Introduction
The cancer stem cell (CSC) theory hypothesizes the
presence of a small population of cells within the heterogenous
cancer cell population termed CSCs or tumor-initiating cells, which
are responsible for therapy failure and tumor recurrence in cancer
(1,2).
Previous studies conducted in several types of cancer reported that
these CSCs exhibit a high potential for differentiation and
self-renewal, as they possess increased expression of stem cell
surface proteins and are highly tumorigenic in vivo and
in vitro (1,2). It has also been reported that aberrantly
regulated Wnt/beta-catenin and Notch1 signaling in CSCs are
involved in multidrug resistance in these cells (3–6). The
multidrug resistance properties of CSCs are due to the
overexpression of the ATP-binding cassette (ABC) transporter
protein ABC sub-family G member 2 (ABCG2), which acts as a drug
efflux pump for DNA-targeting drugs, and causes cancer cells to
escape from conventional cancer treatment strategies (7–9).
Therefore, it is crucial to improve and design novel therapeutic
drugs that could effectively eradicate refractory CSCs.
Head and neck squamous cell carcinoma (HNSCC) is one
of the most common malignancies worldwide, and the life span of
patients following diagnosis at metastatic stage is only 4 months
(10). Despite recent innovations in
cancer treatment strategies (6–8), the
overall survival rate of HNSCC patients has not improved. Previous
studies on HNSCC reported the persistence of a small population of
CSCs that are the major cause for therapy failure (5,6). These
CSCs have a high differentiation potential, are highly tumorigenic,
and express stem cell surface markers, including cluster of
differentiation (CD)44, CD133 and octamer-binding transcription
factor 4 (10–12). Another remarkable feature of these
CSCs is the fact that they are highly resistant to apoptosis and
exhibit multidrug resistance, which confers them immortality
(5–7).
It has been previously reported that increased expression of
stress-inducible transcription factors such as Nrf2 is also
involved in ABC transporter-mediated drug efflux in CSCs (13). The current study attempted to identify
and characterize CD133+ SP cells present in HNSCC
samples, and to evaluate the expression of Nrf2 and ABCG2 in HNSCC
CD133+ CSCs.
Materials and methods
Cell culture from primary HNSCC
samples
Human HNSCC samples were obtained from patients with
HNSCC during surgery performed from March 2015 until December 2015
at The First Affiliated Hospital of Xinxiang Medical University
(Weihui, China). The present study was approved by the ethics
committee of The First Affiliated Hospital of Xinxiang Medical
University. The primary tumor samples were minced with blades into
small pieces, and then enzymatically digested with collagenase,
hyaluronidase and DNase (all obtained from Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), prior to be incubated for 2 h
at 37°C in the presence of 5% CO2. Cells were
disaggregated in phosphate-buffered saline and centrifuged at 350 ×
g for 20 min. The pellet was then resuspended in serum-free
Dulbecco's modified Eagle's medium/F-12 (Gibco; Thermo Fisher
Scientific, Inc.) containing human recombinant epidermal growth
factor (20 ng/ml; Gibco; Thermo Fisher Scientific, Inc.) and human
basic fibroblast growth factor (20 ng/ml; Gibco; Thermo Fisher
Scientific, Inc.).
Fluorescence-activated cell sorting
(FACS) analysis of HNSCC samples
Cells (~106 cells/ml) were subjected to
FACS analysis. For that purpose, cells were divided into two
groups: Group I, corresponding to cells labeled with 5 µl/ml
Hoechst 33342 dye (Sigma-Aldrich, St. Louis, MO, USA) alone (n=7);
and group II, corresponding to cells treated with verapamil drug
(0.8 µl/ml; Sigma-Aldrich) in addition to Hoechst 33342 dye (n=7).
Cells were next counterstained with propidium iodide (Invitrogen;
Thermo Fisher Scientific, Inc.) at a concentration of 2 µg/ml, and
subjected to FACS analysis in a FACSCalibur™ (BD Biosciences,
Franklin Lakes, NJ, USA), using a 610-nm dichroic short-pass
filter, while the red and blue emissions were collected using
670/30-nm and 450/65-nm band-pass filters, respectively. Images of
the cells were obtained using a fluorescence microscope at ×40
magnification (BX63; Olympus Corporation, Tokyo, Japan).
In vitro proliferation,
chemoresistance and sphere formation assay
These assays were performed as previously described
(14). The DNA-targeting drugs used
in these assays, including etoposide, gemcitabine, 5-fluorouracil,
cisplatin, paclitaxel and oxaliplatin, were obtained from
Sigma-Aldrich.
