Introduction
A xanthoma refers to an exogenous mass that is
visible on the body surface. Xanthomas are not tumors but clusters
of foam cells that form within the connective tissue of the skin,
tendons and subcutaneous tissues. Xanthomas comprise important
clinical manifestations of lipid metabolism disorders, and are
commonly encountered in patients with familial hypercholesterolemia
(FH) (1). Xanthomas may be divided
into several categories: Tendinous xanthoma, xanthoma tuberosum,
eruptive xanthoma, xanthoma planum and palmar xanthoma (1). The most commonly observed xanthomas
among patients with FH are tendinous xanthomas (40–50% of all
patients), which are subcutaneous tumors located within the tendons
used for extension, and mainly affect the Achilles, patellar
tendons and extensor tendons of the hands, buttocks, elbows,
eyelids and hand creases (2,3). Tuberous xanthomas are also commonly
observed in FH patients (10–15% of all patients), and manifest as
yellow nodules, often measuring ≤2 cm in diameter, and are located
in pressure areas, including the extensor aspects of the knees,
elbows and buttocks (2–4). The clinical manifestations associated
with xanthomas depend on the duration and severity of
hyperlipoproteinemia (5); therefore,
the presence of multiple xanthomas often indicates severe and
long-term FH and tends to be observed in patients with homozygous
FH (HoFH) (2,6).
FH is an inherited disorder featuring elevated
plasma levels of low-density lipoprotein cholesterol (LDLC),
atherogenesis and xanthomas (7). Two
clinical variants of this disorder exist, the homozygous and
