Introduction
Hepatocellular carcinoma (HCC) is the third leading
cause of cancer mortality worldwide. In 2010, liver cancer resulted
in 754,00 mortalities, representing an increase of 62.4% since
1990. Despite significant advances in the treatment and the
prevention of postoperative metastasis, the 5-year postoperative
recurrence rate of HCC remains high and the recurrence and
metastasis of HCC also remains a problem in clinical practice
(1,2).
Efforts have been made to develop more efficient drugs to inhibit
and prevent tumor metastasis. Cancer metastasis involves tumor
cells dissociating from the primary locus, invading the surrounding
tissue, entering and extravasating from the circulation, and
growing in distant organs (3,4). During this complex process, cell
adhesion is one of the most important events (5). A number of previous studies have focused
on synthesized anti-adhesion peptides (6–8). However,
the application of these short peptides is limited due to their
short half-life and high dosage required. To prolong the half-life
of synthesized peptide, multimers and derivatives were designed
(9–11). It has been demonstrated that the
anti-metastasis effect of multimers of synthesized peptides was
stronger than that of monomer peptides (12,13).
Integrins are a family of adhesion molecules located
on cells and in the extracellular matrix. The expression levels of
integrins are closely associated with the migration ability of
cells (14). The anti-adhesion
peptide β (DLYYLMDLSYSMK, β1) was designed by Liu et al
based on the conserved sequence of the integrin a and β units
(15). In our previous study, this
peptide was shown to block the interaction between HCC cells and
the extracellular matrix, in addition to inhibiting intrahepatic
and pulmonary metastases following carcinosectomy in a nude mouse
model with human HCC that has high metastatic potential (LCI-D20)
(16,17). On the basis of these studies, the
trimeric peptide β (β3) was designed. The present study aimed to
determine the effects of β3 on the adhesive properties of the human
liver cancer cell line HCCLM6 to fibronectin (FN), the invasion of
HCCLM6 cells through reconstituted basement membrane. In addition
the rate of liver cancer recurrence and LCI-D20 mouse survival time
following early carcinosectomy were investigated.
Materials and methods
Design and production of β3
The anti-adhesion trimeric β peptide
(DLYYLMDLSYSMKGGDLYYLMDLSYSMKGGDLYYLMDLSYSMK, β3) was expressed in
E. coli as described previously (18). Briefly, the DNA fragment of β3 (Sangon
Biotech Co., Ltd., Shanghai, China) was cloned into the pET-His
expression vector (Gene Power Lab Ltd., Shenzhen, China) and the
fusion protein His-β3 was expressed in E. coli BL21 (DE3)
plysS (Gene Power Lab Ltd.). Following 1.5 h of induction with
isopropyl-β-D-thiogalactoside (GE Healthcare Bio-Sciences,
Pittsburgh, PA, USA), 20 mg of β3 peptide was obtained from 1 L of
culture medium following purification with metal-chelating
sepharose 6B FF (Vector Gene Technology Company Ltd., Beijing,
China). The purity of β3 was 92.2% according to Gel-Pro Analyzer
3.1 software (Media Cybernetics, Inc., Rockville, MD, USA).
Cell culture
The highly metastatic HCC cell line HCCLM6, was
initially established and preserved by the Liver Cancer Institute,
Fudan University (Shanghai, China) and was cultured in Gibco
Dulbecco's modified eagle's medium (DMEM, Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Thermo Fisher Scientific, Inc.), 100 U/ml penicillin at 37°C under
an atmosphere of 5% CO2. The medium was replenished
every 3 days to maintain cell growth.
Coating the 96-well high binding
microplate with FN
FN (Sigma-Aldrich, St. Louis, MO, USA) solution
(containing 10 µg/ml FN, 20 mmol/l Tris-Cl, pH 7.4, 150 mmol/l
NaCl, 1 mmol/l MgCl2, 1 mmol/l CaCl2, 1
mmol/l MnCl2) was added to a 96-well high binding
microplate (100 ml per well), and incubated at 4°C overnight. The
plate was then incubated with blocking buffer (10 mmol/l Hepes, pH
7.4, 140 mmol/l NaCl, 5.4 mmol/l KCl, 5.56 mmol/l glucose, 3% BSA,
1 mmol/l MgCl2, 2 mmol/l CaCl2, 1 mmol/l
MnCl2) at 37°C for 2 h, and air dried for future
use.
