Introduction
The incidence and mortality of gastric cancer have
decreased in recent years; however, it remains the fourth most
common type of cancer in the world (1,2). In the
treatment of localized resectable cases, surgery is the only
effective approach; however, numerous patients with gastric cancer
are at a very advanced stage when they are diagnosed and do not
clinically benefit from surgical resection (3). Therefore, for such patients, it is
essential to combine surgical treatment with effective chemotherapy
approaches.
Oxaliplatin (L-OHP) is a third-generation platinum
compound that has been developed as an alternative pharmacological
compound to cisplatin (4). Its usage
decreases the tumor resistance, inadequate oral bioavailability and
poisonous side effects associated with the use of cisplatin
(5,6).
LOHP is currently used in combination with other chemotherapy
drugs, including 5-fluorouracil, to treat advanced gastric cancer
(7,8).
The response rate of patients to L-OHP is high (53–59%), and L-OHP
exhibits low toxicity (4–6,9–11). However, although L-OHP is an effective
anti-tumor treatment, it may cause severe adverse reactions in
patients, as it enhances inflammatory activity, thus increasing the
risk of hepatic injury (6,12). This phenomenon may result in the
development of chemotherapy-associated steatohepatitis (CASH),
which is a serious form of non-alcoholic fatty liver disease
(8,13).
Polyenephosphatidylcholine (PPC) is one of the
primary active components of essential phospholipids, and has high
affinity and bioavailability for cellular and subcellular membranes
(14). Furthermore, PPC serves a
crucial role in maintaining the fluidity and function of
biomembranes, and several studies have demonstrated its
hepatoprotective effects (9–11,15–17).
The principal aim of the current study was to
determine the effect of PPC combined with L-OHP on the growth and
apoptosis of SGC-7901 cells. The present study aimed to evaluate
whether PPC acts as a beneficial supplement, which could be used
alongside L-OHP to protect against liver damage but not to
compromise its anti-tumor effects.
Materials and methods
Reagents
L-OHP and PPC were purchased from Sanofi S.A (Paris,
France). Fetal bovine serum (FBS) and RPMI 1640 medium were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Trypsin and MTT were obtained from Amresco, LLC. (Solon, OH,
USA). The kits for glutathione peroxidase (GSH-Px), malondialdehyde
(MDA) and superoxide dismutase (SOD) were all purchased from
Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Cell
culture plates were purchased from Corning Inc. (Corning, NY, USA),
and X-ray film was obtained from Kodak (Rochester, NY, USA). Poly
(ADP-ribose) polymerase and antibodies against cytochrome c
(#11940), B-cell lymphoma-2 (Bcl-2) (#15071), Bcl-2
antagonist/killer (Bak) (#12105), Bcl-2-associated X protein (Bax)
(#5023), and cleaved caspase-3 (#9661), −8 (#8592), and −9 (#7237)
were all purchased from Cell Signaling Technology, Inc. (Danvers,
MA, USA).
Cell culture
Cells from the human gastric adenocarcinoma cell
line SGC-7901 and the human vascular endothelial cell line HMEC-1
were purchased from the Chinese Academy of Sciences (Shanghai,
China). Cells were cultured in RPMI 1640 medium containing 10% FBS,
100 U/ml streptomycin and 100 U/ml penicillin at 37°C in a
humidified atmosphere containing 5% CO2.
Cell viability assay
An MTT assay was used to assess cell viability.
SGC-7901 and HMEC-1 cells were seeded in 96-well plates (200
µl/well containing 6,000 cells/well). After 24 h, the medium was
replaced with complete culture medium supplemented with different
concentrations of L-OHP and/or PPC. Following treatment for 24, 48
or 72 h, the cells were incubated with 0.5 mg/ml MTT for 4 h at
37°C. The MTT solution was discarded, and 150 µl dimethyl sulfoxide
was subsequently added to each well. Absorbance was measured at 570
nm using a Sunrise™ microplate reader (Tecan, Männendorf,
Switzerland) and cell viability was expressed as the ratio of
absorbance of the experimental group to that of the control group.
Each experiment was repeated ≥3 times.
Cell cycle analysis
The cell cycle was analyzed by flow cytometry. Cells
were cultured in complete medium supplemented with 3.5 µg/ml L-OHP
combined with various concentrations of PPC (0–64 µmol/l) for 48 h.
For cell cycle analysis, cells were collected, washed twice with
0.01 M PBS and fixed in 70% ethanol at 4°C overnight. Subsequently,
the cells were washed with PBS, digested with 200 µl trypsin (1
mg/ml) at 37°C for 30 min and stained with 800 µl propidium iodide
(PI; 50 µg/ml) at room temperature for 30 min. Cells were
subsequently washed with PBS and immediately analyzed via flow
cytometry. The percentage of SGC-7901 cells in each phase of the
cell cycle (G0/G1, S and G2/M) was calculated using the MultiCycle
AV software program version 1.0 (Phoenix Flow Systems, San Diego,
CA, USA).
Cell apoptosis assay
Cell apoptosis was evaluated by flow cytometry.
SGC-7901 cells were treated with 3.5 µg/ml L-OHP combined with
various concentrations of PPC (0–64 µmol/l) for 48 h. Cells were
digested by 2.5 g/l trypsin, washed with 0.01 mol/l PBS twice,
fixed with cold 95% alcohol at 4°C for 30 min, stained with PI and
annexin V-fluorescein isothiocyanate, and analyzed with a FACSort
flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The
apoptotic index (AI) was calculated as follows: AI = (number of
apoptotic cells / total number) × 100%. Each experiment was
repeated ≥3 times.
SOD assay
SOD activity in SGC-7901 cells was determined using
a kit that utilizes a tetrazolium salt to detect superoxide
radicals generated by xanthine oxidase and hypoxanthine by forming
a red formazan dye. The optical density of the formazan dye was
then measured at 550 nm by a spectrophotometer. The enzyme activity
was expressed as U/mg protein, and 1 U of enzyme was defined as the
enzymatic activity inhibiting the autoxidation of pyrogallol
(P0381; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) by
50%.
