Introduction
Bronchogenic carcinoma is the leading cause of
tumor-related mortality in developed countries (1). Lung cancer was associated with
~1,590,000 deaths in 2012, and it is currently the leading cause of
cancer-associated mortality worldwide. Non-small cell lung cancer
(NSCLC) accounts for ~85% of all lung cancer cases (2). Women with metastatic ovarian
adenocarcinoma from the lung have been found to have a mean age of
46 years (3), with disease onset at a
young age also being a prominent characteristic of anaplastic
lymphoma receptor tyrosine kinase (ALK) rearrangement-positive
NSCLC (4). The ovaries are an
uncommon location for metastasis from lung cancer, and few such
cases have been reported. The 2 patients in the present study
developed ovarian metastasis at different times in their illnesses,
and 1 patient developed breast metastasis. Ovarian metastasis from
lung cancer represents only 2–4% of all ovarian metastatic masses.
This frequency, however, is increasing due to the rising incidence
of lung cancer in women (3).
Secondary metastatic ovarian tumor occurrence is variable and
depends on a number of factors, including the accuracy of the
pathological diagnosis, the completeness of the staging and
possible genetic patterns. In total, 7 cases of ovarian metastasis
of lung cancer have been reported (5). In order to find a suitable treatment
strategy, an indicator for ovarian metastasis from lung cancer is
essential (5). Although, there are
diverse clinical indicators for cancer prognostic evaluation,
patients who share the same clinical features can have quite
different clinical outcomes. Nowadays, with the development of gene
profiling techniques, such as microRNA microarray and reverse
transcription-quantitative polymerase chain reaction, novel
biomarkers are intended to be used as prognostic factors combined
with traditionally clinical features (6–8).
The current study presents two female NSCLC patients
who developed ovarian metastases during their clinical courses. The
patients' epidermal growth factor receptor (EGFR) or ALK mutation
status was analyzed after the diagnoses of ovarian metastases, and
these two patients received different therapies. This report also
provides a brief review of the literature regarding ovarian
metastases of lung cancers, providing some clues into the clinical