Cell adhesion assay
A total of 100 µl of HCCLM6 cell suspension
(2×105 cells/ml) was plated in each well of an FN-coated
96-well high binding microplate. A total of 100 ml of DMEM medium
containing β3 at concentrations of 20, 40, 100 or 200 µmol/l was
added at the same time. The final concentrations of β3 were 10, 20,
50 or 100 µmol/l, respectively. The same volume of cell culture
medium without β3 was added to the control group and 200 ml of cell
culture medium only was added in the plate for the blank group. The
assay was conducted in quintuplicate for each sample. Following
incubation for 3 h at 37°C in 5% CO2, the unattached
cells were gently washed away with HANKS buffer (Sangon Biotech
Co., Ltd.). The attached cell number in each well was measured
using the MTT assay (see below). The inhibition rate of β3 on cell
adhesion to FN was calculated with the following equation: Cell
adhesion inhibition rate = (average OD of control well - average OD
of β3-treated well) / (average OD of control well - average OD of
blank well) × 100%.
MTT assay
The number of attached cells in each well was
analyzed by the MTT assay, and quantified by a micro-titer plate
reader (Amersham, USA). Briefly, 100 µl DMEM and 20 µl MTT (5
mg/ml; Sigma-Aldrich) were added to each well. After incubation at
37°C for 4 h, the medium and MTT was discarded. A total of 200 µl
of 0.04 mol/l hydrogen chloride-2-propanol solution was added to
each well. The amount of MTT formazan product, which reflects the
number of cells adhering to FN, was determined by measuring
absorbance with a microplate reader at a wavelength of 570 nm and a
reference wavelength of 630 nm.
Invasion assay
The invasion assay was performed as described
previously (19). Briefly, the upper
portions of Transwell chambers (Corning, New York, NY, USA) were
coated with 75 µl of Matrigel (BD Biosciences, Franklin Lakes, NJ,
USA) diluted 1:10 in serum-free DMEM and incubated at 37°C for 2 h.
The supernatants of HCCLM6 cells cultured with DMEM containing 10%
FCS were harvested once the cells had grown to confluence, and
after the addition of FN at a final concentration of 5 µg/ml,
resulting in conditioned medium. The cells were harvested by
trypsinization and diluted to a 2×106/ml cell suspension
with serum-free DMEM. A total of 100 µl of the cell suspension and
100 µl of 200 µmol/l β3 peptide in serum-free DMEM (or serum-free
DMEM only as a control) were added in the upper chambers. Then, 600
µl of conditioned medium was added to the bottom chamber of the
Transwell plate. After incubation at 37°C for 48 h under a 5%
CO2 atmosphere, the non-invading cells and the gel were
gently removed from the upper chamber with cotton-tipped swabs.
After rinsing with PBS, cells on the filters were fixed with
formaldehyde and stained in Giemsa staining solution for 30 min.
The number of invaded cells on the filters were counted in five
randomly selected high-powered (×200) fields per filter under a
microscope (Leica, Heerbrugg, Switzerland). The invasion inhibition
rate was calculated using the following equation: Invasion
inhibition rate = [1 - (invaded cell number in β3 chamber / invaded
cell number in control chamber)] × 100%.
Animal model and treatment
A total of 24 6-week-old male BALB/cA nude mice were
obtained from the Shanghai Institute of Materia Medica, Chinese
Academy of Sciences (Shanghai, China). A tumor block from
tumor-bearing mice was implanted into the left lobe of the nude
mouse liver as described previously (20). Briefly, a left upper abdominal
transverse incision was made under anesthesia. The left lobe of the
liver was exposed and a part of the liver surface was mechanically
injured with scissors. Next, a tumor block of 0.2×0.2×0.2 cm was
fixed within the liver tissue. After the surgery, mice were kept in
laminar-flow cabinets under pathogen-free conditions and given free
access to food and water. Liver cancer early resection was
performed at 0.2 cm from the edge of the tumor at day 10 following
implantation, prior to metastasis.