GSH-Px assay
GSH-Px activity in SGC-7901 cells was evaluated by a
previously described method, through a coupled assay using
H2O2 and dithiobis-nitrobenzoic acid (D8130;
Sigma-Aldrich; Merck Millipore). Enzymatic activity (1 U)
represented a decrease in GSH concentration of 1 mmol/l/min
following subtraction of non-enzymatic mode. All measurements were
performed in triplicate, and the results were normalized per mg
protein.
MDA assay
Lipid peroxidation was assayed by measuring the
concentration of MDA via spectrophotometry. The concentration of
MDA in SGC-7901 cells was measured using thiobarbituric acid
(T5500; Sigma-Aldrich; Merck Millipore) in conjunction with
commercially available kits following the manufacturer's protocol
(Nanjing Jiancheng Bioengineering Institute). The samples were
detected by dual wavelength in order to eliminate the influence of
glycation and other lipidic aldehydes. All measurements were
performed in triplicate and the results were expressed as nmol MDA
per mg protein.
Western blot analysis
Following treatment with 3.5 µg/ml L-OHP and
different concentrations of PPC (0–64 µmol/l) for 48 h, cells were
washed twice with cold PBS at 4°C, extracted into
radioimmunoprecipitation assay lysis buffer on ice for 30 min and
then sonicated at 3 W for 15 sec. The cell lysates were centrifuged
at 12,000 × g for 10 min at 4°C. The total protein content
was determined using a Pierce BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.). Lysate aliquots were diluted with 6X SDS sample
buffer and boiled for 5 min. A total of 30 µg protein from each
treatment group was separated by 12% SDS-PAGE and then
electrophoretically transferred onto nitrocellulose membranes from
Pall Gelman Sciences (Port Washington, NY, USA). After being
blocked at room temperature for 2 h with 5% non-fat milk in TBS
with 0.1% Tween-20 (TBST), the membranes were incubated overnight
at 4°C with antibodies against cytochrome c, Bcl-2, Bak,
Bax, cleaved caspase-3, −8 and −9, cyclin D1 (#9661; Cell Signaling
Technology, Inc.) and cyclin E (#9376; Cell Signaling Technology,
Inc.) at a dilution of 1:1,000, or with anti-β-actin antibody
(#3700; Cell Signaling Technology, Inc.) at a dilution of
1:5,000.
After being washed with TBST, the membranes were
incubated with horseradish Px-conjugated goat anti-rabbit
immunoglobulin G (#7076; Cell Signaling Technology, Inc.) at a
dilution of 1:5,000 at room temperature for 2 h. Following
additional washes with double distilled water (5 min for three
times), the membranes were visualized using SuperSignal West Femto
Maximum Sensitivity Substrate (#34094; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol, and exposed to
X-ray film in the dark room. The densities of the bands were
determined by standard scanning densitometry with normalization of
the densitometry measurements to that of β-actin.
Drug combination analysis
The combination index (CI) was calculated based on
the Chou-Talalay equation (12,18). CI