management of this disease. Written informed consent was obtained
from the patient or the patient's family for the publication of
this study.
Case report
Case 1
A 38-year-old female was admitted to the Cancer
Center of Union Hospital (Tongji Medical College, Huazhong
University of Science and Technology, Wuhan, China) in May 2011 due
to lower back pain that had persisted for ~4 months. At 1 month
prior to admission, the patient reported worsening of this symptom,
with pain radiating to the left hip and reduced movement of the
left leg, which was not associated with numbness or relieved by
lying down. There was no history of trauma, fever, abdominal pain,
vomiting, headaches, coughing or chest pain. The patient had no
history of smoking, and there was no family history of a similar
presentation or a significant past medical history.
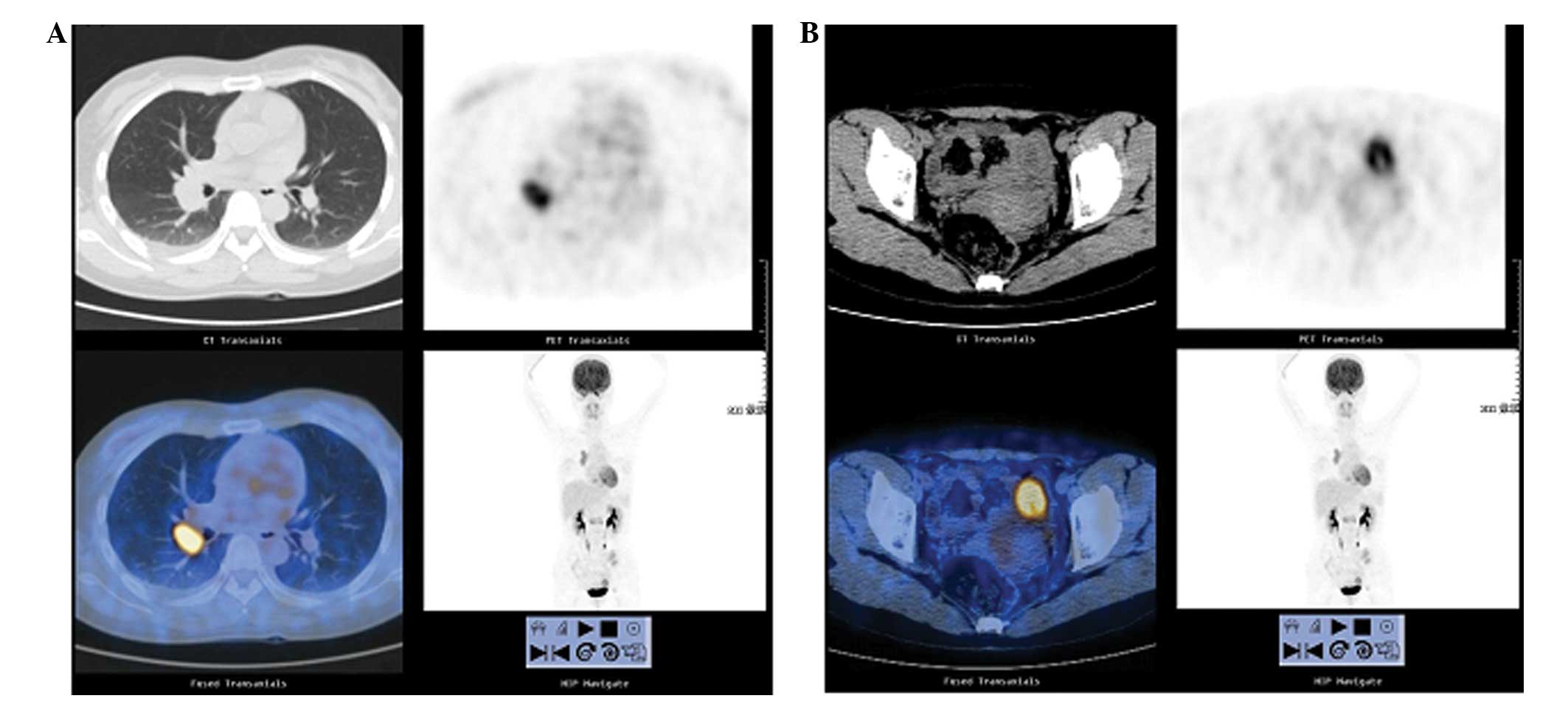
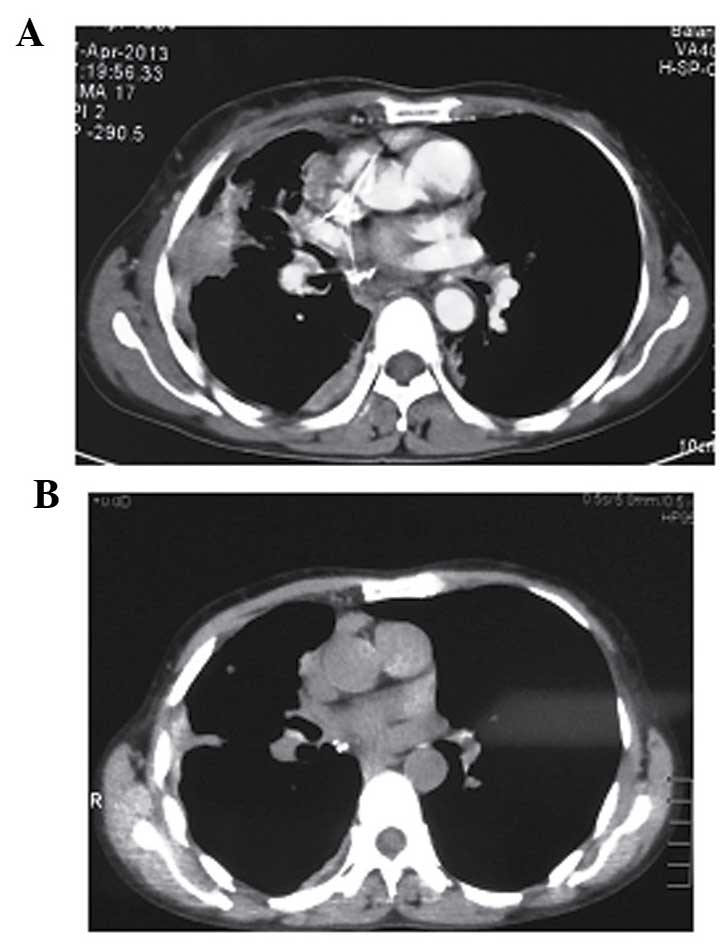
Positron emission tomography-computed tomography
(PET-CT) of the whole body revealed a high metabolic signal at the
right upper lobe of the lung (Fig.
1A), at the left ovary (with enlarged right hilar lymph nodes)
(Fig. 1B), and in multiple bone
areas. The patient also underwent a CT-guided biopsy of the mass on
the right upper lobe of the lung, which showed a middle-grade
differentiated, glandular structure indicative of primary lung
adenocarcinoma. Furthermore, EGFR mutation analysis was performed
using scorpion/amplified refractory mutation system technology,
revealing 21 exon mutations. The patient was finally staged as
T4N1M1 according to the tumor-node-metastasis classification system
(9). The patient was started on 150
mg/day Tarceva, with concurrent radiation therapy to lumbar
vertebrae 1 and 2, and to the left iliac bone to a dose equivalent
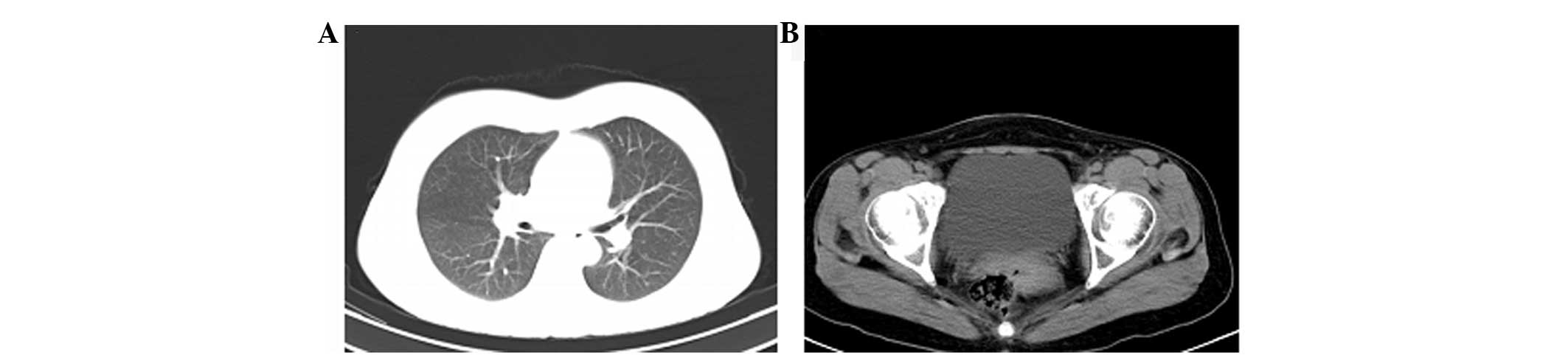
to 40 Gy in 20 fractions. After ~5 months, the mass in the right
upper lobe of the lung had decreased in size until near complete
remission (CR) was achieved (Fig. 2),
with normal tumor marker levels. The patient was discharged to
continue with Tarceva therapy and was advised to attend regular
follow-up clinics. At ~9 months after discharge, follow-up chest
and pelvic CT scans showed a relapse on the right ovary, but stable
disease (SD) on the upper right lung (Fig. 3).