Tumor cell implantation
A total of ~2×104 SP and non-SP cells
were administered to non-obese diabetic (NOD)/severe combined
immunodeficiency (SCID) mice by subcutaneous injection, as
previously described (13,15). All mice (6–8 week-old males, n=7) were
maintained in dedicated housings at a temperature of 25±1°C, with
constant access to food pellets and water ad libitum. Mice
were sacrificed 4–5 weeks later, and the tumor size was measured
according to the following formula: V=(ab2/2), where a
is the long diameter and b the short diameter of the tumor
(13). The maximum diameter noted was
~2.1 cm.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted using TRIzol (Life
Technologies; Thermo Fisher Scientific, Inc.) and subjected to RT
with a Reverse Transcriptase kit (Fermentas; Thermo Fisher
Scientific, Inc.). The sequences of the human specific primers used
for PCR were as follows: ABCG2, forward
5′-TCAATCAAAGTGCTTCTTTTTTATG-3′ and reverse
5′-TTGTGGAAGAATCACGTGGC-3′; Nrf2, forward 5′-ACACGGTCCACAGCTCATC-3′
and reverse 5′-TGCCTCCAAAGTATGTCAATCA-3′; and glyceraldehyde
3-phosphate dehydrogenase, forward 5′-ATGTCGTGGAGTCTACTGGC-3′ and
reverse 5′-TGACCTTGCCCACAGCCTTG-3′ (IDT Shanghai Co. Ltd.,
Shanghai, China). The PCR parameters were as follows: Initial
denaturation at 95°C for 2 min, followed by 35 cycles of annealing
at 58°C for 45 sec, extension at 72°C for 2 min and a final