values were calculated using the formula: CI = (D)1 / (Dx)1 + (D)2
/ (Dx)2 for mutually exclusive drugs, where D refers to the drug
dose. In the denominator of the equation, (Dx)1 represents the D1
‘alone’ that inhibits a system by certain percentage and (Dx)2
represents the D2 ‘alone’ that inhibits a system by certain
percentage. In the numerator of the equation, (D)1 + (D)2 ‘in
combination’ also inhibit a system by certain percentage. The. CI
values were calculated according to the different percentages of
inhibition, from 0.05 to 0.95 (which represents 5–95% cell death).
Briefly, CI<0.85, 0.85<CI<1.15 and CI>1.15 indicated a
synergistic, additive and antagonistic effect, respectively.
Statistical analysis
Results were represented as the mean ± standard
deviation. Significance was assessed by one-way analysis of the
variance following appropriate transformation to normalized data
and equalized variance where necessary. Differences in cell
viability were compared by F-test. Statistical analysis was
performed using SPSS statistical software version 17.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Cell viability
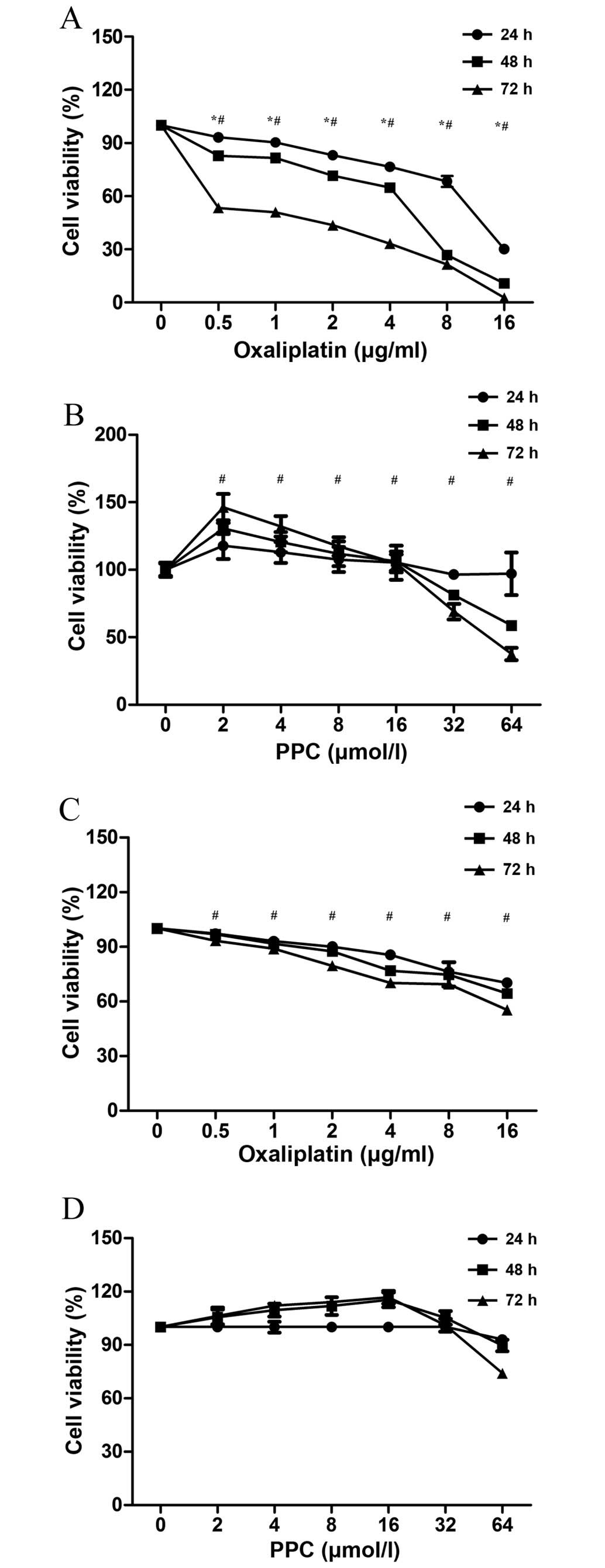
The effect of L-OHP on SGC-7901 cell viability is
presented in Fig. 1A. The growth of
gastric cancer cells was inhibited by L-OHP in a time- and
dose-dependent manner (F=194.193, P=0.0027 and F=12.428, P=0.01,
respectively). The effect of PPC on the viability of SGC-7901 cells
is presented in Fig. 1B. Low
concentrations of PPC (2–16 µmol/l) increased cell viability,
whereas high concentrations of PPC (32–64 µmol/l) decreased cell
viability. All effects were dose-related (F=373.769, P<0.01) but
not time-related (F=0.077, P=0.782). L-OHP also decreased HMEC-1
cell viability in a dose-dependent manner (F=6.23, P=0.032;
Fig. 1C). However, PPC did not affect
HMEC-1 cell viability (P=0.76; Fig.
1D), and therefore, it did not affect the growth of vascular
endothelial cells.
Combined treatment
The half maximal inhibitory concentration
(IC50) of L-OHP was calculated as 5.16, 3.89 and 0.89
µg/ml following 24, 48 and 72 h treatment, respectively.
Calculations were performed by Graphpad Prism software version 6
(Graphpad Software, Inc., La Jolla, CA, USA). Therefore, for the
combination groups, 3.5 µg/ml L-OHP was selected, which was
slightly lower than the IC50 for 48 h. Combination
groups were divided into three groups: i) Control group (I); ii)
L-OHP group (II); and iii) L-OHP + different concentrations of PPC
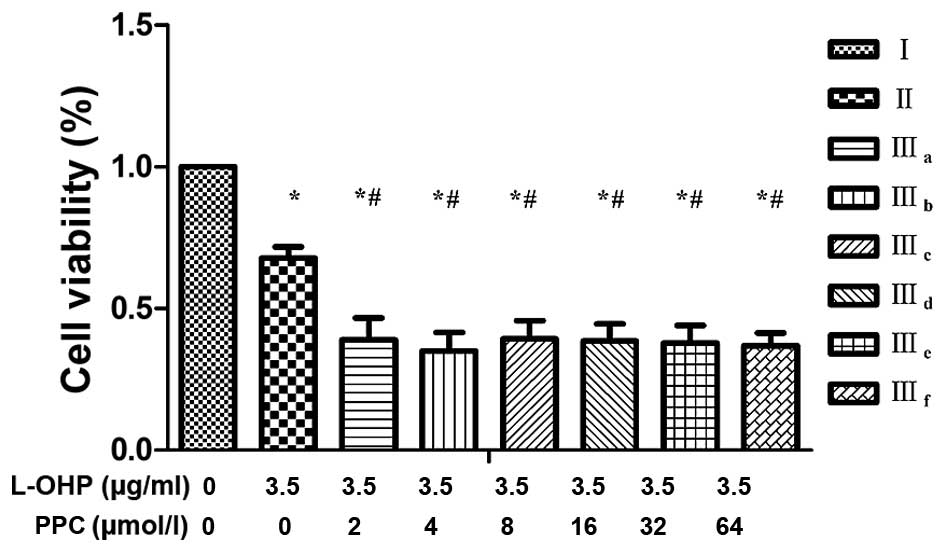
groups (IIIa-IIIf), as indicated in Table I. A low concentration of PPC combined
with L-OHP inhibited cell viability in a synergistic manner, which
suggested that low concentrations of PPC may enhance the
anti-proliferative effect of L-OHP in SGC-7901 cells. However, high
concentrations of PPC reduced this anti-proliferative effect
slightly (Table I and Fig. 2).
 | Table I.Drug concentrations and CI of
experimental groups IIIa-IIIf. |
Table I.
Drug concentrations and CI of
experimental groups IIIa-IIIf.