heterozygous variants. Heterozygous FH (HeFH) accounts for the
majority of FH cases, with an approximate incidence of 1 per 500
individuals; whereas the homozygous variant of the disease is
considerably rare, with an approximate incidence of 1 per 1,000,000
individuals (3). The clinical
features of HeFH are mild, and include a blood LDLC concentration
of >4.9 mmol/l, total cholesterol (TC) levels >7.5 mmol/l and
an age of onset of >20 years (8).
By contrast, HoFH is characterized by a greater incidence rate of
cutaneous xanthomas, enlarged Achilles tendons, atherosclerosis,
corneal arcus, a blood LDLC concentration of >13 mmol/l, TC
levels >14.95 mmol/l and disease onset in early childhood
(7).
The present study reports the case of a 23-year-old
male patient with HoFH, who presented with multiple large tuberous
and tendinous xanthomas within various dermal tissues.
Case report
A 23-year-old male patient presented at Zhejiang
Provincial People's Hospital (Hangzhou, China) in May 2008 with
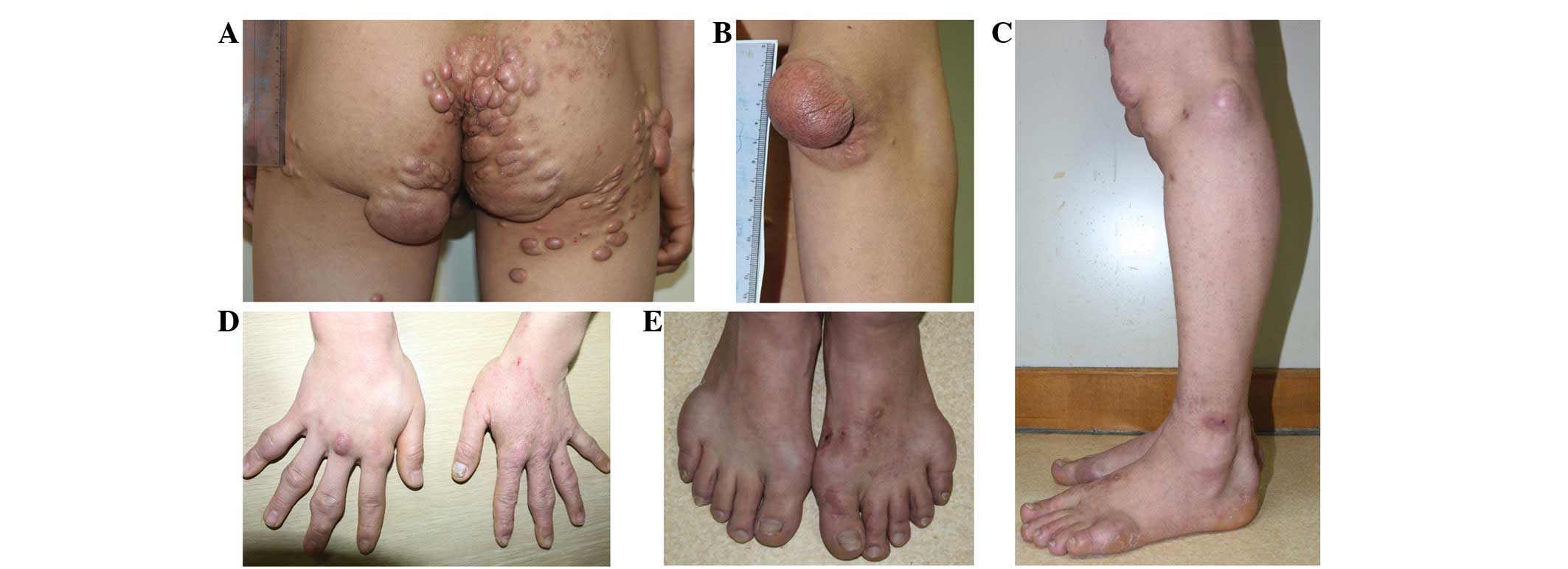
multiple yellowish elevated masses over the dorsum of the elbows,
knees, buttocks and hands. The size of the masses varied between
1×1×1 cm (over the dorsum of the hands) and 7×5×4 cm (over the
dorsum of the elbows) (Fig. 1). The
lesions were originally asymptomatic; they appeared at 2 years of
age and then progressively increased in size and extent. The
patient had symptoms of discomfort and pain in the elbows and
buttocks, which were due to the large size of the masses.
The plasma TC levels of the patient were 14.95