extension at 72°C for 7–10 min. The reaction was performed in an
Applied Biosystems thermocycler (Thermo Fisher Scientific, Inc.).
The amplified products were analyzed on 1.5% agarose gel
electrophoresis and visualized with ethidium bromide, using a gel
image documentation device (Bio Basic Canada, Inc., Markham, ON,
Canada). The intensity of the DNA bands from three independent
experiments was measured with ImageJ version 3.2 (https://imagej.nih.gov/ij/).
Statistical analysis
One-way analysis of variance and Student's
t-test were performed with GraphPad Prism version 6
(GraphPad Software, Inc., La Jolla, CA, USA) in order to evaluate
the significance of the differences between SP and non-SP cells
when comparing various or a single parameter, respectively.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Identification and characterization of
CD133+ cancer stem-like cells from HNSCC
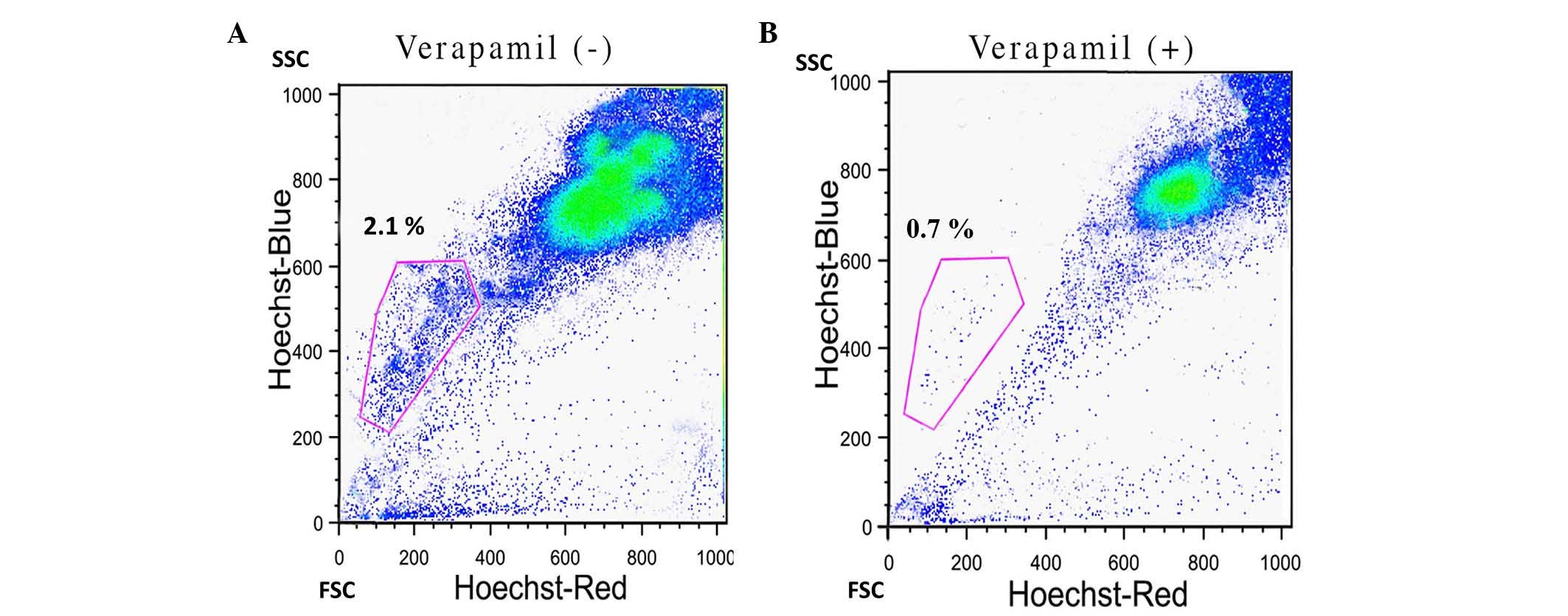
FACS analysis identified ~2.1% SP cells in the HNSCC
samples (Fig. 1A). In order to
confirm the presence of SP cells, the samples were treated with the
ABC transporter inhibitor verapamil. As indicated in Fig. 1B, the percentage of SP cells was
significantly reduced to 0.7% upon treatment with verapamil
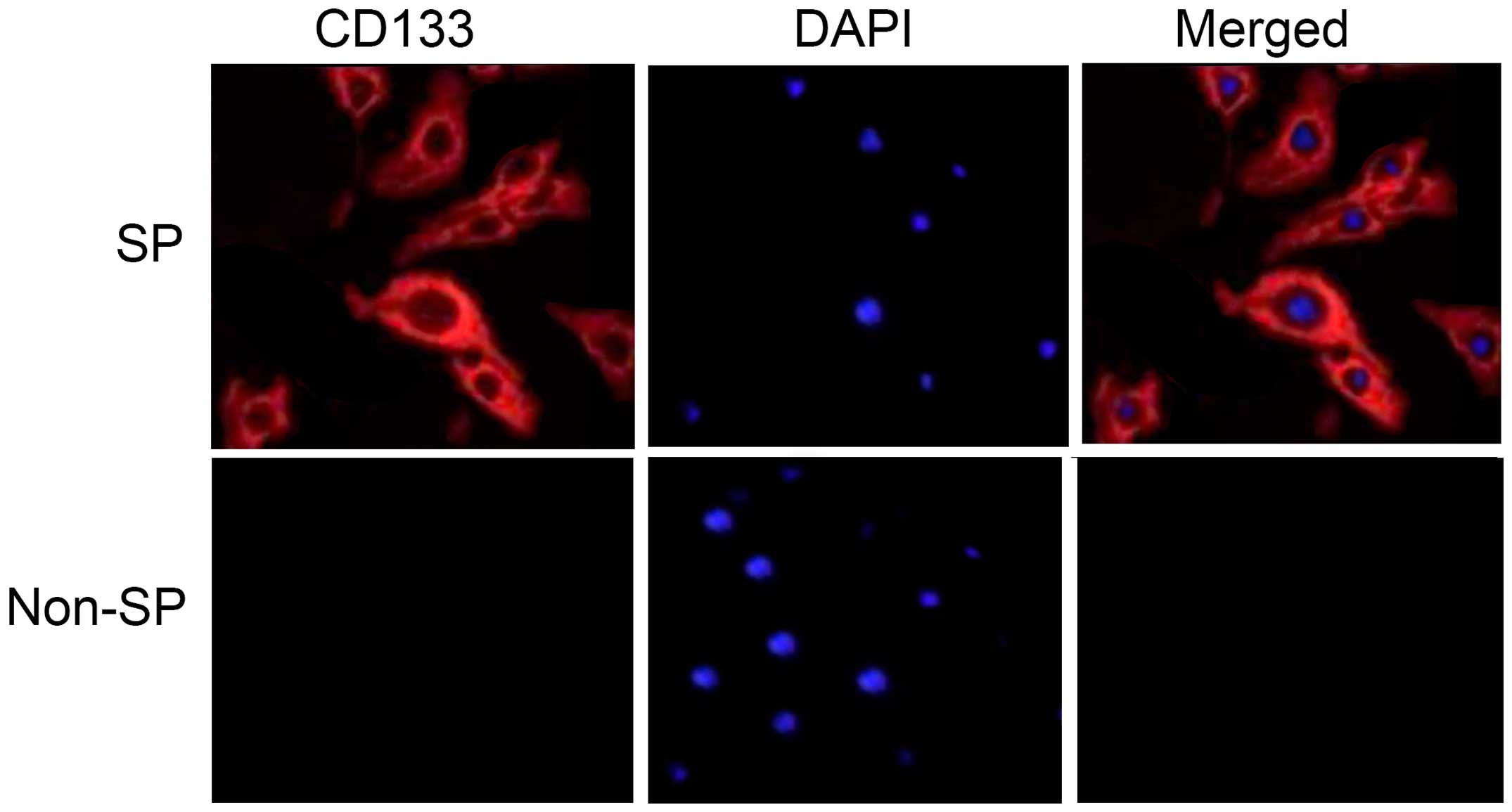
(P=0.024). Furthermore, the FAC-sorted SP cells were positive for
the stem cell surface protein CD133 (Fig.