| Groups | L-OHP, µg/m | PPC, µmol/l | CI |
|---|
| I (control) | 0.0 | 0 | N/A |
| II | 3.5 | 0 | N/A |
|
IIIa | 3.5 | 2 | 3.10 |
|
IIIb | 3.5 | 4 | 2.89 |
|
IIIc | 3.5 | 8 | 1.70 |
|
IIId | 3.5 | 16 | 1.41 |
|
IIIe | 3.5 | 32 | 0.94 |
|
IIIf | 3.5 | 64 | 0.94 |
Cell apoptosis
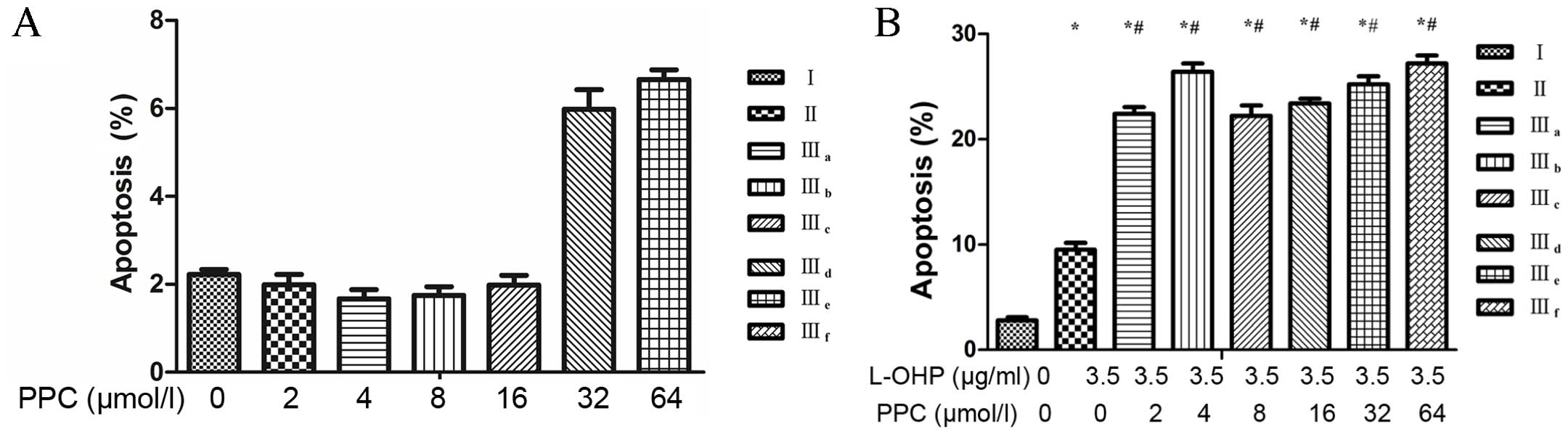
Treatment with L-OHP alone resulted in a slight
increase in the number of apoptotic cells compared with untreated
cells. Treatment with varying concentrations of PPC resulted in a
slight increase in the number of apoptotic cells compared with
untreated cells; however, this effect was not dose-related (P=0.07;
Fig. 3A). By contrast, the
combination of L-OHP and PPC treatment resulted in a significant
increase in the rate of apoptosis compared with L-OHP treatment
alone (Fig. 3B). These results
demonstrated that PPC enhanced the apoptosis induced by L-OHP;
however this effect was not dose-related (P=0.46; Fig. 3B and C).
Apoptotic proteins expression
The levels of cytochrome c, Bcl-2, Bax,
caspase-9 and caspase-3 expression were analyzed by western
blotting (Table II and Fig. 4) in order to characterize the
signaling pathways involved in L-OHP-induced apoptosis. The release
of cytochrome c from mitochondria activates downstream
caspases, and is a critical step in the apoptotic cascade (19). The results indicated that there was a
significant increase in cytochrome c levels in the cytosol
following 48-h treatment with L-OHP. The expression of the
anti-apoptotic protein Bcl-2 was decreased, whereas the expression
of the pro-apoptotic protein Bax was increased, following L-OHP
treatment. In addition, caspase-9 and caspase-3 activation was also
examined in the present study. Upon apoptotic stimulation,
caspase-9 and caspase-3 are cleaved into active fragments (20). The results of the western blot
analysis indicated that levels of the cleaved activated forms of
caspase-9 and caspase-3 significantly increased following L-OHP
treatment and that PPC greatly promoted the apoptotic effect
induced by L-OHP via a similar mechanism.
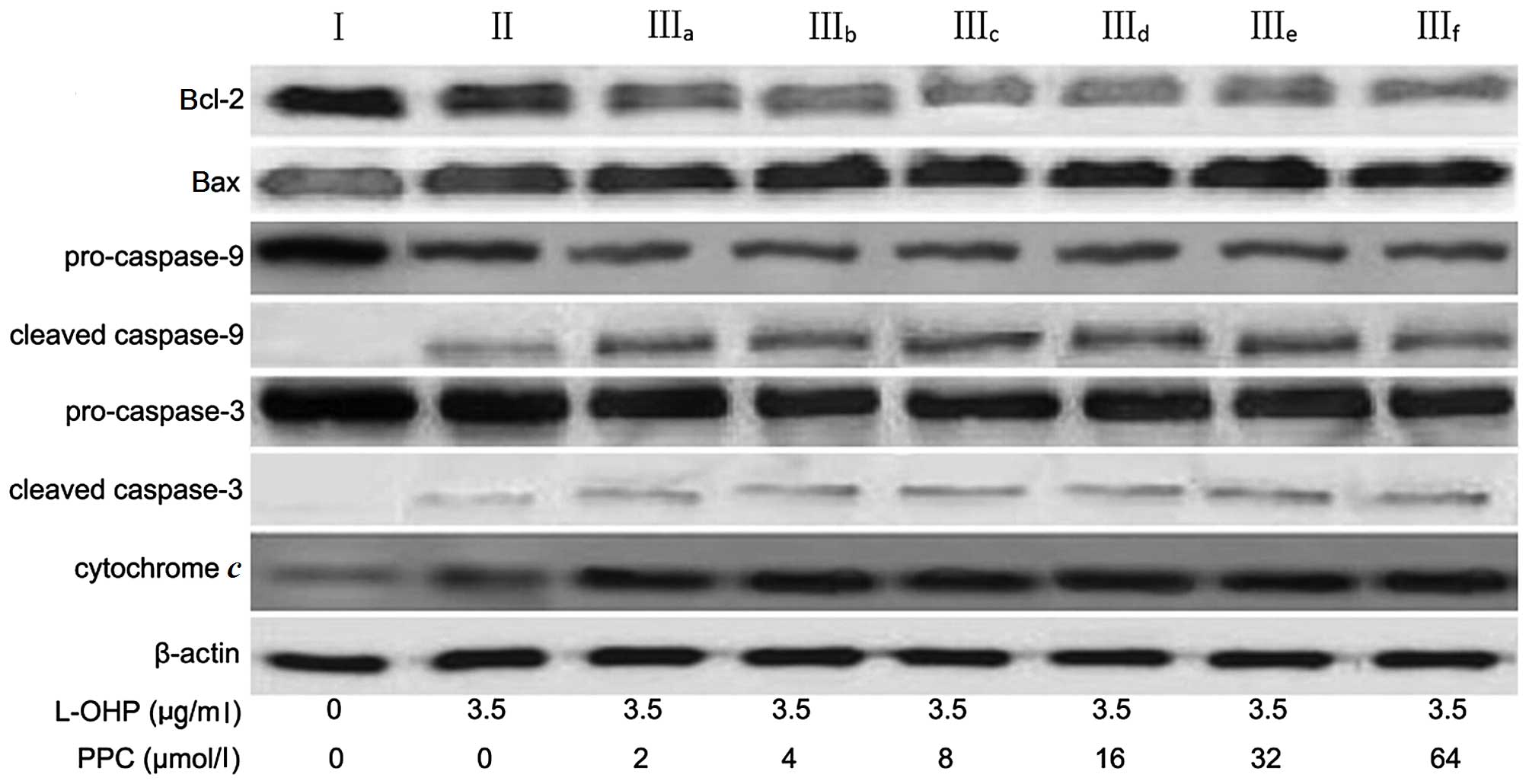 | Figure 4.Expression levels of cytochrome
c, Bcl-2, Bax, caspase-9 and caspase-3 in SGC-7901 cells, as
