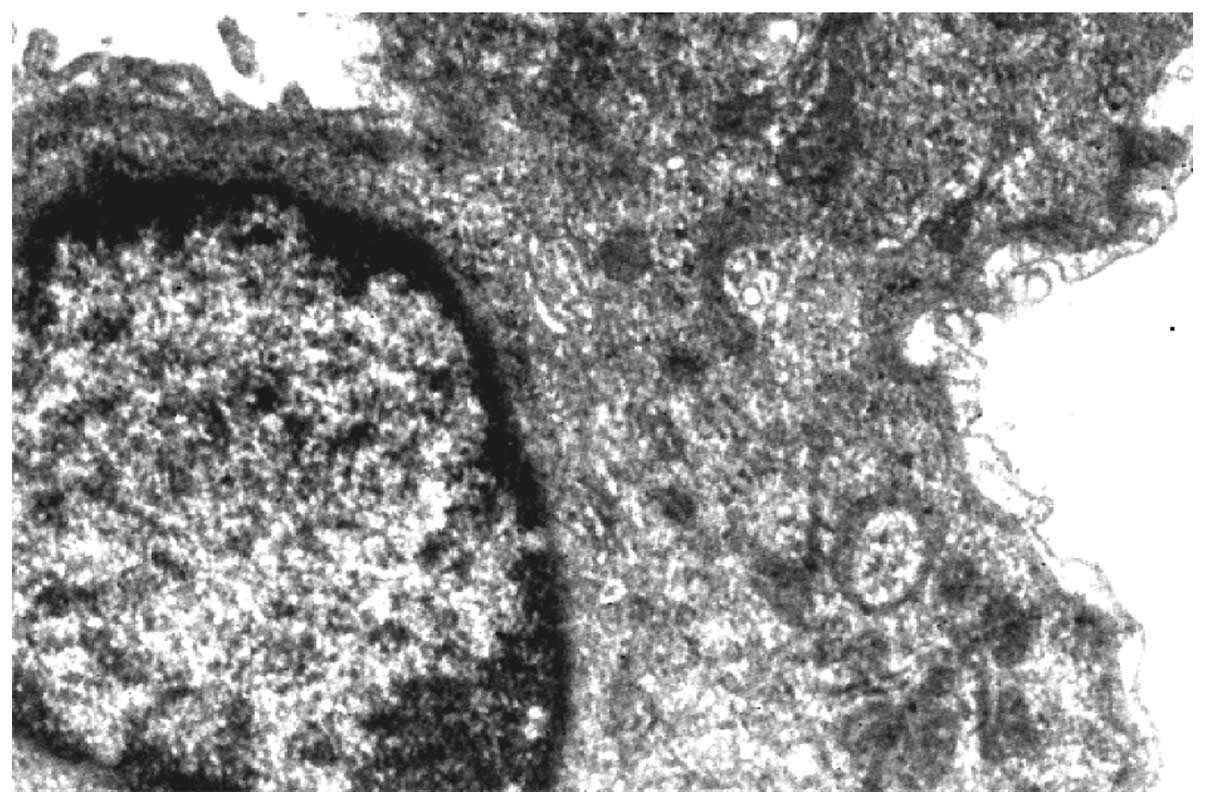
Changes in the ultrastructural appearance of lung
cells (Figs. 2 and 3) were evident in carcinogenesis following
benzo[a]pyrene treatment. The most obvious changes were seen
in the shape of nucleus along with the disruption of nuclear
envelope and lamina. Mitochondria were found to be swollen,
elongated and with flocculent material, giving a denser appearance.
The cytoplasm of cells was granulated and the vacuolization of
inclusion bodies was also observed. Separation of endothelium and
epithelium from the basement membrane of the alveolar was also
observed.