Measurement of tumor recurrence and
mouse survival time
Animals were randomly assigned to 2 groups (each
group, n=12). At day 1 following resection, the animals were
subcutaneously administrated 100 µl of 1 mg/ml of β3 or normal
saline (NS) as a control every other day for 10 doses. Half of the
mice (n=6) in each group were sacrificed at day 55
post-implantation with an overdose of 6% chloral hydrate (0.5
ml/100 g of body weight; Sigma-Aldrich). At autopsy, the viscera of
the animals were examined macroscopically. If recurrence of tumors
at the incisal margin was observed, the lesions were resected and
weighed.
The other 6 mice of each group were housed in
pathogen-free cages. Each animal was examined daily until death.
The number of days that each animal survived following resection
was recorded. Survival curves were constructed using the
Kaplan-Meier method.
All animal experiments were conducted in strict
accordance with the National Institute of Health Guide for the Care
and Use of Laboratory Animals. This study was approved by the
Experimental Animal Ethics Committee of Shanghai Medical College,
Fudan University (approval no. 20021101).
Statistical analysis
All data were entered into Excel spreadsheets
(Excel, Microsoft, Seattle, USA). Statistical analysis was
performed using Student's t-test, or the Mann Whitney U test
when the data were not normally distributed. Values of P<0.05 in
a two-tailed fashion were considered to indicate a statistically
significant difference. All analyses were performed using SAS (SAS
Institute Inc., Cary, NC, USA).
Results
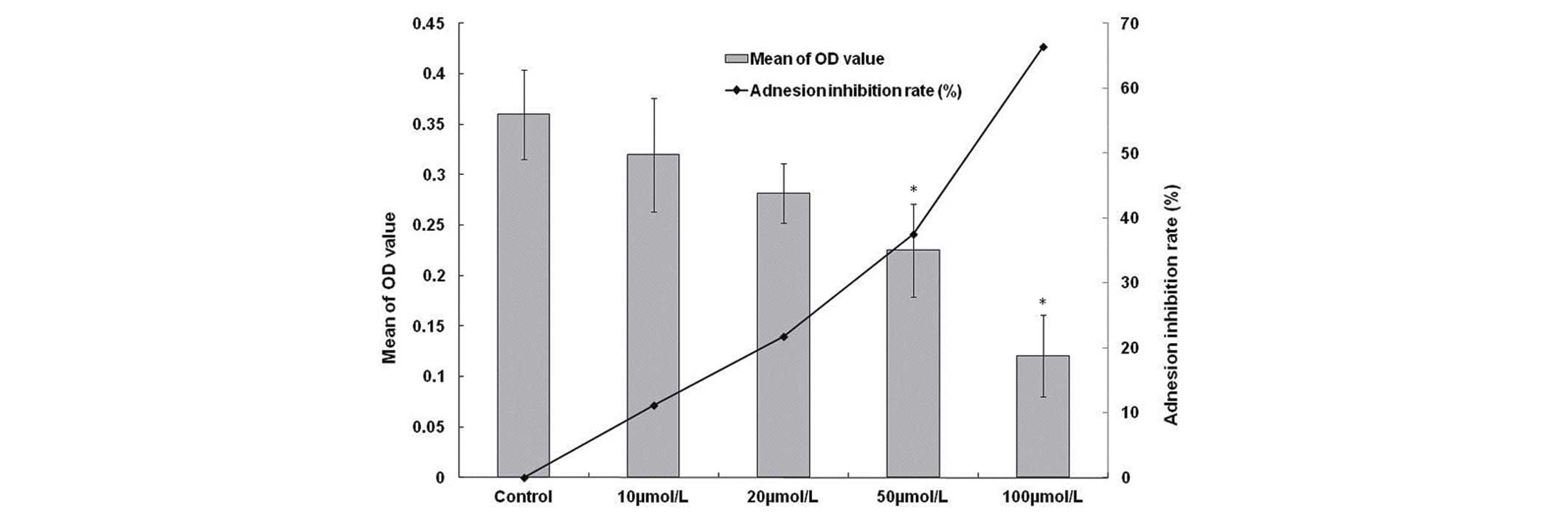
The inhibitory effect of β3 on the
adhesion of HCCLM6 cells to FN
HCCLM6 cells incubated with 10, 20, 50 and 100
µmol/l β3 for 3 h exhibited a marked reduction in cell adhesion.