2). These data suggest that HNSCC stem cells are able to resist
the effect of chemotherapeutic drugs, and the overexpression of ABC
transporter proteins is critical for the process of drug expulsion
from the cell.
HNSCC CSCs are multidrug resistant and
tumorigenic
To further characterize the HNSCC CD133+
SP cells, the FAC-sorted SP and non-SP cells were subjected to an
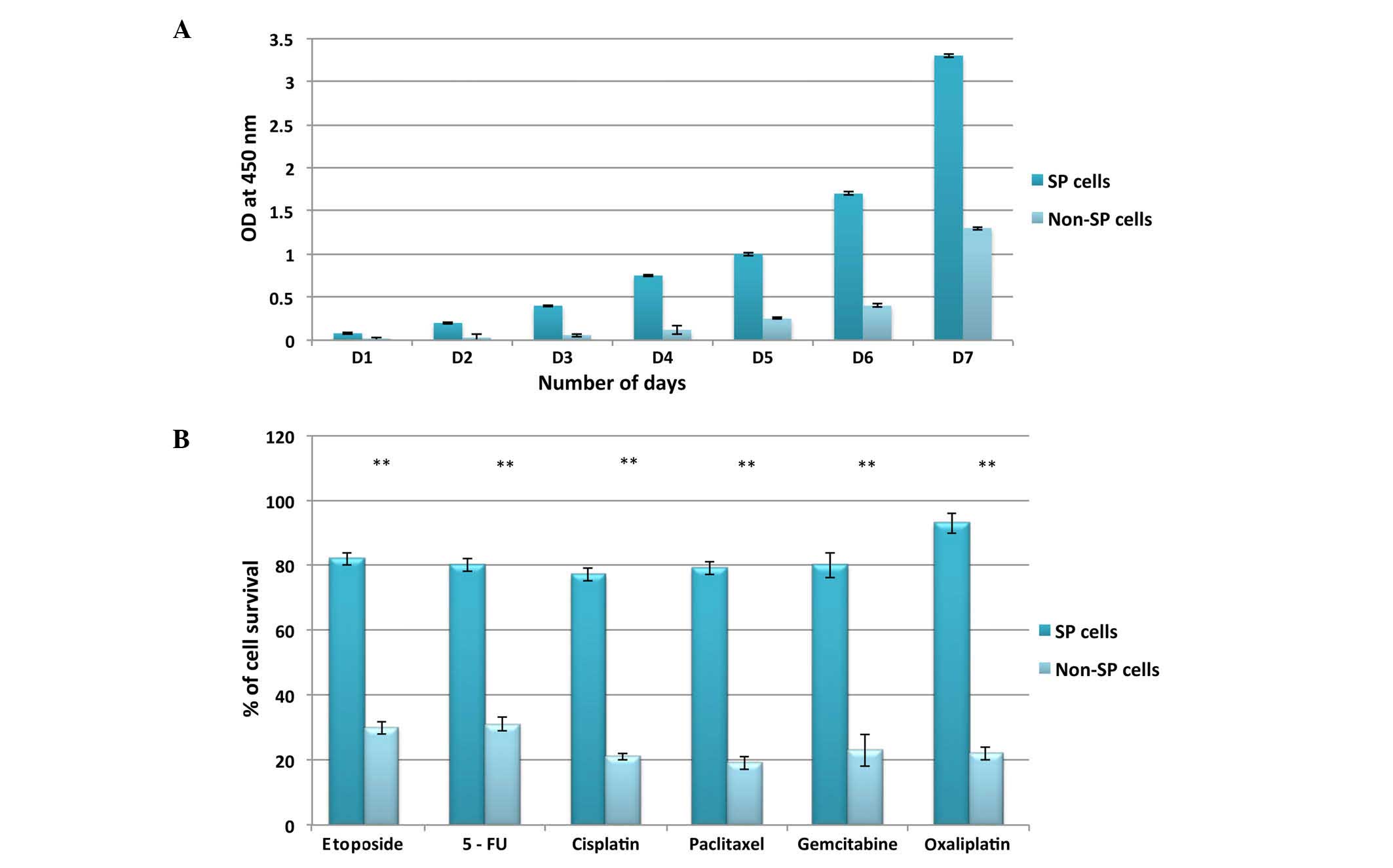
in vitro cell proliferation assay in order to determine the
rate of cell proliferation. As revealed in Fig. 3A, the growth rate of SP cells was
significantly higher, and the cells became confluent more rapidly
(on day 6), than non-SP cells (day 15). Notably, the
chemoresistance assay demonstrated that FAC-sorted SP cells were
not susceptible to DNA-targeting drugs, including etoposide,
gemcitabine, 5-fluorouracil, cisplatin, paclitaxel and oxaliplatin
(Fig. 3B). The survival rate of SP
cells was >70% following treatment with the above drugs, whereas
the survival rate of non-SP cells was <30%.