evaluated by western blotting. After being treated with 3.5 µg/ml
L-OHP combined with different concentrations of PPC for 48 h, the
cells were lysed, and the cellular extracts were separated on
SDS-PAGE and transferred to a nitrocellulose membrane. The
membranes were probed with different antibodies. β-actin was used
as an internal control. Blots were representative images of three
independent experiments. L-OHP, oxaliplatin; PPC,
polyenephosphatidylcholine; Bcl-2, B-cell lymphoma-2; Bax,
Bcl-2-associated X protein. |
 | Table II.Relative intensity of Bcl-2, Bax,
pro-caspase-9, cleaved caspase-9, pro-caspase-3, cleaved caspase-3
and cytochrome c. |
Table II.
Relative intensity of Bcl-2, Bax,
pro-caspase-9, cleaved caspase-9, pro-caspase-3, cleaved caspase-3
and cytochrome c.
| Protein | Group I | Group II | Group
IIIa | Group
IIIb | Group
IIIc | Group
IIId | Group
IIIe | Group
IIIf |
|---|
| Bcl-2 | 100 |
82a |
41a,b |
38a,b |
39a,b |
40a,b |
41a,b |
43a,b |
| Bax | 100 | 132a | 178a,b | 180a,b | 180a,b | 183a,b | 179a,b | 177a,b |
| Pro-caspase-9 | 100 |
73a |
47a,b |
51a,b |
49a,b |
51a,b |
47a,b |
50a,b |
| Cleaved
caspase-9 | 100 | 125a | 152a,b | 155a,b | 155a,b | 153a,b | 157a,b | 152a,b |
| Pro-caspase-3 | 100 |
93a |
73a,b |
70a,b |
69a,b |
70a,b |
69a,b |
72a,b |
| Cleaved
caspase-3 | 100 | 123a | 151a,b | 153a,b | 155a,b | 155a,b | 153a,b | 157a,b |
| Cytochrome
c | 100 | 143 | 189a,b | 188a,b | 192a,b | 194a,b | 188a,b | 190a,b |
Cell cycle
FACScan (BD Biosciences) analysis of SGC-7901 cells
stained with PI demonstrated that exposure to 3.5 µg/ml L-OHP for
48 h significantly induced cell cycle arrest in the G0/G1 phase
compared with control cells (P=0.012), and that PPC enhanced the
cell cycle arrest induced by L-OHP (P=0.035). However, treatment
with different concentrations of PPC had no significant effect on
cell cycle distribution (P=0.75; Fig.
5).
Cell cycle regulatory proteins
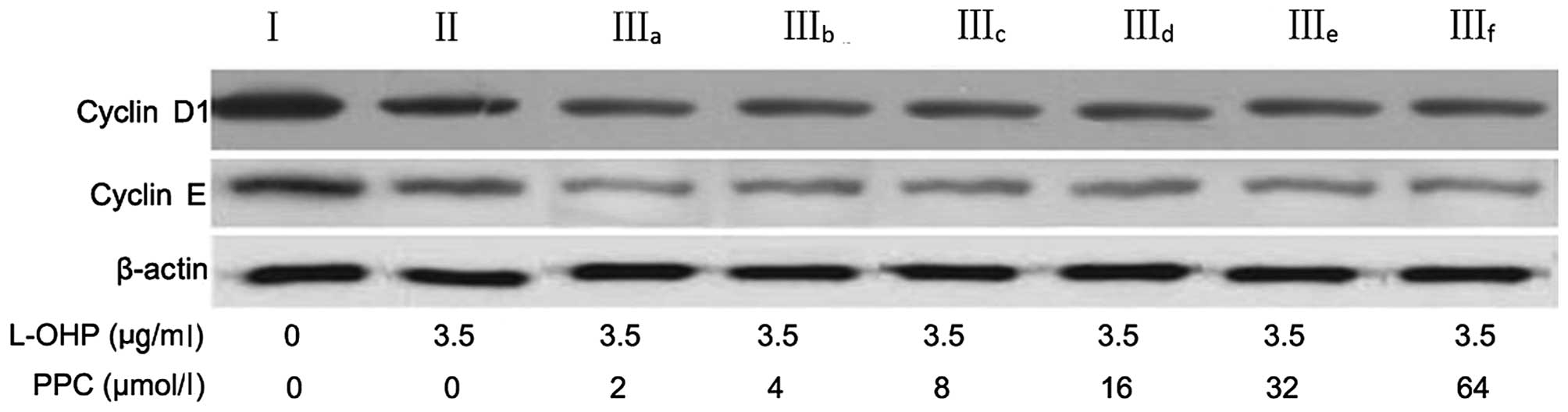
To determine the mechanism by which L-OHP induces
G0/G1 arrest, the expression of the relevant regulatory proteins,
cyclins D1 and E of the G0/G1 phase, was examined. As presented in
Table III and Fig. 6, L-OHP downregulated the expression of
cyclins D1 and E and this effect was enhanced by PPC (P=0.027).