The patient was started on 500 mg/m2
pemetrexed plus 75 mg/m2 cisplatin (both administered on
day 1 of the cycle), with a partial response (PR) after 6 cycles,
followed by radiotherapy to the left ovary consisting of 50 Gy in
25 fractions. The patient was then discharged to attend follow-up
clinics at a local hospital. In February 2013, the patient reported
to the local hospital with headaches, nausea, vomiting and fainting
episodes. A CT scan was performed, which revealed brain metastasis.
The patient was referred back to the Cancer Center and readmitted.
Magnetic resonance imaging (MRI) revealed multiple brain metastasis
and whole-brain radiation was consequently performed to 36 Gy in 12
fractions. Following completion of the therapy, the patient
returned home, where she succumbed 2 months later. Written informed
consent was obtained from the patient for the publication of this
study.
Case 2
In August 2010, a 47-year-old female presented at
the Cancer Center of Union Hospital due to a cough that had
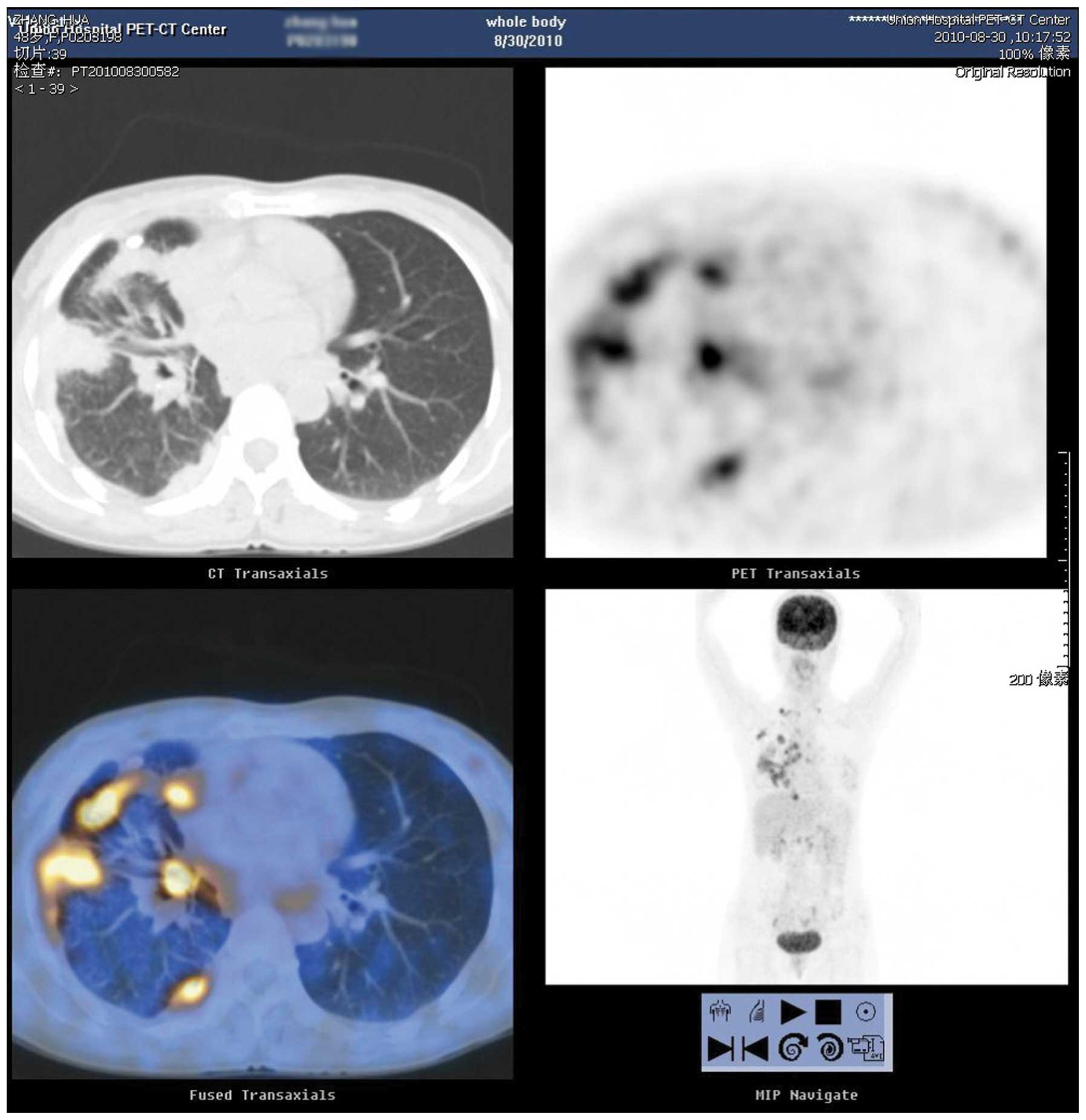
persisted for 7 months. A chest CT scan revealed a mass on the
right lung. PET-CT of the whole body was also performed, which
showed high metabolic signals on the right lung and right
supraclavicular lymph node (Fig. 4).