mmol/l (reference value, 3.11–5.96 mmol/l), whereas the LDLC level
was 12.69 mmol/l (reference value, 2.10–3.10 mmol/l). The
apolipoprotein (Apo) A1 level was 0.74 g/l (reference value,
1.10–1.76 g/l), the Apo B level 2.84 g/l (reference value,
0.63–1.14 g/l) and the Apo A1/Apo B ratio was 3.84 (reference
value, 0.40–1.96). Test results for liver enzymes, renal function,
blood glucose, uric acid, free thyroxin and thyroid-stimulating
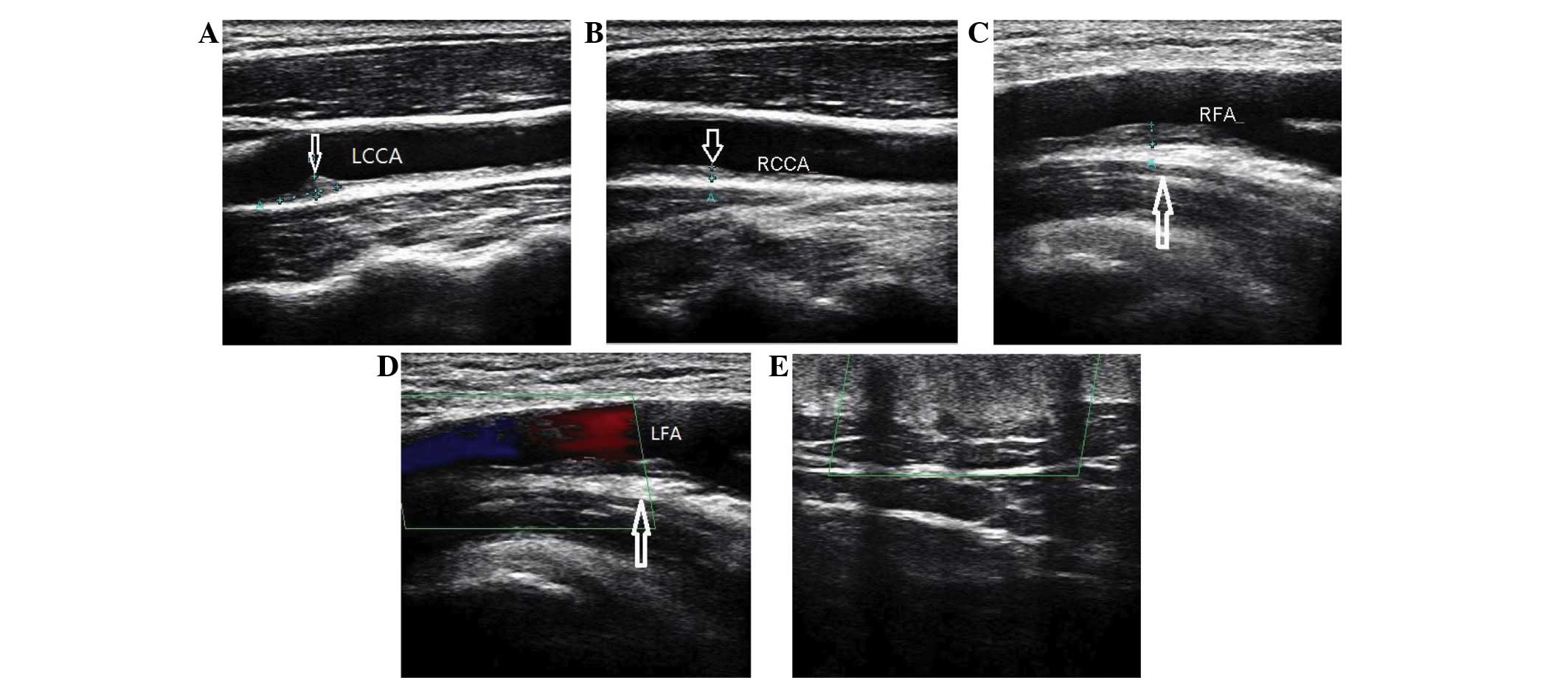
hormone were normal. A Doppler ultrasound scan (TA700; Toshiba,
Tokyo, Japan) revealed that the two carotid arteries and the lower
extremity arteries presented with progressive atherosclerosis
(Fig. 2). The carotid arteries had
accumulated atheromatous plaques, and the lower extremity arteries
had progressive calcific sclerosis. Patient chest X-ray, abdominal
ultrasound, electrocardiogram and echocardiogram were normal.
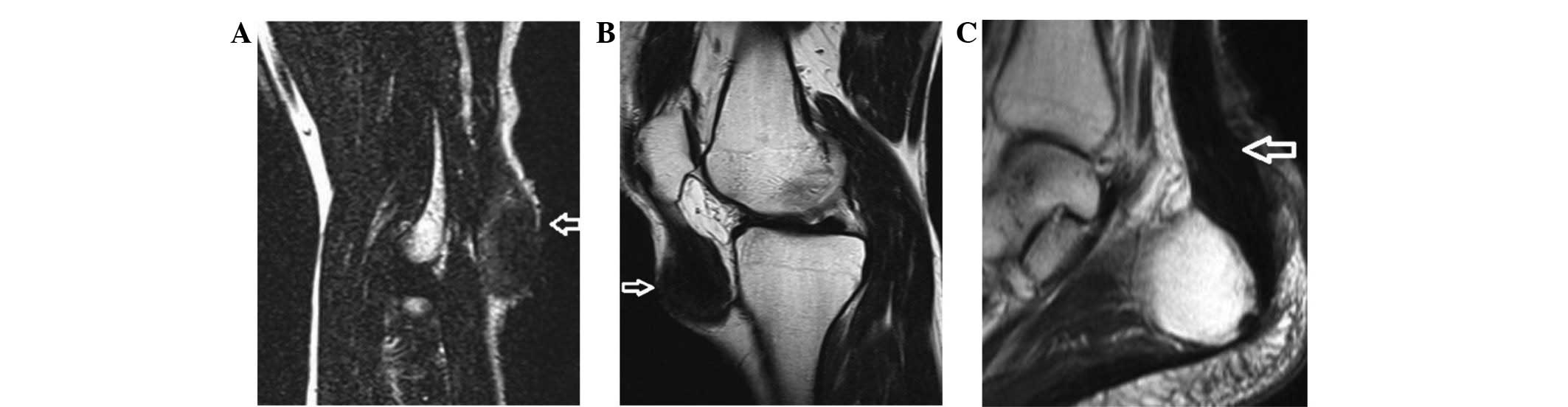
Magnetic resonance imaging (MRI) scans (Siemens AG, Munich,
Germany) revealed decreased signal intensity on T1-weighted and
T2-weighted spin-echo images of the masses in the elbows, knees and
posterior malleolus. Xanthomas had infiltrated the triceps tendon
and the patellar ligament. MRI scan also revealed a thickened
Achilles tendon and patellar ligament (Fig. 3). Neurological examination for central
nervous system function and brain MRI scan were performed to
determine the presence or absence of cerebrotendinous xanthomatosis
(9), a rare autosomal-recessive
familial lipid metabolic disease caused by mutations in sterol 27
hydroxylase; however, no abnormality was identified.