However, these effects were not dose-dependent (P=0.38).
 | Table III.Relative intensity of cyclin D1 and
cyclin E expression. |
Table III.
Relative intensity of cyclin D1 and
cyclin E expression.
| Protein | Group I | Group II | Group
IIIa | Group
IIIb | Group
IIIc | Group
IIId | Group
IIIe | Group
IIIf |
|---|
| Cyclin D1 | 100 | 72a | 53a,b | 51a,b | 49a,b | 48a,b | 50a,b | 47a,b |
| Cyclin E | 100 | 83a,b | 53a,b | 57a,b | 56a,b | 51a,b | 54a,b | 51a,b |
MDA levels, SOD activity and GSH-Px
activity
To assess the intracellular oxidant and antioxidant
status, the MDA levels, SOD activity and GSH-Px activity were
evaluated in SGC-7901 cells (Fig. 7).
The MDA levels in the combined groups (treated with L-OHP and
varying concentrations of PPC) were significantly higher, and the
SOD and GSH-Px activities were significantly lower, compared with
those in the control group. However, there were no significant
differences in MDA levels, SOD activity or GSH-Px activity between
the combined groups and those treated with L-OHP alone
(P=0.88).
Discussion
General improvements in health and quality of life
mean that life expectancy continues to increase. However, the
incidence of gastric cancer is also gradually increasing,
particularly in elderly people (13,21). The
majority of patients with gastric cancer are already at a very
advanced stage when they are diagnosed (22). The palliative treatment accepted for
advanced gastric cancer, including recurrent and metastatic cancer,
is chemotherapy, which is much better than the best supportive care
in improving the survival and quality of life of patients (14,23).
Furthermore, it is generally accepted that chemotherapy decreases
the probability of relapse and improves patient survival rates, due
to the high sensitivity of gastric cancer to chemotherapy (15,24). Thus,
it is necessary to develop effective chemotherapy drugs to treat
patients with advanced gastric cancer.
L-OHP is a new-generation platinum compound that
exhibits low toxicity and lacks cross-drug resistance with
cis-diammineglycolatoplatinum, and has expanded the range of
effective treatment options currently available for patients with
advanced gastric cancer (16,17,25,26). It
has been reported that platinum causes mitochondrial dysfunction,
possibly by inhibiting the electron transfer system, resulting in
the increased production of hydrogen peroxide, superoxide anions
and hydroxyl radicals (18,27), which generate reactive oxygen species
(ROS) (28). The majority of cells in
the body contain enzymes that act as antioxidants and remove ROS
(19,29). There are two primary antioxidant
enzymes, GSH-Px and SOD (20,30). It is generally considered that MDA is
an indicator of lipid peroxidation, as it is an oxidative
degradation product of cell membrane lipids (21,31).
Therefore, changes in GSH-Px and SOD activities or MDA levels may
indicate production or elimination of ROS caused by anti-cancer
drugs. In the present study, treatment of the gastric cancer cell
line SGC-7901 with L-OHP resulted in a significant increase in MDA
levels and a marked decrease in GSH-Px and SOD activities, which
demonstrated its anti-tumor effect. However, several randomized
studies have determined that chemotherapy treatment with L-OHP may
cause liver injury followed by progressive steatohepatitis, which
is associated with neurotoxicity and severe pain (22,23,32,33).
Therefore, the present study assessed whether treatment with a
combination of L-OHP and a liver-protective compound may reduce
such side effects.
PPC is a major active ingredient in essential
phospholipids, and is used in the treatment of CASH (34). It normalizes the metabolism of lipids
and proteins, improves the detoxification function of cells and
restores the structure of cells (35). In the current study, although PPC
increased the growth and proliferation of SGC-7901 cells when used
alone, it also greatly promoted their apoptosis induced by L-OHP.
This suggests that PPC would not compromise the anti-tumor effects
of L-OHP.
In order to understand the precise molecular
mechanism, the production of ROS and the activity of
ROS-eliminating enzymes stimulated by L-OHP were evaluated in the
current study. PPC did not influence SOD activity, GSH-Px activity
or MDA levels, which were altered by L-OHP. Thus, it is likely that
PPC does not alter L-OHP activity when administered alongside it as
a combination treatment. L-OHP triggers cancer cell apoptosis by
damaging the cell DNA and the main apoptotic pathway involves
cytochrome c, which is present in mitochondria (12,18).
Anti-cancer agents cause DNA damage and induce the transmission of
death signals via the activation of the suppressor gene p53, which
subsequently inhibits Bcl-2 expression in mitochondria (24,25,36,37).
This inhibition leads to the release of cytochrome c, which
triggers the apoptosis of cancer cells (26,38).
During this process, cytochrome c activates caspases 9 and
3, with the help of apoptotic protease-activating factor 1
(27,39), and activated caspase 3 subsequently
suppresses caspase-activated DNase, inducing DNA fragmentation
(28,40). The present study suggested that L-OHP
may activate this apoptotic pathway in mitochondria, where it
functions primarily by inducing DNA fragmentation and chromatin
condensation.