The cytology of the lymph node revealed metastatic adenocarcinoma
and the patient was diagnosed with right lung adenocarcinoma,
clinical stage T4N3M0. Chemotherapy with 1,000 mg/m2
gemcitabine (on days 1 and 8) plus 75 mg/m2 cisplatin
(on day 1) was initiated. After 2 cycles, the response was SD and
therefore, the chemotherapy was changed to 500 mg/m2
pemetrexed and 75 mg/m2 cisplatin (both administered on
day 1 of the cycle). After 4 more cycles of chemotherapy, the
patient was treated with maintenance therapy of 150 mg/day
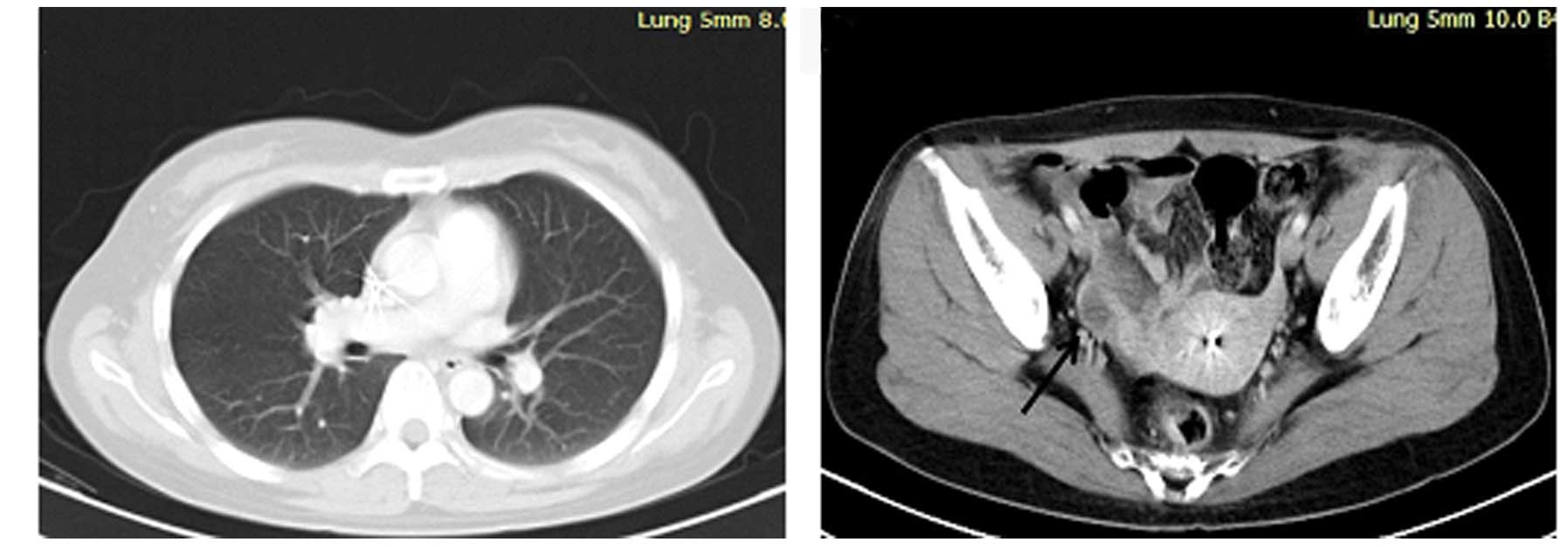
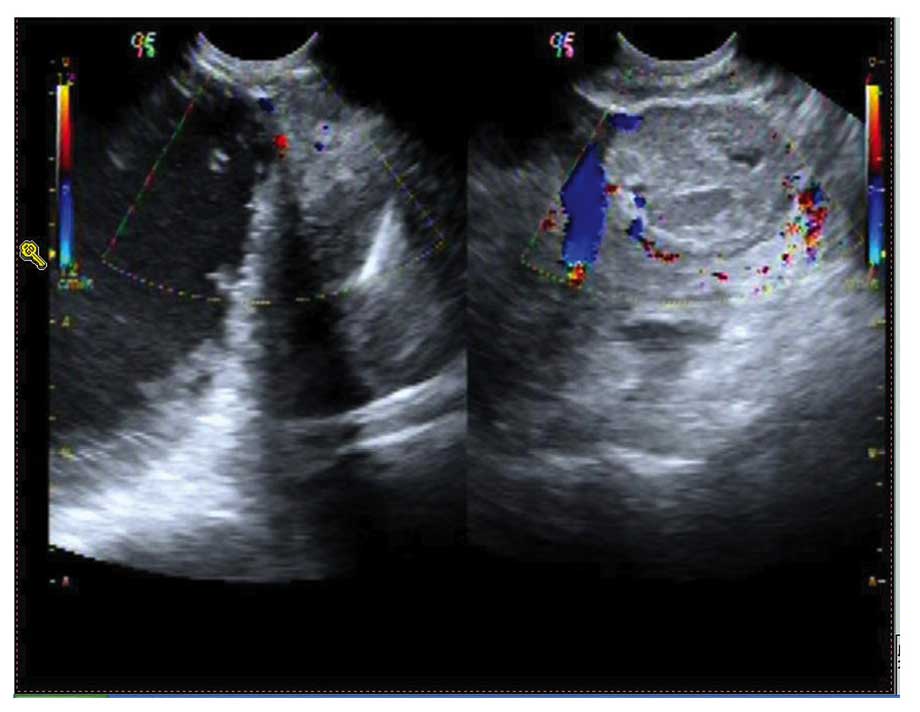
erlotinib. In April 2012, a follow-up ultrasonic pelvic examination
revealed a mass in the right ovary (Fig.