The father of the patient had a history of coronary
heart disease (CHD) and was treated with percutaneous coronary
intervention 2 years previously. The mother and sister had no
reported symptoms of CHD or similar skin lesions. The two parents
demonstrated elevated TC and LDLC levels, a typical characteristic
of HeFH, whereas the sister did not. Secondary
hypercholesterolemia, which may be caused by hypothyroidism,
diabetes and renal or hepatic disease, were excluded. The lipid
profiles of the patient and his family are described in Table I.
 | Table I.Lipid profiles of the patient, the
patient's parents and the patient's sister. |
Table I.
Lipid profiles of the patient, the
patient's parents and the patient's sister.
|
|
|
| Cholesterol |
|
|---|
|
|
|
|
|
|
|---|
| Individual | Age, years | TC, mmol/l | HDL, mmol/l | LDL, mmol/l | TG, mmol/l |
|---|
| Patient | 23 | 14.95 | 1.90 | 12.69 | 1.06 |
| Father | 57 | 7.18 | 1.13 | 5.41 | 0.68 |
| Mother | 49 | 7.53 | 1.14 | 6.65 | 0.69 |
| Sister | 27 | 3.92 | 1.34 | 2.38 | 0.56 |
| Normal range |
| 3.11–5.96 | 1.04–2.05 | 2.10–3.10 | 0.34–1.69 |
The patient was diagnosed with HoFH and multiple
xanthomas. The patient subsequently abstained from alcohol and
tobacco use, and followed a vegetarian diet that was low in
saturated fat and cholesterol, as advised by physicians. In
addition, the patient was treated with a combined treatment regimen
of rosuvastatin (10 mg/day) and ezetimibe (10 mg/day) for a year.
Following a year of conservative treatment, no marked decrease in
the plasma TC levels (controlled at 13.05 mmol/l) or substantial
regression of the multiple xanthomas was observed. Due to the
symptomatic nature of the lesions and for cosmetic reasons,
surgical removal of the large xanthomas was considered necessary.
Surgical excision of the masses over the elbows and buttocks was
performed. Intraoperatively, the yellowish xanthomatous masses in
each elbow were found to closely adhere to the triceps tendon,
which prevented the complete excision of these lesions. Excision
was not performed for all lesions, since the extent of xanthomatous
involvement had not been clearly determined. Resected tissues were
sent to the Department of Pathology, Zhejiang Provincial People's
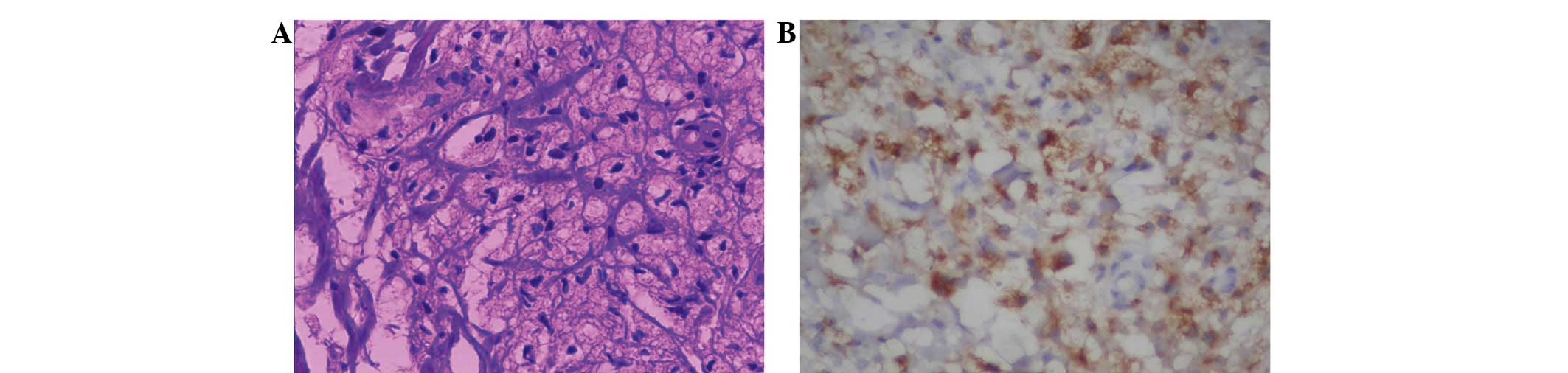
Hospital for tissue examination. Microscopic examination of the
surgical specimens (Olympus BX50; Olympus Corporation, Tokyo,
Japan) revealed nests composed of xanthoma tissue, which consisted
of connective tissue and foam cells, containing cholesterol,
cholesterol esters, triglycerides and phospholipids (Fig. 4A). These findings confirmed the
diagnosis of HoFH. Immunohistochemical staining showed that the
foam cells strongly expressed cluster of differentiation 68 (CD68)
(Fig. 4B). Treatment with
rosuvastatin and ezetimibe was continued postoperatively, and the
dose of rosuvastatin was adjusted to 20 mg/day. Four weeks
subsequent to surgery, the symptoms had largely resolved. At that
point, apheresis therapy was recommended for a better control of
the high LDLC levels, but the patient declined due to the high cost
of the procedure. Although the patient had no symptoms of CHD, he
was advised to take note of any chest tightness and other symptoms
of CHD and undergo a cardiac examination at least twice a year. One