Apart from stimulating the apoptosis of cancer
cells, the suppressive effect of L-OHP on SGC-7901 cell growth may
also be caused by cell cycle arrest at the G0/G1 phase. Cell cycle
proteins, including cyclins D1 and E, which control the transition
of cell cycle phases, have previously been examined (29,41). These
two cell cycle proteins regulate the activity of cyclin-dependent
kinases 4 and 6 by activating the transcription factor E2F and
inactivating retinoblastoma protein to induce DNA synthase
expression (30,42). In the present study, suppression of
cyclins D1 and E was observed in SGC-7901 cells, which indicated
that L-OHP may arrest the cell cycle in the G0/G1 phase.
PPC does not affect the anti-tumor activity of
L-OHP; however, it may serve a synergistic role by inhibiting the
growth and promoting the apoptosis of SGC-7901 cells. One possible
mechanism of PPC action is that it may induce the redistribution of
the primary phospholipid components in the bilayer of the membrane,
particularly in cancer cells, which have a higher metabolism than
healthy cells (43). This may then
lead to a critical rearrangement of membrane components and even
result in further architectural alterations of the membrane. These
alterations could modify the diffusion properties of membranes,
which are important in the development of drug resistance (31,44).
Cytotoxic compounds, including L-OHP, must reach their targets
inside cancer cells through their membrane. Thus, PPC may inhibit
the growth and promote the apoptosis of SGC-7901 cells by boosting
the accumulation of L-OHP in cancer cells.
In conclusion, the present study identified an
association between PPC and L-OHP in the treatment of SGC-7901
cells. These data raise the possibility that, by combining PPC and
L-OHP treatment, the dose of L-OHP administered to patients could
be reduced, which would decrease the side effects associated with
its use. Further studies are necessary to confirm the combinational
efficacy of PPC and L-OHP. Future studies performed by the authors
of the present study will focus on the fluidity and permeability of
the membrane altered by PPC.
References
|
1
|
Dong CX, Fu JF, Ye XY, Li XF, Zhong X and
Yuan Y: Surgical resection of advanced gastric cancer following
trastuzumab/oxaliplatin/capecitabine combination therapy. World J
Gastroenterol. 20:12355–12358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choi IJ, Lee JH, Kim YI, Kim CG, Cho SJ,
Lee JY, Ryu KW, Nam BH, Kook MC and Kim YW: Long-term outcome
comparison of endoscopic resection and surgery inearly gastric
cancer meeting the absolute indication for endoscopic resection.
Gastro intest Endosc. 81:333.e1–341.e1. 2015. View Article : Google Scholar
|
|
4
|
Fiteni F, Nguyen T, Vernerey D, Paillard
MJ, Kim S, Demarchi M, Fein F, Borg C, Bonnetain F and Pivot X:
Cisplatin/gemcitabine or oxaliplatin/gemcitabine in the treatment
of advanced biliary tract cancer: A systematic review. Cancer Med.
3:1502–1511. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schumacher JD and Guo GL: Mechanistic
review of drug-induced steatohepatitis. Toxicol Appl Pharmacol.
289:40–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang Tao, Song Hao, Zhao Yuan-Yuan, Liang
Jun, Zhang Hong-Jun and Liu Xi-Guang: Effect of oxaliplatin
combined with polyenephosphatidylcholine on proliferation of
gastric cancer cells. Chinese Journal of Cancer Prevention and
Treatment. 13:41–44. 2014.
|
|
7
|
Cao M, Li X, Zhang B, Han S, Yang Y, Zhou
B and Zhang Y: The effect of polyene phosphatidyl choline
intervention on nonalcoholic steatohepatitis and related mechanism.
Am J Transl Res. 8:2325–2330. 2016.PubMed/NCBI
|
|
8
|
Kim HS, Kim JH, Kim HJ, Jang HJ, Kim JB,
Kim JW, Jung SY, Kim BC, Yang DH, Park S, et al: Oxaliplatin,
5-fluorouracil and leucovorin (modified FOLFOX-6) as first-line
chemotherapy for advanced gastric cancer patients with poor
performance status. Oncol Lett. 3:425–428. 2012.PubMed/NCBI
|
|
9
|
Rosati G, Ferrara D and Manzione L: New
perspectives in the treatment of advanced or metastatic gastric
cancer. World J Gastroenterol. 15:2689–2692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koizumi W, Takiuchi H, Yamada Y, Boku N,
Fuse N, Muro K, Komatsu Y and Tsuburaya A: Phase II study of
oxaliplatin plus S-1 as first-line treatment for advanced gastric
cancer (G-SOX study). Ann Oncol. 21:1001–1005. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park I, Lee JL, Ryu MH, Chang HM, Kim TW,
Sym SJ, Lee SS, Jang G, Yoo C, Bae KS and Kang YK: Phase I/II and
pharmacokinetic study of S-1 and oxaliplatin in previously
untreated advanced gastric cancer. Cancer Chemother Pharmacol.
65:473–480. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choti MA: Chemotherapy-associated
hepatotoxicity: Do we need to be concerned? Ann Surg Oncol.
16:2391–2394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Malaguarnera M, Di RM, Nicoletti F and
Malaguarnera L: Molecular mechanisms involved in NAFLD progression.
J Mol Med (Berl). 87:679–695. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okyama W, Tanaka N, Nakajima T, Tanaka E,
Kiyosawa K, Gonzalez FJ and Aoyama T: Polyenephosphatidylcholine
prevents alcoholic liver disease in PPARalpha-null mice through
attenuation of increases in oxidative stress. J Hepatol.
50:1236–1246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lieber CS, Robins SJ, Li J, DeCarli LM,
Mak KM, Fasulo JM and Leo MA: Phosphatidylcholine protects against
fibrosis and cirrhosis in the baboon. Gastroenterology.