Ultrastructure of lung of
benzo[a]pyrene+quercetin-treated mice,
benzo[a]pyrene+curcumin-treated mice, and
benzo[a]pyrene+curcumin+quercetin-treated mice
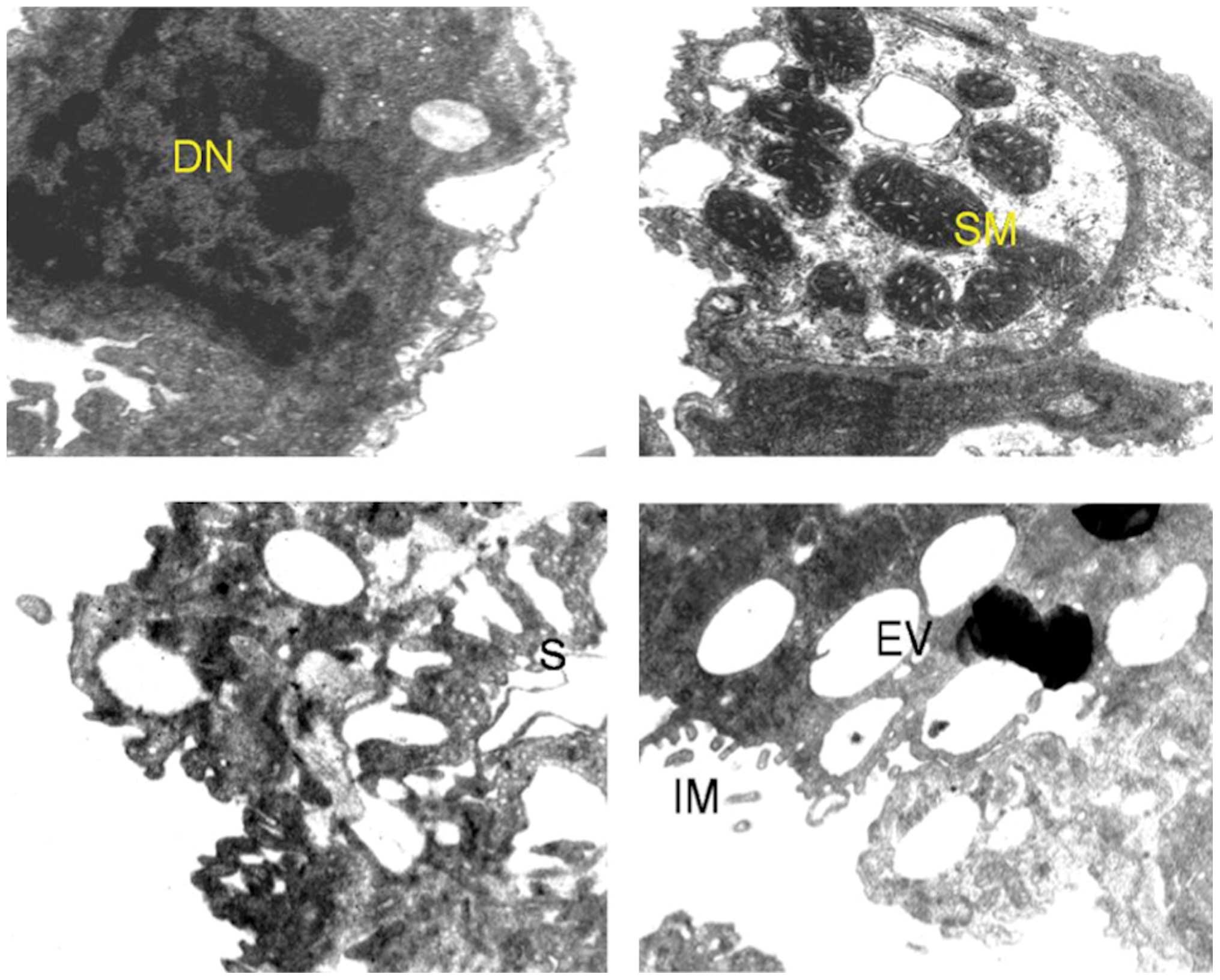
Supplementation with curcumin (Fig. 4) and quercetin (Fig. 5) alone and in combination (Fig. 6) with benzo[a]pyrene-treated
mice appreciably moderated the ultrastructural changes with regard
to integrity of the cells as a whole as well as the cell
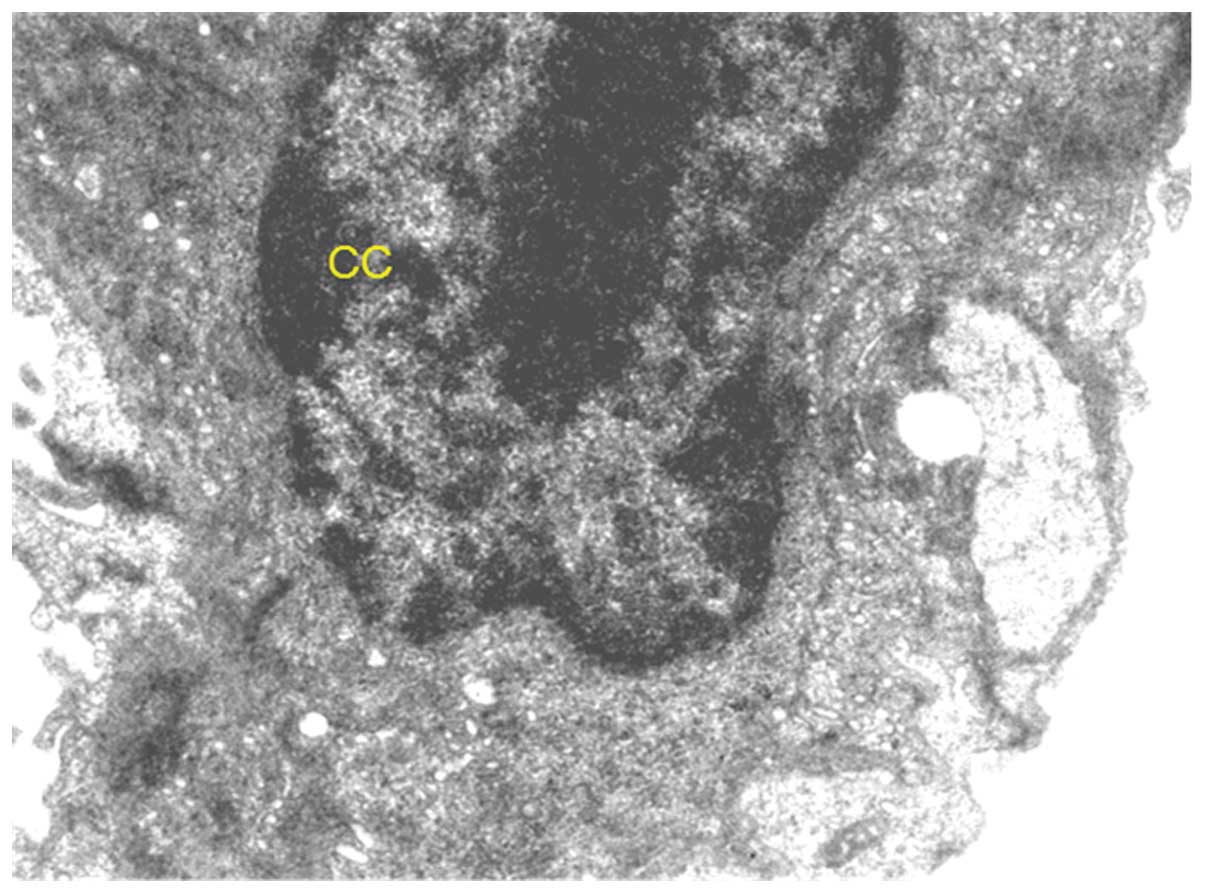
organelles. However, chromatin condensation in nucleus and some
vacuoles in the cytoplasm were evident.
Discussion
The results of the present study showed the
synergistic potential of curcumin and quercetin in moderating
alterations in ultra-structure in lung cells during
experimentally-induced lung carcinogenesis in mice. Curcumin and
quercetin collectively improved the majority of ultrastructural
changes in lungs of BP-treated mice.
In the normal control mice, type I and II alveolar
cells were recognized (6). The
alveolar epithelium of type I consisted of cells forming a thin
layer of lining of the alveolar air spaces with nuclei occasionally
bulging into the alveolar lumen. Usually, the inter-alveolar septa
is formed by alveolar epithelium of type I cells on either side of
the lumen along with the capillary sandwiched between them and this
arrangement was also visible in the ultrastructure of lung
(7). The basement membrane of the
three epithelia seems to be forged into one unit to form a
continuous pattern of the inter-alveolar septa. This arrangement is
necessary for the proper exchange of respiratory molecules of type