year following surgery, the serum LDLC levels of the patient were
controlled at 8.50 mmol/l with no symptoms of CHD or postoperative
recurrence of xanthomas.
Written informed consent was obtained from the
patient for the publication of this study, and the study was
approved by the Ethics Review Committee of Zhejiang Provincial
People's Hospital.
Discussion
Xanthomas are palpable masses that are typically
located within the skin or subcutaneous tissue and consist of
cholesterol, cholesterol esters, triglycerides, phospholipids, and
numerous lipid-laden foamy macrophages (10). Xanthomas have been indicated to
commonly occur in patients with FH (1). A previous study has shown that the most
frequent site for xanthomas is the Achilles tendon (11). Other frequent sites include the
extensor tendons of the hands and feet, as the extensor tendon
areas are subject to mechanical stress (11). The mechanical stress encountered in
tendons is similar to recurrent trauma, which is thought to
predispose these sites to the development of xanthomas (12). Courtice (13,14)
indicated that the concentration of LDLs increased in the lymphatic
drainage system of a rabbit paw that was subjected to mild heat
injury of the skin. Courtice also argued that increased capillary
permeability to various subclasses of LDL resulted in an increased
extravascular LDL concentration, and that a portion of the
extracellular LDL may move into the lymphatic system, which is
responsible for draining the area of the paw. Mechanical stress,
similarly to the effect of mild heat injury to the skin, also
increases capillary permeability around the mechanical
stress-exposed areas, which may lead to the accumulation of LDLC.
This theory may explain why tuberous xanthomas are predominantly
encountered in mechanical stress-exposed regions.
The present patient presented with multiple
xanthomas in the mechanical stress-exposed parts of the body,
including the buttocks, extensor aspect of the knee, elbow and
ankle. The occurrence of multiple large xanthomas is rare. Although
the appearance is similar to tumors, xanthomas have distinct
characteristics that allow for the differentiation of the two. In
the majority of cases, xanthomas are asymptomatic; unless they grow
to be large in size and cause compression to adjacent structures,
which may cause pain and mobility problems. Histologically,
xanthomas consist of foamy macrophages with fibrosis and
cholesterol clefts. A high level of LDLC is the most common
clinical manifestation and the main cause of xanthomas in patients
with FH. In these patients, markedly elevated LDLC levels are
secondary to LDL receptor defects and result in lipid leakage from
the vasculature into the surrounding tissue. This results in the
uptake of lipids by macrophages, which, in turn, leads to the
increased accumulation of undegraded lipids and formation of foam
cells (1,15). Extracellular cholesterol crystallizes
into clefts, inducing an inflammatory reaction with giant cells and
resulting in fibrosis. CD68 is particularly useful as a marker for
the various cells of the macrophage lineage, including monocytes,
histiocytes, giant cells, Kupffer cells and osteoclasts (16). In addition, foam cells, which are
widely prevalent in xanthomas, are strongly immunopositive for CD68
(16).