5). A bilateral salpingo-oophorectomy (BSO) was performed, with
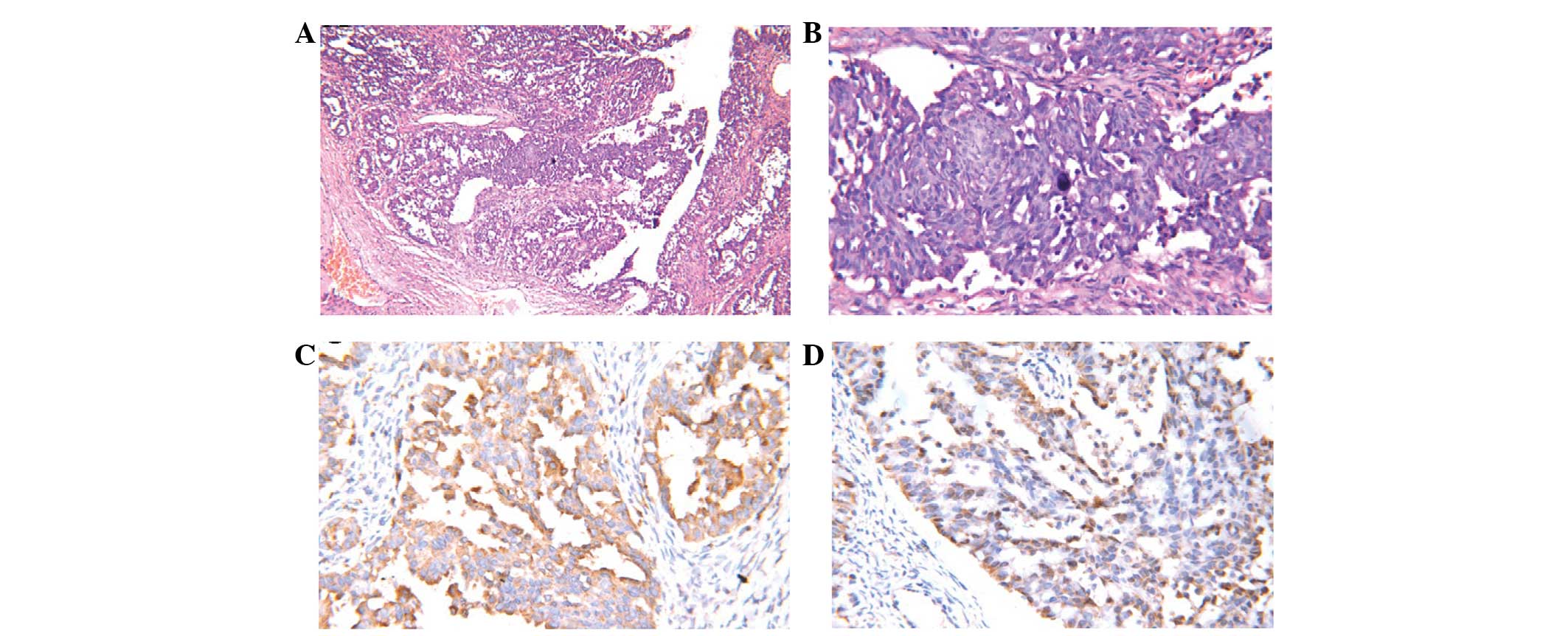
the biopsy result showing moderately-differentiated adenocarcinoma
of the right ovary (Fig. 6A and B)
with EGFR wild-type. Immunohistochemistry showed strong reactivity
for cytokeratin (CK)7, napsin A (Fig.
6C) and thyroid transcription factor 1 (TTF-1) (Fig. 6D), while the tumor was negative for
cancer antigen 125. A diagnosis of metastatic adenocarcinoma of the
lung was formed.
Subsequent to the BSO, the patient received 2 cycles
of pemetrexed alone, followed by 150 mg/day erlotinib. After 3
months, the patient began experiencing back pain, which worsened
within 1 month. Upon visiting a local hospital, bone emission
computed tomography was performed showing multiple bone metastases.