II cells and for the presence fo microvilli on the surface of the
alveolar epithelium of the mouse lung (8).
Mitochondria were numerous in number and they
appeared to have approximate roundness and be elliptical in shape.
The inner matrix of mitochondria was of medium density and the
cell-to-cell contact was clearly distinct. Additionally, inclusion
bodies were present in the type II cells. Type II cells are
responsible for the release of surfactant, which covers the
alveolar surface to reduce surface tension in order that alveolar
surfaces do not collapse onto one another on expiration. In the
BP-treated group, the shapes of the nuclei were altered together
with the disruption of nuclear envelope and lamina. This may be
correlated with the formation of micronuclei as be evident in the
micronucleus assay (9). Mitochondria
were found to be swollen, elongated and filled with flocculent
material, giving a denser appearance. The cytoplasms of cells were
granulated and the vacuolization of the inclusion bodies was also
observed. Separation of endothelium and epithelium from the
basement membrane of the alveoli was also observed, which is
indicative of injury (10). This
result may well be correlated with the increase in the levels of
tumor marker enzymes, especially lactate dehydrogenase (LDH), which
is also a marker of tissue injury (11). The derangement of microvilli in the
type II cells may be postulated to occur due to neoplastic changes
occurring in the cells, which may be included the formation of
intermediate forms of types I and II (12). In addition, there may be a replacement
of normal type II cells by the hyperplastic type II cells.