Numerous types of imaging examinations aid the
diagnosis of xanthomas. Sonography is a simple, widely available
and economical modality used for the identification of
xanthomatosis, rendering it superior to gross clinical assessment.
Sonography has been reported as an effective method for the
investigation of tendinous xanthomas (17,18). In
the present case, Doppler ultrasound revealed multiple hypoechoic
foci in the masses of each elbow.
An MRI scan is a practical imaging modality that
distinguishes xanthomas from tumors. On an MRI scan, xanthomas
exhibit morphological and signal intensity abnormalities. In the
presence of a xanthoma, the normally flat or concave margins of
tendons or other normal tissues may change and appear convex on
axial MR images (11). Furthermore,
tendons with xanthomas tend to a have increased signal intensity on
T1- and T2-weighted spin-echo images compared with normal tendons
(19). Tumors often exhibit a marked
increase in signal intensity compared with xanthomas.
The patient in the present study presented with
multiple large xanthomas with a wide-ranging distribution, and an
onset at 2 years of age. The patient had an LDLC level of 12.69
mmol/l, suggesting a high likelihood of HoFH. The parents of the
patient had mildly elevated levels of LDLC (father, 5.41 mmol/l;
mother, 6.45 mmol/l), which, when combined with the absence of
xanthomas, suggests that the parents suffered from HeFH. The
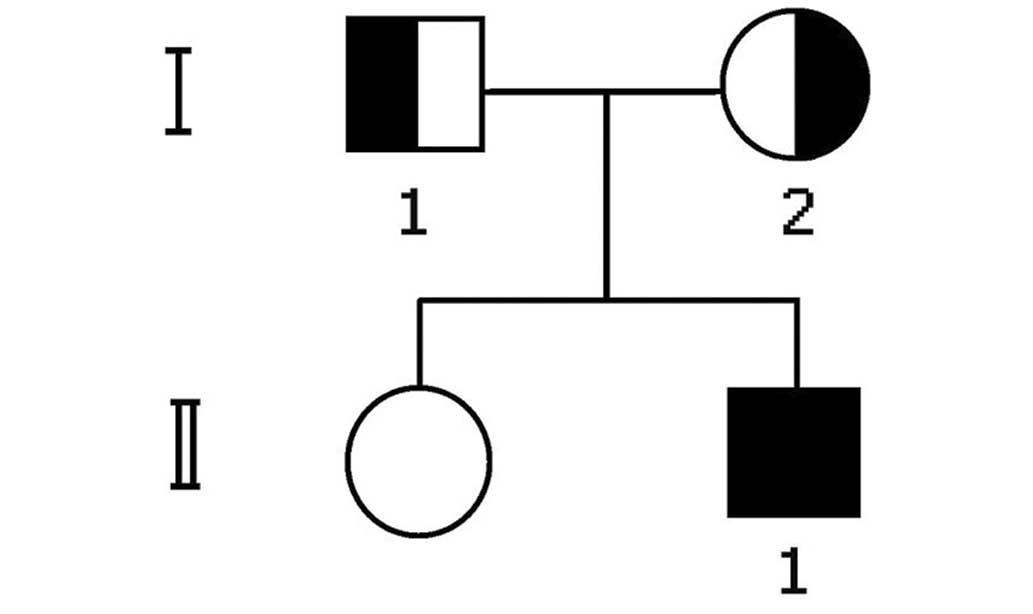
patient was the offspring of two parents with HeFH, and appeared to
have inherited an HoFH phenotype associated with an increased level
of serum LDLC and more severe symptoms than the parents (Fig. 5).
A high level of plasma LDLC is a risk factor for
atherosclerosis. Tendinous and tuberous xanthomas may indicate FH
and increased cardiovascular risk, as xanthomas are associated with
elevated plasma concentrations of LDLC. The presence of xanthomas
increases the risk of cardiovascular disease in patients with FH by
as much as three-fold, indicating that xanthomas and
atherosclerosis may share a certain etiology (20,21).