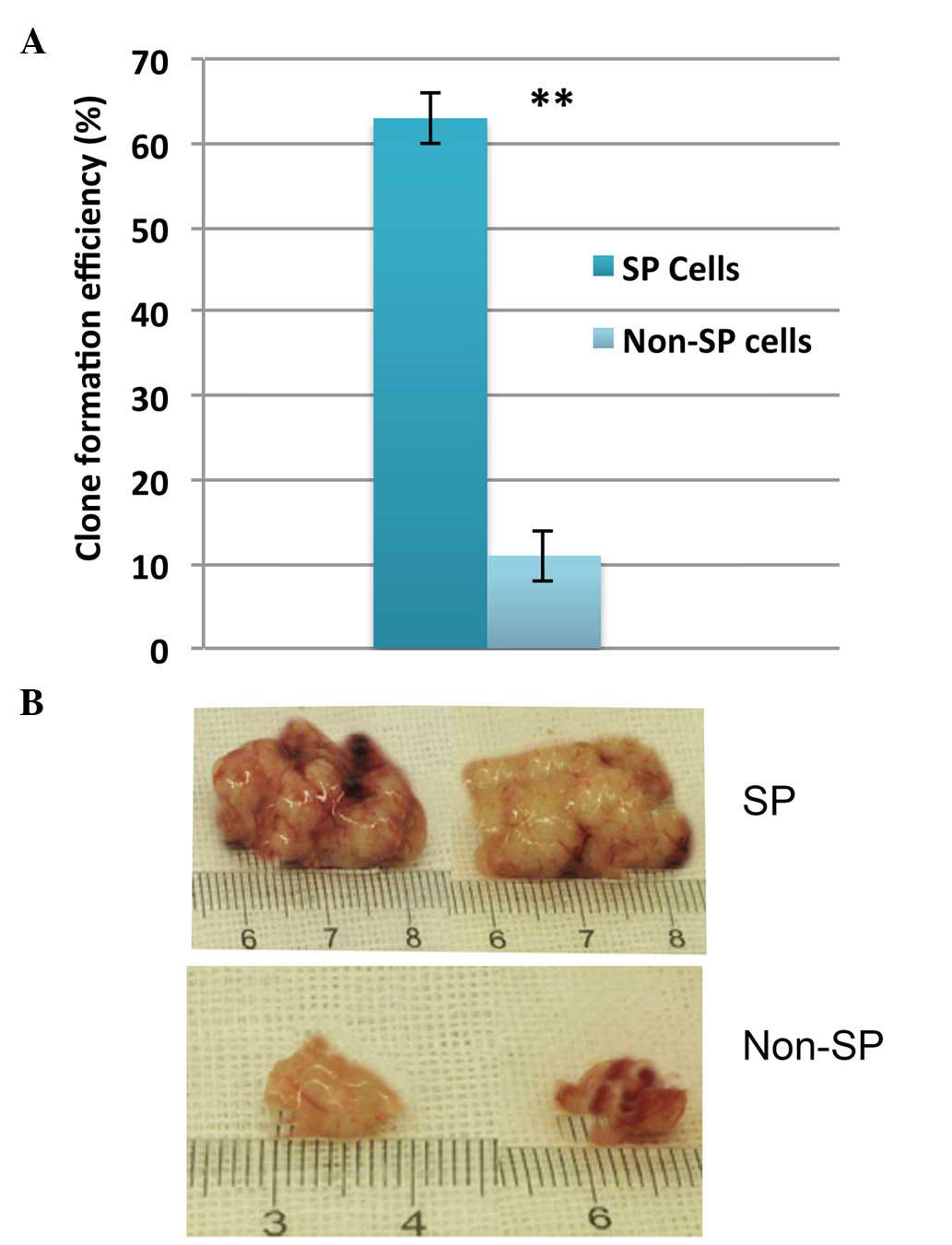
Next, the self-renewal capacity of SP cells was
investigated. HNSCC CD133+ SP cells were able to
generate larger tumor spheres (Fig.
4A), whose size markedly increased in a time-dependent manner
(data not shown). In addition, injection of the lowest cell density
of SP cells tested (4×103 cells) into NOD/SCID mice
could efficiently induce tumor growth in vivo (Fig. 4B). Taken together, these data suggest
that enhanced cell proliferation and self-renewal, alongside high
multidrug resistance, contribute to therapy failure, tumor
recurrence and invasion.