The adhesion inhibition rates were 11.8, 21.7, 37.5 and 66.4%,
respectively (Fig. 1). These findings
indicate that β3 is able to inhibit the adhesion of HCCLM6 cells to
FN, and β3 may obstruct the invasion of HCC cells to paratumor
liver parenchyma.
The inhibitory effect of β3 on the
invasion ability of HCCLM6 cells
Following incubation with 100 µmol/l β3, the number
of invaded HCCLM6 cells was reduced significantly, with an
inhibition rate of 51.3% (Table I).
Therefore, β3 may block HCC cells from invading the surrounding
tissue and entering and extravasating from the circulation in
vivo.
 | Table I.The inhibitory effects of β3 on the
invasion of HCCLM6 cells (n=5). |
Table I.
The inhibitory effects of β3 on the
invasion of HCCLM6 cells (n=5).
| Group | Invaded cells (mean ±
SD) | Invasion inhibitory
rate (%) |
|---|
| Control group | 22.6±4.77 | – |
| β3 group |
11±2.74a | 51.3 |
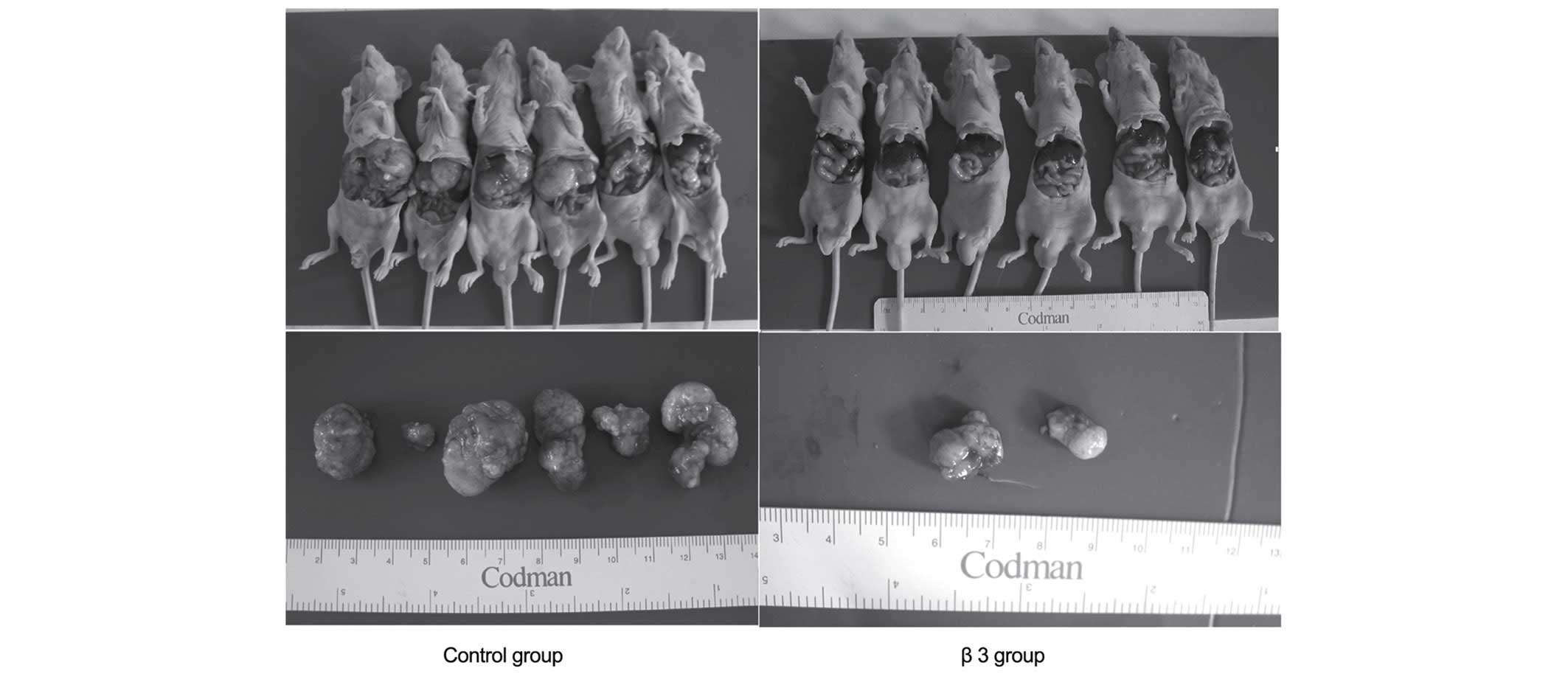
The influence of β3 on the
intrahepatic recurrence of the LCI-D20 model following early
resection
On the 10th day post-tumor-implantation, LCI-D20
tumors were resected, and β3 or the same volume of NS was
subcutaneously injected. On day 55, mice were sacrificed to check
for intrahepatic recurrence. The recurrent tumors were located
around the incisal margins. There were 2 (2/6) mice with