Supplementation with phytochemicals in individual
and in combined form appreciably moderated the ultra-structural
changes with regard to the integrity of the cells as a whole as
well as the cell organelles. The presence of chromatin condensation
observed in the nuclei is indicative of apoptosis, which is well
substantiated by an increase in apoptotic cells (9). The nuclear envelope was intact, which
may be correlated well with the decrease in the formation of
micronuclei as observed by the micronucleus assay (13). A number of vacuoles decreased and the
microvilli arrangement showed order signifying the protective role
played by the phytochemicals. There was an improved arrangement of
capillary endothelium with reduced separation of endothelium from
the basement membrane of the alveoli that could well be linked with
a decrease in the levels of LDH. This improvement in the
ultrastructure of lung by the phytochemicals may have led to
decreased lung weights by reducing the levels of pro-inflammatory
enzyme COX-2 that in turn also assisted in reducing the tumor
incidence as well as multiplicity (14).
Therefore, curcumin and quercetin show great
prospects in dealing with the condition of lung carcinogenesis and
greater improvement was observed in the combined treatment group
more frequently. The results of the present study suggest these
phytochemicals hold great potential to be used as an effective
preventive measure against the occurrence of lung cancer in a
section of human population with a family history of lung cancer,
as well those who are constantly exposed to carcinogens from
different sources.
In conclusion, the present findings show that a
combination approach in the supplementation of phytochemicals can
effectively produce ultrastructural changes at the transmission
electron microscopic level during lung carcinogenesis.
References
|
1
|
He Z, Xia Y, Tang S, Chen Y and Chen L:
Detection of occult tumor cells in regional lymph nodes is
associated with poor survival in pN0 non-small cell lung cancer: A
meta-analysis. J Thorac Dis. 8:375–385. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamamoto S, Huang D, Du L, Korn RL,
Jamshidi N, Burnette BL and Kuo MD: Radiogenomic analysis
demonstrates associations between 18F-fluoro-2-deoxyglucose PET,
prognosis, and epithelial-mesenchymal transition in non-small cell
lung cancer. Radiology. Apr 15–2016.(Epub ahead of print).
View Article : Google Scholar
|
|
4
|
Orfali G, Duarte AC, Bonadio V, Martinez
NP, de Araújo ME, Priviero FB, Carvalho PO and Priolli DG: Review
of anticancer mechanisms of isoquercitin. World J Clin Oncol.
7:189–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Malhotra A, Nair P and Dhawan DK: Study to
evaluate molecular mechanics behind synergistic chemo-preventive
effects of curcumin and resveratrol during lung carcinogenesis.
PLoS One. 9:e938202014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun N, Sun P, Lv H, Sun Y, Guo J, Wang Z,
Luo T, Wang S and Li H: Matrine displayed antiviral activity in
porcine alveolar macrophages co-infected by porcine reproductive
and respiratory syndrome virus and porcine circovirus type 2. Sci
Rep. 6:244012016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morales AG, Stempinski ES, Xiao X, Patel
A, Panna A, Olivier KN, McShane PJ, Robinson C, George AJ, Donahue
DR, et al: Micro-CT scouting for transmission electron microscopy
of human tissue specimens. J Microsc. Feb 8–2016.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Flodby P, Kim YH, Beard LL, Gao D, Ji Y,
Kage H, Liebler JM, Minoo P, Kim KJ, Borok Z and Crandall ED:
Knockout mice reveal a major role for alveolar epithelial type I
cells in alveolar fluid clearance. Am J Respir Cell Mol Biol. Apr
11–2016.(Epub ahead of print). View Article : Google Scholar
|
|
9
|
El-Zein RA, Lopez MS, D'Amelio AM Jr, Liu
M, Munden RF, Christiani D, Su L, Tejera-Alveraz P, Zhai R, Spitz
MR, et al: The cytokinesis-blocked micronucleus assay as a strong
predictor of lung cancer: Extension of a lung cancer risk
prediction model. Cancer Epidemiol Biomarkers Prev. 23:2462–2470.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bantikassegn A, Song X and Politi K:
Isolation of epithelial, endothelial, and immune cells from lungs
of transgenic mice with oncogene-induced lung adenocarcinomas. Am J
Respir Cell Mol Biol. 52:409–417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ahn H, Lee K, Kim JM, Kwon SH, Lee SH, Lee
SY and Jeong D: accelerated lactate dehydrogenase activity
potentiates osteoclastogenesis via NFATc1 signaling. PLoS One.
11:e01538862016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Crystal RG: Airway basal cells. The
‘smoking gun’ of chronic obstructive pulmonary disease. Am J Respir
Crit Care Med. 190:1355–1362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhatia A and Kumar Y: Cancer cell
micronucleus: an update on clinical and diagnostic applications.
APMIS. 121:569–581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Said-Elbahr R, Nasr M, Alhnan MA, Taha I
and Sammour O: Nebulizable colloidal nanoparticles co-encapsulating
a COX-2 inhibitor and a herbal compound for treatment of lung
cancer. Eur J Pharm Biopharm. 103:1–12. 2016. View Article : Google Scholar : PubMed/NCBI
|