Elevated expression of Nrf2 and ABCG2
contributes to chemoresistance in HNSCC SP cells
In order to explore the molecular mechanism and
signaling pathways involved in the enhanced expression profile of
ABC transporters and the phenomenon of multidrug resistance, the
expression of molecules associated with Nrf2 signaling was
evaluated. Previous reports have indicated that Nrf2-dependent
ABCG2 expression in SP cells is responsible for the chemoresistance
exhibited by these cells. Furthermore, depletion of Nrf2 expression
suppressed the ABCG2-mediated multidrug resistance of CSCs
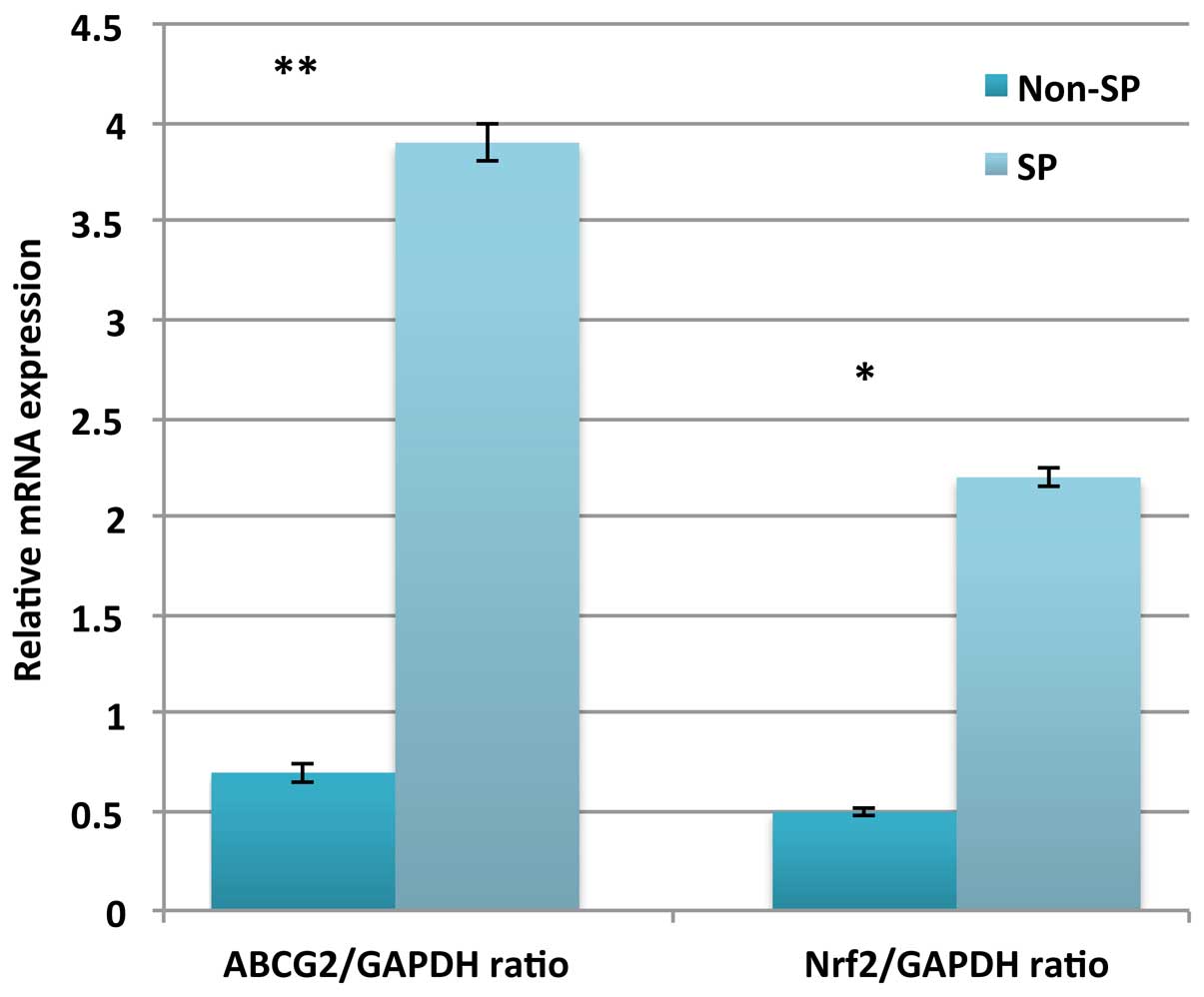
(16–18). In the present study, the relative
messenger (m)RNA expression of Nrf2 was significantly upregulated
in SP cells (P=0.018), which also displayed upregulation of the
ABCG2 gene (Fig. 5). Therefore, these
data suggest that a link exists between Nrf2 and ABC transporters
regarding their contribution to the phenomenon of multidrug
resistance in SP cells.