intrahepatic recurrent tumors in the β3 group, while there were 6
(6/6) mice with intrahepatic recurrent tumors in the control group
(Fig. 2 and Table II). Compared with the control group,
the weight of recurrent tumors of the β3 group were markedly
reduced. These results indicate that β3 may have inhibitory effects
on tumor recurrence at the incisal margin.
 | Table II.Liver cancer recurrence after early
resection. |
Table II.
Liver cancer recurrence after early
resection.
| Group | Number of mice
examined | Weight of recurrent
lesions (mean ± SD) | Number of mice with
recurrent lesions |
|---|
| Control group | 6 |
2.31±1.57a | 6 |
| β3 group | 6 |
0.17±0.26ab | 2 |
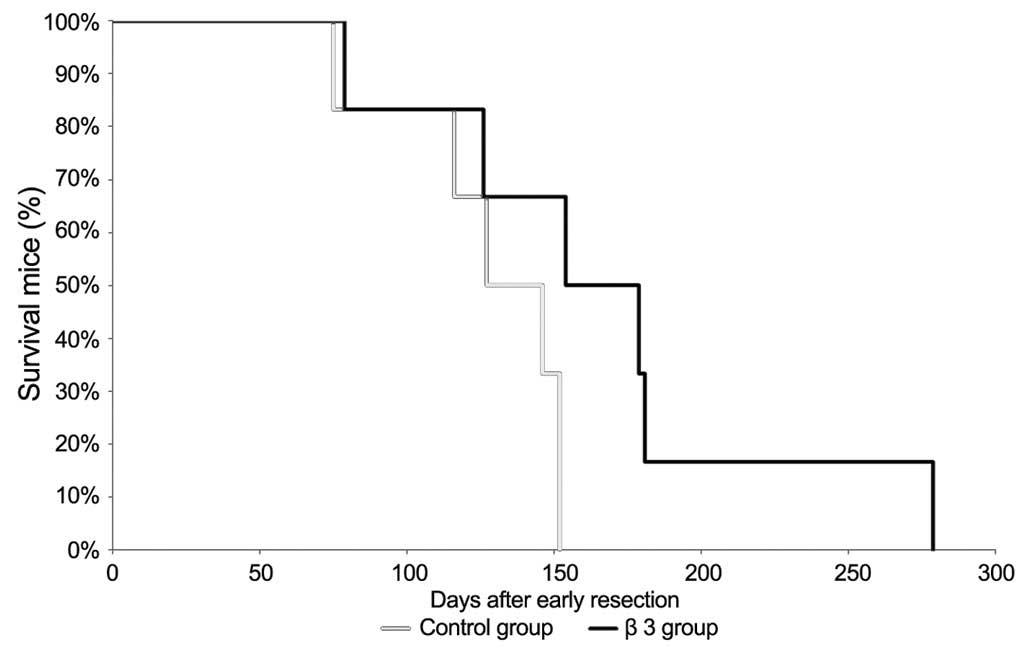
Survival analysis of LCI-D20 mice
after early resection
Twelve animals were randomly assigned to 2 groups
(each group, n=6). At day 1 following resection, the animals were
subcutaneously administrated 100 µl of 1 mg/ml of β3 or NS as a
control every other day for 10 doses. The mice were housed in
pathogen-free cages, and given free access to autoclaved food and
water. The survival curves were constructed using the Kaplan-Meier
method (Fig. 3). The median survival
times after early resection were 136.5 days (control group) and
166.5 days (β3 group), respectively. This result indicates that β3
treatment prolonged the survival time of mice following early
resection.