106:152–159. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao Q, Mak KM and Lieber CS:
Dilinoleoylphosphatidylcholine decreases acetaldehyde-induced
TNF-alpha generation in Kupffer cells of ethanol-fed rats. Biochem
Biophys Res Commun. 299:459–464. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao Q, Mak KM and Lieber CS: DLPC and SAMe
combined prevent leptin-stimulated TIMP-1 production in LX-2 human
hepatic stellate cells by inhibiting HO-mediated signal
transduction. Liver Int. 26:221–231. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Esmaeili MA, Abagheri-Mahabadi N,
Hashempour H, Farhadpour M, Gruber CW and Ghassempour A: Viola
plant cyclotide vigno 5 induces mitochondria-mediated apoptosis
viacytochrome C release and caspases activation in cervical cancer
cells. Fitoterapia. 109:162–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saito H, Osaki T, Murakami D, Sakamoto T,
Kanaji S, Tatebe S, Tsujitani S and Ikeguchi M: Effect of age on
prognosis in patients with gastric cancer. ANZ J Surg. 76:458–461.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoon H and Kim N: Diagnosis and management
of high risk group for gastric cancer. Gut Liver. 9:5–17. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Glimelius B, Ekström K, Hoffman K, Graf W,
Sjödén PO, Haglund U, Svensson C, Enander LK, Linné T, Sellström H
and Heuman R: Randomized comparison between chemotherapy plus best
supportive care with best supportive care in advanced gastric
cancer. Ann Oncol. 8:163–168. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oba K, Paoletti X, Bang YJ, Bleiberg H,
Burzykowski T, Fuse N, Michiels S, Morita S, Ohashi Y, Pignon JP,
et al: Role of chemotherapy for advanced/recurrent gastric cancer:
An individual-patient-data meta-analysis. Eur J Cancer.
49:1565–1577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Falcone A, Ricci S, Brunetti I, Pfanner E,
Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W,
Fanchini L, et al: Phase III trial of infusional fluorouracil,
leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with
infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as
first-line treatment for metastatic colorectal cancer: The Gruppo
Oncologico Nord Ovest. J Clin Oncol. 25:1670–1676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Al-Batran SE, Hartmann JT, Probst S,
Schmalenberg H, Hollerbach S, Hofheinz R, Rethwisch V, Seipelt G,
Homann N, Wilhelm G, et al: Phase III trial in metastatic
gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus
either oxaliplatin or cisplatin: A study of the Arbeitsgemeinschaft
Internistische Onkologie. J Clin Oncol. 26:1435–1442. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nordberg J and Arnér ES: Reactive oxygen
species, antioxidants and the mammalian thioredoxin system. Free
Radic Biol Med. 31:1287–1312. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeng L, Li Y, Li T, Cao W, Yi Y, Geng W,
Sun Z and Xu H: Selenium-platinum coordination compounds as novel
anticancer drugs: Selectively killing cancer cells via a reactive
oxygen species (ROS)-mediated apoptosis route. Chem Asian J.
9:2295–2302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee DJ and Kang SW: Reactive oxygen
species and tumor metastasis. Mol Cells. 35:93–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Atmaca M, Kuloglu M, Tezcan E and Ustundag
B: Antioxidant enzyme and malondialdehyde levels in patients with
social phobia. Psychiatry Res. 159:95–100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Singh N, Zaidi D, Shyam H, Sharma R and
Balapure AK: Polyphenols sensitization potentiates susceptibility
of MCF-7 and MDA MB-231 cells to Centchroman. PLoS One.
7:e377362012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Culy CR, Clemett D and Wiseman LR:
Oxaliplatin. A review of its pharmacological properties and
clinical efficacy in metastatic colorectal cancer and its potential
in other malignancies. Drugs. 60:895–924. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fernandez FG, Ritter J, Goodwin JW,
Linehan DC, Hawkins WG and Strasberg SM: Effect of steatohepatitis
associated with irinotecan or oxaliplatin pretreatment on
resectability of hepatic colorectal metastases. J Am Coll Surg.
200:845–853. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Horejsová M and Urban J: The effect of
polyene phosphatidylcholine (Essentiale forte) in the treatment of
liver steatosis and ultrasound findings-preliminary study. Cas Lek
Cesk. 133:366–369. 1994.(In Czech). PubMed/NCBI
|
|
35
|
Su HL, Zhu YX, Gao ZJ, Dong XY, Zhu JY,
Lei WR, Zhang Y and Han Y: Efficacy comparison between bicyclol and
polyene phosphatidylcholine treatments for the patients with
nonalcoholic fatty liver disease. Zhonghua Gan Zang Bing Za Zhi.
9:552–553. 2011.(In Chinese).
|
|
36
|
Dragovich T, Rudin CM and Thompson CB:
Signal transduction pathways that regulate cell survival and cell
death. Oncogene. 17:3207–3213. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu X, Kim CN, Yang J, Jemmerson R and
Wang X: Induction of apoptotic program in cell-free extracts:
Requirement for dATP and cytochrome c. Cell. 86:147–157. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zou H, Henzel WJ, Liu X, Lutschg A and
Wang X: Apaf-1, a human protein homologous to C. elegans CED-4,
participates in cytochrome c-dependent activation of caspase-3.
Cell. 90:405–413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Enari M, Sakahira H, Yokoyama H, Okawa K,
Iwamatsu A and Nagata S: A caspase-activated DNase that degrades
DNA during apoptosis, and its inhibitor ICAD. Nature. 391:43–50.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Almeida A: Regulation of APC/C-Cdh1 and
its function in neuronal survival. Mol Neurobiol. 46:547–554. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang K and Kumar R: Interferon-alpha
inhibits cyclin E- and cyclin D1-dependent CDK-2 kinase activity
associated with RB protein and E2F in Daudi cells. Biochem Biophys
Res Commun. 200:522–528. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hossain Z, Konishi M, Hosokawa M and
Takahashi K: Effect of polyunsaturated fatty acid-enriched
phosphatidylcholine and phosphatidylserine on butyrate-induced
growth inhibition, differentiation and apoptosis in Caco-2 cells.
Cell Biochem Funct. 24:159–165. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Raffy S and Teissié J: Control of lipid
membrane stability by cholesterol content. Biophys J. 76:2072–2080.
1999. View Article : Google Scholar : PubMed/NCBI
|