Discussion
Currently, the major challenge in the treatment of
cancer is the presence of a small population of CSCs that could
escape the current conventional treatment strategies and is capable
of reinitiating tumor growth, metastasis and invasion following
treatment (19). Therefore, the
development of novel anticancer drugs to prevent tumor recurrence
is an ultimate essential goal in the field of cancer therapy.
Numerous studies concerning the characterization of CSCs revealed
that these cells undergo rapid proliferation and generation of
tumor spheres, possess a high potential for differentiation, and
exhibit multidrug and apoptosis resistance (19,20).
In the present study, the most common Hoechst dye
exclusion assay (21) was used to
purify CSCs from HNSCC specimens, and identified ~2.1% SP cells in
the samples. Similar to previous findings, the present
characterization experiments demonstrated that HNSCC cancer
stem-like SP cells were capable of generating rapid tumor spheres,
displayed an enhanced cell proliferation rate and were highly
resistant to DNA-targeting drugs (17–19).
Notably, these HNSCC SP cells possessed enhanced expression of the
stem cell surface protein CD133, compared with non-SP cells.
Previously, it was reported that the HNSCC cell lines M3a2 and M4e
also contained SP cells, which were highly tumorigenic and
chemoresistant (22). In the current
study, it was also demonstrated that HNSCC SP cells were able to
induce tumor growth rapidly in vivo in NOD/SCID mice.
These findings suggest that overexpression of ABC
transporter genes and the stem cell surface protein CD133 may be
involved in chemoresistance and maintenance of self-renewal in
HNSCC SP cells. These observations were further confirmed by the
reduction in the number of SP cells following treatment with
verapamil. These data clearly indicates that ABC transporter genes
are actively involved in pumping the above drug out of the
cells.
Nrf2 is a basic leucine zipper domain transcription
factor, which is known to protect cells from oxidative stress and
other foreign pathogens by accelerating the expression of several
antioxidant enzymes and ABC transporter proteins (23–25). Nrf2
is subjected to proteasomal degradation by Kelch-like
ECH-associated protein 1 (KEAP1), and previous studies in primary
cell cultures demonstrated that this ocurred either by loss of
Nrf2-KEAP1 interaction or by mutations in the KEAP1 or Nrf2 genes
(16–18); however, the mechanism of proteasomal
degradation of Nrf2 requires further investigation. Recent studies
in lung cancer cells reported that overexpression of Nrf2 leads to
transcriptional upregulation of ABC transporter genes such as
ABCG2, which contributes to drug resistance (13). Similarly, the present study also
demonstrated that the relative mRNA expression of Nrf2 in HNSCC
stem cells was significantly elevated compared with non-SP cells.
Therefore, it is possible to speculate that the multidrug
resistance properties of HNSCC SP cells are regulated by the
Nrf2-mediated overexpression of ABCG2. However, it is worth
investigating whether the depletion of Nrf2 by small interfering
RNA could lead to the attenuation of ABCG2 expression, thus
enhancing the sensitivity of SP cells towards drug treatment. In
addition, the precise molecular mechanism involved in the
regulatory pathways and other causative factors that result in
Nrf2-mediated multidrug resistance remain to be studied in
detail.
In summary, the present data suggest that HNSCC
contains CD133+ cancer stem-like SP cells that are
highly resistant to a variety of drugs due to the overexpression of
drug efflux pumps (such as ABCG2), which is induced by the
stress-inducible factor Nrf2 and is ultimately responsible for
treatment failure and tumor recurrence. Therefore, developing novel
therapeutic drugs that efficiently suppress the function of Nrf2
and its downstream activating factors will aid to prevent
Nrf2-mediated resistance to chemotherapeutic drugs and tumor
recurrence in HNSCC.
Acknowledgements
The authors would like to thank Dr Xu-Yang
(Department of Molecular Medicine, The Fifth Affiliated Hospital of
Xinjiang Medical University, Urumqi, China) and Dr Ying Zheng
(Department of Otolaryngology, Head and Neck Surgery, Jilin
Provincial Cancer Hospital, Changchun, China) for sharing RT-PCR
strategies, primers and other protocols used in the present
study.
References
|
1
|
Schoenhals M, Kassambara A, De Vos J, Hose
D, Moreaux J and Klein B: Embryonic stem cell markers expression in
cancers. Biochem Biophys Res Commun. 383:157–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chiou SH, Wang ML, Chou YT, Chen CJ, Hong
CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS and Wu CW:
Coexpression of Oct4 and Nanog enhances malignancy in lung
adenocarcinoma by inducing cancer stem cell-like properties and
epithelial-mesenchymal transdifferentiation. Cancer Res.