Discussion
The adhesion molecules on the surface of both tumor
cells and endothelial cells are associated with tumor metastasis
and recurrence. Blocking the interaction between tumor cell
adhesion molecules and their ligands is a major strategy to prevent
cancer metastasis (21,22). A number of previous studies have
focused on the synthesized anti-adhesion peptides (13,23). One
such peptide is RGD, derived from the common conserved sequence of
the main matrix proteins such as fibronectin, collagen and
fibrinogen (24,25). A second peptide is YIGSR, which
originates from the basement membrane protein laminin (26). The third peptide is EILDV, which stems
from the core sequence of fibronectin (27). The application of these short peptides
is limited due to their short half-life, ease of degradation and
the requirement for a high dosage. To prolong the peptides'
half-lives, multimers and derivatives of these peptides were
designed (28). The anti-metastasis
effect of multimers of synthesized peptides was stronger compared
to peptide monomers. The more times the sequence is repeated, the
stronger the anti-metastasis effect is.
FN is an important cell adhesion molecule in the
extracellular matrix. It mediates cell adhesion and migration, and
serves a significant role in tumor invasion and metastasis
(29). Examination of tumor cell
adhesion to FN is a common method for studying tumor cell
metastasis. In the present study, the extracellular matrix was
simulated by coating cell culture plates with FN, after which the
inhibitory effects of β3 peptide on liver cancer cell adhesion to
FN were investigated. The results demonstrated that after treatment
of HCCLM6 cells with β3 peptide for 3 h, an inhibitory effect on
cell adhesion to FN was observed. There are two possible mechanisms
by which β3 peptide blocked tumor cell adhesion to FN. First, the
β3 peptide may occupy the integrin binding site through binding to
the RGD sequence of the matrix protein. Second, β3 may also
interact with integrin because β3 was designed based on the
conserved sequence of the integrin α and β units.
During metastasis, tumor cells must penetrate the
basement membrane through three steps: Dislodging from the original
site, entering blood circulation, and migrating from blood flow
into remote sites (30). Matrigel,
used as an artificial basement membrane matrix, is produced from
mouse Engelbreth-Holm-Swarm sarcoma rich in extracellular matrix
protein (31). The artificial
basement membrane is plated on a Millipore filter in Transwell
culture chambers, and forms a membrane structure similar to natural
basement membrane. Invasive tumor cells can penetrate the membrane
under the induction of chemotactics, simulating the invasion of
tumor cells through the basement membrane in vivo. The
results indicated that β3 exerted significant inhibitory effects on
the invasion of HCCLM6 cells.
In addition, the anti-tumor effect of β3 was
observed in vivo. In LCI-D20 mice, β3 treatment was shown to
reduce the weights of recurrent tumors at the incisal margins, in
addition to the number of mice with intrahepatic recurrent tumors
after early resection. Notably, β3 prolonged the survival time of
LCI-D20 mice after early hepatectomy.
Metastasis and recurrence of liver cancer are major
determinants for the prognosis and long-term survival of liver
cancer patients. Polypeptide therapy is a newly developed treatment
for tumors. Taken together, these cell and animal studies
demonstrated that the β3 peptide had anti-adhesion and
anti-recurrence effects, in addition to the capability to prolong
survival time after early resection. Therefore, the β3 peptide is
worthy of further investigation as a potential drug for blocking
tumor metastasis and recurrence.