The patient was readmitted to the Cancer Center 1 month later where
radiotherapy was administered to the T6-T8 vertebrae at a dosage of
30 Gy/10 fractions. This was followed by 3 cycles of 75
mg/m2 docetaxel (on day 1), but the disease progressed
to brain metastasis according to MRI of the brain. The patient
underwent whole-brain radiotherapy with a total dose of 36 Gy/12
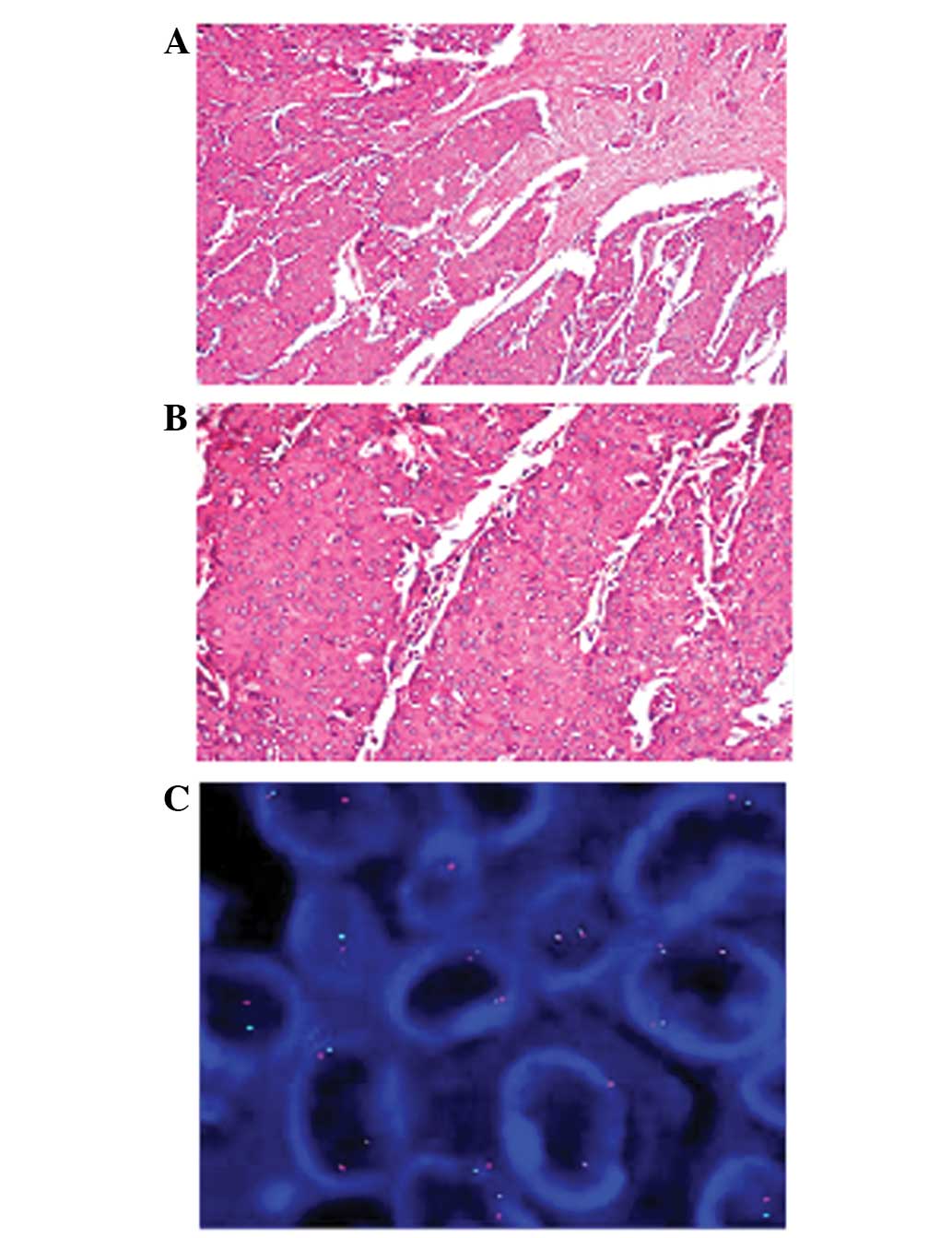
fractions. After 2 weeks, multiple right breast masses were
detected and a biopsy was performed, which showed metastatic
adenocarcinoma (Fig. 7A and B).
Immunohistochemistry showed strong reactivity for CK7, TTF-1 and
CK, while the tumor was negative for estrogen receptor,
progesterone receptor, human epidermal growth factor receptor-2,
napsin A, cluster of differentiation 56, CK20 and villin. In
addition, fluorescence in situ hybridization analysis
revealed the presence of ALK rearrangement (Fig. 7C), confirming a diagnosis of ALK
rearrangement-positive NSCLC with breast metastasis. Moreover, EGFR
analysis revealed the presence of the wild-type gene. The patient
achieved a PR once started on twice daily 250 mg crizotinib
(Fig. 8). The patient has shown no
evidence of progression during regular follow-up visits for 1 year
since crizotinib treatment. Written informed consent was obtained
from the patient for the publication of this study.