70:10433–10444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lino MM, Merlo A and Boulay JL: Notch
signaling in glioblastoma: A developmental drug target. BMC Med.
8:722010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang XP, Zheng G, Zou L, Liu HL, Hou LH,
Zhou P, Yin DD, Zheng QJ, Liang L, Zhang SZ, et al: Notch
activation promotes cell proliferation and the formation of neural
stem cell-like colonies in human glioma cells. Mol Cell Biochem.
307:101–108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawaguchi-Ihara N, Murohashi I, Nara N and
Tohda S: Promotion of the self-renewal capacity of human acute
leukemia cells by Wnt3A. Anticancer Res. 28:2701–2704.
2008.PubMed/NCBI
|
|
6
|
Khan NI, Bradstock KF and Bendall LJ:
Activation of Wnt/beta-catenin pathway mediates growth and survival
in B-cell progenitor acute lymphoblastic leukaemia. Br J Haematol.
138:338–348. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Salnikov AV, Gladkich J, Moldenhauer G,
Volm M, Mattern J and Herr I: CD133 is indicative for a resistance
phenotype but does not represent a prognostic marker for survival
of non-small cell lung cancer patients. Int J Cancer. 126:950–958.
2010.PubMed/NCBI
|
|
8
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ho MM, Ng AV, Lam S and Hung JY: Side
population in human lung cancer cell lines and tumors is enriched
with stem-like cancer cells. Cancer Res. 67:4827–4833. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burkert J, Wright NA and Alison MR: Stem
cells and cancer: An intimate relationship. J Pathol. 209:287–297.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rehman AO and Wang CY: CXC12/SDF-1 alpha
activates NF-kappaB and promotes oral cancer invasion through the
Carma3/Bcl10/Malt1 complex. Int J Oral Sci. 1:105–118. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Prince ME, Sivanandan R, Kaczorowski A,
Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF and Ailles
LE: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci USA. 104:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh A, Wu H, Zhang P, Happel C, Ma J and
Biswal S: Expression of ABCG2 (BCRP) is regulated by Nrf2 in cancer
cells that confers side population and chemoresistance phenotype.
Mol Cancer Ther. 9:2365–2376. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang B, Ma YF and Liu Y: Elevated
Expression of Nrf-2 and ABCG2 involved in multidrug resistance of
lung cancer SP cells. Drug Res (Stuttg). 65:526–531.
2015.PubMed/NCBI
|
|
15
|
Shi Y, Fu X, Hua Y, Han Y, Lu Y and Wang
J: The side population in human lung cancer cell line NCI-H460 is
enriched in stem-like cancer cells. PLoS One. 7:e333582012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kobayashi A, Kang MI, Okawa H, Ohtsuji M,
Zenke Y, Chiba T, Igarashi K and Yamamoto M: Oxidative stress
sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to
regulate proteasomal degradation of Nrf2. Mol Cell Biol.
24:7130–7139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang DD, Lo SC, Cross JV, Templeton DJ
and Hannink M: Keap1 is a redox-regulated substrate adaptor protein
for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol.
24:10941–10953. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh A, Misra V, Thimmulappa RK, Lee H,
Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E,
et al: Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung
cancer. PLoS Med. 3:e4202006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morrison SJ and Kimble J: Asymmetric and
symmetric stem-cell divisions in development and cancer. Nature.
441:1068–1074. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song J, Chang I, Chen Z, Kang M and Wang
CY: Characterization of side populations in HNSCC: Highly invasive,
chemoresistant and abnormal Wnt signaling. PLoS One. 5:e114562010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bunting KD: ABC transporters as phenotypic
markers and functional regulators of stem cells. Stem Cells.
20:11–20. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Itoh K, Chiba T, Takahashi S, Ishii T,
Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et
al: An Nrf2/small Maf heterodimer mediates the induction of phase
II detoxifying enzyme genes through antioxidant response elements.
Biochem Biophys Res Commun. 236:313–322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K,
Yamamoto M, Talalay P and Kensler TW: Sensitivity to carcinogenesis
is increased and chemoprotective efficacy of enzyme inducers is
lost in nrf2 transcription factor-deficient mice. Proc Natl Acad
Sci USA. 98:3410–3415. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kwak MK, Kensler TW and Casero RA Jr:
Induction of phase 2 enzymes by serum oxidized polyamines through
activation of Nrf2. Effect of the polyamine metabolite acrolein.
Biochem Biophys Res Commun. 305:662–670. 2003. View Article : Google Scholar : PubMed/NCBI
|