However, cardiovascular disease caused by atherosclerosis in
individuals with HoFH typically appears during childhood and may
result in mortality by 20–30 years of age (3). Measuring the intima-media thickness by
ultrasound aids the identification of the preclinical stages of
atherosclerosis (22). In addition,
large-scale epidemiological studies have shown an association
between increased intima-media thickness and future CHD and stroke
(22,23). In the present case, ultrasound showed
an increase in the intima-media thickness of the bilateral internal
carotid and femoral arteries, indicating FH, and a high risk of
developing cardiovascular disease.
The management of patients with HoFH poses a medical
challenge. If left untreated, and occasionally despite maximum
medical therapy, HoFH often rapidly leads to atherosclerotic
changes that cause aortic stenosis and coronary artery disease
(24). The first step for the
treatment of HoFH is a change in lifestyle and diet regulation. The
diet of HoFH patients must be low in saturated fats and cholesterol
(8,25). Combined drug therapy, including
statins plus ezetimibe or bile acid resin, is recommended for
patients with severe hypercholesterolemia (1,26). Studies
have shown that statins have the ability to soften xanthomas
(1,27). Massive xanthomas may require surgical
intervention (28). Surgery is only
suggested for xanthomas that are extremely large and painful and
cause mobility problems. Certain studies, however, have reported a
high postoperative recurrence rate of xanthomas (29). Postoperative cholesterol-lowering
therapy may reduce the likelihood of recurrence (27); therefore, for multiple, massive
tendinous and tuberous xanthomas, local surgical excision combined
with postoperative cholesterol-lowering therapy appears to be the
most effective treatment option.
Acknowledgements
The present study was supported by a grant awarded
to Professor Qing Bi by the Medical Science and Technology Project
In Zhejaing Province (grant no. Y2012ZDA003).
References
|
1
|
Zak A, Zeman M, Slaby A and Vecka M:
Xanthomas: Clinical and pathophysiological relations. Biomed Pap
Med Fac Univ Palacky Olomouc Czech Repub. 158:181–188.
2014.PubMed/NCBI
|
|
2
|
Sethuraman G, Sugandhan S, Sharma G,
Chandramohan K, Chandra NC, Dash SS, Komal A and Sharma VK:
Familial homozygous hypercholesterolemia: Report of two patients
and review of the literature. Pediatr Dermatol. 24:230–234. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alves C and Braid Z: Homozygous familial
hypercholesterolemia: Case report of a rare cause of dyslipidemia.
Pediatr Endocrinol Diabetes Metab. 17:162–165. 2011.PubMed/NCBI
|
|
4
|
Cruz PD Jr, East C and Bergstresser PR:
Dermal, subcutaneous and tendon xanthomas: Diagnostic markers for
specific lipoprotein disorders. J Am Acad Dermatol. 19:95–111.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maher-Wiese VL, Marmer EL and Grant-Kels
JM: Xanthomas and the inherited hyperlipoproteinemias in children
and adolescents. Pediatr Dermatol. 7:166–173. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moyle M and Tate B: Homozygous familial
hypercholesterolaemia presenting with cutaneous xanthomas: Response
to liver transplantation. Australas J Dermatol. 45:226–228. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Raal FJ and Santos RD: Homozygous familial
hypercholesterolemia: Current perspectives on diagnosis and
treatment. Atherosclerosis. 223:262–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poustie VJ and Rutherford P: Dietary
treatment for familial hypercholesterolaemia. Cochrane Database
Syst Rev. 2001:CD0019182001.
|
|
9
|
Moghadasian MH: Cerebrotendinous
xanthomatosis: Clinical course, genotypes and metabolic
backgrounds. Clin Invest Med. 27:42–50. 2004.PubMed/NCBI
|
|
10
|
Szalat R, Arnulf B, Karlin L, Rybojad M,
Asli B, Malphettes M, Galicier L, Vignon-Pennamen MD, Harel S,
Cordoliani F, et al: Pathogenesis and treatment of xanthomatosis
associated with monoclonal gammopathy. Blood. 118:3777–3784. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dagistan E, Canan A, Kizildag B and Barut
AY: Multiple tendon xanthomas in patient with heterozygous familial
hypercholesterolaemia: Sonographic and MRI findings. BMJ Case Rep
2013. 2013.