References
|
1
|
Fang WQ, Li SP, Zhang CQ, Xu L, Shi M,
Chen MS and Li JQ: Prophylaxis and clinical treatment for surgical
margin recurrence of small primary hepatocellular carcinoma. Ai
Zheng. 24:834–836. 2005.PubMed/NCBI
|
|
2
|
Zhi X, Lin L, Yang S, Bhuvaneshwar K, Wang
H, Gusev Y, Lee MH, Kallakury B, Shivapurkar N, Cahn K, et al:
βII-spectrin (SPTBN1) suppresses progression of hepatocellular
carcinoma and Wnt signaling by regulation of Wnt inhibitor
kallistatin. Hepatology. 61:598–612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wyke JA: Overview-burgeoning promise in
metastasis research. Eur J Cancer. 36:1589–1594. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: An imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chu XY and Chen LB: Cellular adhesive
molecular and the invasion and metastasis of neoplasm. Yixue
Yanjiusheng Xuebao. 13:42–45. 2000.
|
|
6
|
Li FH: The inhibitory effect of bioactive
peptides on neoplasm metastasis. Kouqiang Hemian Waike Zazhi.
9:231–234. 1999.
|
|
7
|
Liu LY, Chen ZY and Zhao TH:
Investigations of a peptide with RGD and YIGSR fragments: Synthesis
and its anti-tumor invasion activities. Zhongguo Xinyao Zazhi.
14:729–731. 2005.
|
|
8
|
Saiki I, Yoneda J, Kobayashi H, Igarashi
Y, Komazawa H, Ishizaki Y, Kato I and Azuma I: Antimetastatic
effect by anti-adhesion therapy with cell-adhesive peptide of
fibronectin in combination with anticancer drugs. Jpn J Cancer Res.
84:326–335. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu LY, Chen ZY and Zhao TH: Synthesis of
RGD identical-fork-peptide derivative with inhibitive effecton
adhesiveness of advanced metastatic tumor cells. Zhongguo Xinyao
Zazhi. 15:1661–1663. 2006.
|
|
10
|
Zhang HQ, Shinohara H, Gu N, Sasaki H and
Sisido M: Cell adhesion inhibition by RGD peptides linked with a
photoisomerizable nonnatural amino acid. J Southeast Univ.
17:22–26. 2001.
|
|
11
|
Zhao M, Wang C, Jiang X and Pen S:
Synthesis of RGD containing peptides and their bioactivities. Prep
Biochem Biotechnol. 32:363–380. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao K, Zhao TH, Chen ZY, Gao W, Yang HS
and Shi B: The invasive capacity of human lung great cellular
xancerous PG cells on reformed basement membrane and inhibition of
synthetic peptides. Zhongliu Fangzhi Yanjiu. 29:20–22. 2002.
|
|
13
|
Okrój M, Dobrzańska-Paprocka Z, Rolka K
and Bigda J: In vitro and in vivo analyses of the biological
activity of RGD peptides towards Ab Bomirski melanoma. Cell Mol
Biol Lett. 8:873–884. 2003.PubMed/NCBI
|
|
14
|
Liu J, Guo SX and Tang JG: Research
progress of RGD-peptide for cancer therapy. Guowai Yixue
(Zhongliuxue Fence). 30:193–197. 2003.
|
|
15
|
Liu YK, Nemoto A, Feng Y and Uemura T: The
binding ability to matrix proteins and the inhibitory effects on
cell adhesion of synthetic peptides derived from a conserved
sequence of integrins. J Biochem. 121:961–968. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uemura T, Nemoto A and Liu YK: Synthetic
peptide derived from a conserved sequence of integrin β subunit.