Discussion
Bronchogenic carcinoma is the foremost cause of
tumor-associated mortality in developed countries (1). Women with metastatic ovarian
adenocarcinoma from the lung have been reported to have a mean age
of 46 years (3), with disease onset
at a young age also being a prominent characteristic of ALK
rearrangement-positive NSCLC (4). The
ovaries are an uncommon location for metastasis from lung cancer,
and few such cases have been reported. The patients in the present
study developed ovarian metastasis at different stages during their
illnesses, and 1 patient developed breast metastasis. Ovarian
metastasis from lung cancer represents only 2–4% of all ovarian
metastatic masses; however, this rate is increasing due to the
rising occurrence of lung cancer in women (3). Secondary metastatic ovarian tumor
occurrence is variable and depends on a number of factors,
including the accuracy of the pathological diagnosis, the
completeness of staging and possible genetic patterns. In the study
by Young and Scully (5), 7 cases of
ovarian metastasis of lung cancer. The study consisted of cases of
ovarian tumors that were detected prior to (n=3), concurrent with
(n=3) or <1 year after (n=1) the primary lung cancer diagnosis.
It was also indicated that the pathological features and clinical
characteristics of the disease may be of use in the diagnosis of
ovarian metastasis from lung cancer.
The occurrence rate of ovarian metastases derived
from non-gynecological sites is 11 times greater than that of
metastases from the female genital tract organs, with
adenocarcinomas of the gastrointestinal tract being the most common
(6). Kim et al discussed the
cases of 166 patients with non-gynecologic malignancies and adnexal
tumors, in which ovarian metastatic tumors were detected in 68%
(7). Another large study reported
that only 10% of 10,288 malignant ovarian neoplasms were metastatic
(8).
Adenocarcinoma accounts for ~33% of the lung
carcinomas that metastasize to the ovary. Metastatic lung
adenocarcinoma mimic ovarian surface epithelial-stromal tumors
(particularly those large ovarian masses mimicking primary tumors),
such as serous, endometrioid, mucinous and clear cell type tumors
(3). Therefore, staining for CK-7 and
CK-20 has widely been used to differentiate between primary and
secondary ovarian malignancies. Yeh et al were the first to
point out the importance of immunohistochemical staining (10). Positive TTF-1 and CK-7 staining, and
negative CK-20 staining is diagnostically required to determine the
correct diagnosis. The lungs and thyroid gland express TTF-1, a
member of the NKx2 homeodomain transcription factor family, and
this knowledge is widely used in surgical pathology, as well as in
the determination of whether an adenocarcinoma of unknown primary
originates from the pulmonary system (11). Napsin A may be a potential addition to
the immunohistochemical panel for identifying lung cancer
metastases. Napsin A is an aspartic proteinase detected in alveolar
macrophages and type 2 pneumocytes, and a putative marker for
pulmonary adenocarcinomas. Therefore, it may be of use in
differentiating between primary lung adenocarcinoma and
adenocarcinomas of other organs at the primary and metastatic sites
(12). In particular, the combined
use of napsin A and TTF-1 may be of great assistance due to the
resultant increased sensitivity and specificity for identifying the
lung origin of a metastatic adenocarcinoma (13).
In NSCLC patients with activating EGFR
mutations, EGFR-specific tyrosine kinase inhibitors (TKI) are known
to be an effective mode of therapy that is well tolerated. However,
progression or relapse is inevitable during treatment in EGFR-TKI
patients with EGFR-mutated NSCLC due to the appearance of
drug resistance. This was evident in case 1 in the present study,
where the patient developed progressive disease in the
contralateral ovary following erlotinib treatment for 16 months. In
half of all cases, a T790M point mutation is the cause of acquired
resistance, which is believed to increase the affinity of EGFR for
adenosine triphosphate (14).
Patients with ALK rearrangements tend to be
younger than the majority of patients with NSCLC (15). Echinoderm microtubule-associated
protein-like 4 (EML4)-ALK rearrangements also occur more
often in adenocarcinomas of individuals who have never smoked or
those who are light smokers with tumors lacking EGFR and
KRAS proto-oncogene, GTPase (KRAS) mutations. The incidence
of ALK rearrangement is only 3–5% in randomly selected NSCLC
patients (16). EML4-ALK is a
fusion-type protein tyrosine kinase found in 4–5% of NSCLC cases
(4,17,18). The
ALK gene arrangements are largely mutually exclusive with
EGFR or KRAS mutations (19). Screening for this fusion gene in NSCLC
is important, as ALK-positive tumors are highly sensitive to
therapy with ALK-targeted inhibitors. Crizotinib is the
first Food and Drug Administration (FDA)-approved ALK TKI. The drug
has been sanctioned for the treatment of locally advanced or
metastatic NSCLC in those individuals with ALK-positive tumors, as
determined using an FDA-approved test (20). Patients who present with advanced
NSCLC that is positive for ALK have been shown to exhibit
objective response rates of 50–61% in single-arm clinical studies
(20,21). One case of bilateral ovarian
metastasis of NSCLC with ALK rearrangement has previously
been reported, in which the patient benefited from crizotinib
therapy, as determined by a lack of progression on regular
follow-up examinations for 4 years after the initial surgery for
the primary lung cancer (22). The
patient in case 2 of the present study also benefited from
crizotinib following multiline treatment modalities. The purpose of
the present study, therefore, is to present two rare cases of
ovarian metastasis with EGFR mutation and ALK
rearrangement-positive NSCLC, and to show how individual treatment
can prolong the progression-free survival time.