|
|
12
|
Cruz PD Jr, East C and Bergstresser PR:
Dermal, subcutaneous and tendon xanthomas: Diagnostic markers for
specific lipoprotein disorders. J Am Acad Dermatol. 19:95–111.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Courtice FC: Permeability of normal and
injured skin capillaries to lipoproteins in the rabbit. Aust J Exp
Biol Med Sci. 37:451–463. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Courtice FC: The transfer of proteins and
lipids from plasma to lymph in the leg of the normal and
hypercholesterolaemic rabbit. J Physiol. 155:456–469. 1961.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Artieda M, Cenarro A, Junquera C, Lasierra
P, Martínez-Lorenzo MJ, Pocoví M and Civeira F: Tendon xanthomas in
familial hypercholesterolemia are associated with a differential
inflammatory response of macrophages to oxidized LDL. FEBS Lett.
579:4503–4512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Perrone G, Zagami M, Casale M, Salvinelli
F, Morini S and Rabitti C: Immunohistochemistry and differential
diagnosis of a solitary flat laryngeal xanthoma: A case report. In
Vivo. 21:119–121. 2007.PubMed/NCBI
|
|
17
|
Bureau NJ and Roederer G: Sonography of
achilles tendon xanthomas in patients with heterozygous familial
hypercholesterolemia. AJR Am J Roentgenol. 171:745–749. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bude RO, Adler RS and Bassett DR:
Diagnosis of achilles tendon xanthoma in patients with heterozygous
familial hypercholesterolemia: MR vs. sonography. AJR Am J
Roentgenol. 162:913–917. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liem MS, Leuven JA, Bloem JL and Schipper
J: Magnetic resonance imaging of achilles tendon xanthomas in
familial hypercholesterolemia. Skeletal Radiol. 21:453–457. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamashita S, Hbujo H, Arai H, Harada-Shiba
M, Matsui S, Fukushima M, Saito Y, Kita T and Matsuzawa Y:
Long-term probucol treatment prevents secondary cardiovascular
events: A cohort study of patients with heterozygous familial
hypercholesterolemia in Japan. J Atheroscler Thromb. 15:292–303.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Civeira F, Castillo S, Alonso R,
Meriño-Ibarra E, Cenarro A, Artied M, Martín-Fuentes P, Ros E,
Pocoví M and Mata P: Spanish Familial Hypercholesterolemia Group:
Tendon xanthomas in familial hypercholesterolemia are associated
with cardiovascular risk independently of the low-density
lipoprotein receptor gene mutation. Arterioscler Thromb Vasc Biol.
25:1960–1965. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bots ML, Hoes AW, Koudstaal PJ, Hofman A
and Grobbee DE: Common carotid intima-media thickness and risk of
stroke and myocardial infarction: The rotterdam study. Circulation.
96:1432–1437. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chambless LE, Heiss G, Folsom AR, Rosamond
W, Szklo M, Sharrett AR and Clegg LX: Association of coronary heart
disease incidence with carotid arterial wall thickness and major
risk factors: The atherosclerosis risk in communities (ARIC) study,
1987–1993. Am J Epidemiol. 146:483–494. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Beigel R and Beigel Y: Homozygous familial
hypercholesterolemia: long term clinical course and plasma exchange
therapy for two individual patients and review of the literature. J
Clin Apher. 24:219–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Selvan JP, Uthaman B, Abushaban L and
Jebaraj R: Homozygous familial hypercholesterolemia with
generalized arterial disease. Med Princ Pract. 16:75–78. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rader DJ, Cohen J and Hobbs HH: Monogenic
hypercholesterolemia: New insights in pathogenesis and treatment. J
Clin Invest. 111:1795–1803. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ahn JH, Chun TJ and Lee S: Nodular
excision for painful localized achilles tendon xanthomas in type II
hyperlipoproteinemia: A case report. J Foot Ankle Surg. 50:603–606.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moroney PJ and Besse JL: Resection of
bilateral massive achilles tendon xanthomata with reconstruction
using a flexor hallucis longus tendon transfer and bosworth
turndown flap: A case report and literature review. Foot Ankle
Surg. 18:e25–e28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fahey JJ, Stark HH, Donovan WF and Drennan
DB: Xanthoma of the achilles tendon. Seven cases with familial
hyperbetalipoproteinemia. J Bone Joint Surg Am. 55:1197–1211. 1973.
View Article : Google Scholar : PubMed/NCBI
|