Res Adv in Biosci & Bioeng. 23:65–83. 2000.
|
|
17
|
Sun JJ, Zhou XD, Liu YK, Tang ZY, Sun RX,
Zhao Y and Uemura T: Inhibitory effects of synthetic beta peptide
on invasion and metastasis of liver cancer. J Cancer Res Clin
Oncol. 126:595–600. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang SM, Zhu J, Li Y, Pan LF, Zha XL and
Liu YK: Molecular cloning and expression of anti-tumor adhesion
peptide (beta3). Sheng Wu Gong Cheng Xue Bao. 21:558–562. 2005.(In
Chinese). PubMed/NCBI
|
|
19
|
Knutson JR, Lida J, Fields GB and McCarthy
JB: CD44/chondroitin sulfate proteoglycan and alpha 2beta 1
integrin mediate human melanoma cell migration on type IV collagen
and invasion of basement membranes. Mol Biol Cell. 7:383–396. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun FX, Tang ZY, Lui KD, Ye SL, Xue Q, Gao
DM and Ma ZC: Establishment of a metastatic model of human
hepatocellular carcinoma in nude mice via orthotopic implantation
of histologically intact tissues. Int J Cancer. 66:239–243. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Syrigos KN and Karayiannakis AJ: Adhesion
molecules as targets for the treatment of neoplastic diseases. Curr
Pharm Des. 12:2849–2861. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang CG and Xu HM: Research and
application of anti-adhesion therapy in cancer metastasis. Guowai
Yixue (Zhongliuxue Fence). 32:31–34. 2005.
|
|
23
|
Wang YH, Liu YK, Li WC, Ye SL and Tang ZY:
Inhibitory effect of anti-adhesion peptides on invasion/metastasis
ability of hepatocellular carcinoma cells. Zhonghua Shiyan Waike
Zazhi. 21:1168–1169. 2004.
|
|
24
|
Liu YK, Wu WZ, Wu X, Jiang Y and Zhou XD:
Liver cancer metastasis and signal transduction. In: Tang ZY.
Metastasis and recurrence of hepatocellular carcinoma-basic and
clinical studies. Shanghai Shanghai Scientific and technological
education public house. 93–104. 2003.
|
|
25
|
Maeda M, Izuno Y, Kawasaki K, Kaneda Y, Mu
Y, Tsutsumi Y, Nakagawa S and Mayumi T: Amino acids and peptides.
XXXI. Preparation of analogs of the laminin-related peptide YIGSR
and their inhibitory effect on experimental metastasis. Chem Pharm
Bull (Tokyo). 46:347–350. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kaneda Y, Yamamoto Y, Okada N, Tsutsuml Y,
Nakagawa S, Kakiuch M, Maeda M, Kawasaki K and Mayumi T:
Antimetastatic effect of synthetic Glu-Ile-Leu-Asp-Val peptide
derivatives containing D-amino acids. Anticancer Drugs. 8:702–707.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feng ZH, Huang B, Zhang GM, Li D and Wang
HT: Inducement of antitumor-immunity by DC activated by Hsp70-H22
tumor antigen peptide. Chinese Journal of Cancer Research.
15:79–85. 2003. View Article : Google Scholar
|
|
28
|
Wang SM, Zhu J, Li Y, Pan LF, Zha XL and
Liu YK: Inhibitory effects of β peptide and polymeric β peptide on
adhesion of hepatocellular carcinoma cells to fibronectin. Shiyong
Zhongliu Zazhi. 21:223–226. 2005.
|
|
29
|
Li NF, Gemenetzidis E, Marshall FJ, Davies
D, Yu Y, Frese K, Froeling FE, Woolf AK, Feakins RM, Naito Y, et
al: RhoC interacts with integrin α5β1 and enhances its trafficking
in migrating pancreatic carcinoma cells. PLoS One. 8:e815752013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sengupta N and MacDonald TT: The role of
matrix metalloproteinases in stromal/epithelial interactions in the
gut. Physiology (Bethesda). 22:401–409. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Benton G, Arnaoutova I, George J, Kleinman
HK and Koblinski J: Matrigel: From discovery and ECM mimicry to
assays and models for cancer research. Adv Drug Deliv Rev 79–80.
3–18. 2014. View Article : Google Scholar
|