Patients who have ovarian metastasis of NSCLC are
rare, and those with EGFR mutation and ALK
rearrangement-positive ovarian metastasis are even rarer. The
present study will aid our understanding of this type of
metastasis, and will make physicians aware of the possibility of
breast and ovarian metastasis in ALK-positive NSCLC
patients.
References
|
1
|
Rial M Botana, Fernández-Villar A,
González Piñeiro A and Leiro Fernandez V: Primary lung
adenocarcinoma with ovarian metastasis: A rare presentation of
bronchogenic carcinoma. Arch Bronconeumol. 45:571–572. 2009.(In
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Islami F, Torre LA and Jemal A: Global
trends of lung cancer mortality and smoking prevalence. Transl Lung
Cancer Res. 4:327–338. 2015.PubMed/NCBI
|
|
3
|
Irving JA and RH Y: Lung carcinoma
metastatic to the ovary: A clinicopathologic study of 32 cases
emphasizing their morphologic spectrum and problems in differential
diagnosis. Am J Surg Pathol. 29:997–1006. 2005.PubMed/NCBI
|
|
4
|
Soda M, Choi YL, Enomoto M, Takada S,
Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K,
Hatanaka H, et al: Identification of the transforming EML4-ALK
fusion gene in non-small-cell lung cancer. Nature. 448:561–566.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Young RH and Scully RE: Ovarian metastases
from cancer of the lung: Problems in interpretation - a report of
seven cases. Gynecol Oncol. 21:337–350. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vang R and Ronnett BM: Metastatic and
miscellaneous primary tumors of the ovaryGynecologic Pathology.
Nucci MR and Oliva E: Elsevier Churchill Livingstone; Philadelphia:
pp. 539–614. 2009, View Article : Google Scholar
|
|
7
|
Kim K, Cho SY, Park SI, Kang HJ, Kim BJ,
Kim MH, Choi SC, Ryu SY and Lee ED: Risk of metastatic ovarian
involvement in nongynecologic malignancies. Int J Gynecol Cancer.
22:3–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi Y, Ye D, Lu W, Zhao C, Xu J and Chen
L: Histological classification in 10 288 cases of ovarian malignant
tumors in China. Zhonghua Fu Chan Ke Za Zhi. 37:97–100. 2002.(In
Chinese). PubMed/NCBI
|
|
9
|
American Joint Committee on Cancer, . Lung
Cancer Staging. 7th edition. http://cancerstaging.org/references-tools/quickreferences/documents/lungmedium.pdfAccessed
March 7, 2015.
|
|
10
|
Yeh KY, Chang JW, Hsueh S, Chang TC and
Lin MC: Ovarian metastasis originating from bronchioloalveolar
carcinoma: A rare presentation of lung cancer. Jpn J Clin Oncol.
33:404–407. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ordóñez NG: Thyroid transcription factor-1
is a marker of lung and thyroid carcinomas. Adv Anat Pathol.
7:123–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suzuki A, Shijubo N, Yamada G, Ichimiya S,
Satoh M, Abe S and Sato N: Napsin A is useful to distinguish
primary lung adenocarcinoma from adenocarcinomas of other organs.
Pathol Res Pract. 201:579–586. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bishop JA, Sharma R and Illei PB: Napsin A
and thyroid transcription factor-1 expression in carcinomas of the
lung, breast, pancreas, colon, kidney, thyroid, and malignant
mesothelioma. Hum Pathol. 41:20–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soria JC, Mok TS, Cappuzzo F and Jänne PA:
EGFR-mutated oncogene-addicted non-small cell lung cancer: Current
trends and future prospects. Cancer Treat Rev. 38:416–430. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shaw AT, Yeap BY, Mino-Kenudson M,
Digumarthy SR, Costa DB, Heist RS, Solomon B, Stubbs H, Admane S,
McDermott U, et al: Clinical features and outcome of patients with
non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol.
27:4247–4253. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Solomon B, Varella-Garcia M and Camidge
DR: ALK gene rearrangements: A new therapeutic target in a
molecularly defined subset of non-small cell lung cancer. J Thorac
Oncol. 4:1450–1454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mano H: Non-solid oncogenes in solid
tumors: EML4-ALK fusion genes in lung cancer. Cancer Sci.
99:2349–2355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Horn L and Pao W: EML4-ALK: Honing in on a
new target in non-small-cell lung cancer. J Clin Oncol.
27:4232–4235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takahashi T, Sonobe M, Kobayashi M,
Yoshizawa A, Menju T, Nakayama E, Mino N, Iwakiri S, Sato K,
Miyahara R, et al: Clinicopathologic features of non-small-cell
lung cancer with EML4-ALK fusion gene. Ann Surg Oncol. 17:889–897.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
XALKORI®: (crizotinib) (package
insert). Pfizer, NY: 2012, http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202570s002lbl.pdf
|
|
21
|
Kwak EL, Bang YJ, Camidge DR, Shaw AT,
Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB, et al:
Anaplastic lymphoma kinase inhibition in non-small-cell lung
cancer. N Engl J Med. 363:1693–1703. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujiwara A, Higashiyama M, Kanou T,
Tokunaga T, Okami J, Kodama K, Nishino K, Tomita Y and Okamoto I:
Bilateral ovarian metastasis of non-small cell lung cancer with ALK
rearrangement. Lung Cancer. 83:302–304. 2014. View Article : Google Scholar : PubMed/NCBI
|