Introduction
As one of the most aggressive and lethal diseases,
pancreatic ductal adenocarcinoma is the fourth leading cause of
cancer-related mortality worldwide, despite accounting for only
2.2% of all cancers (1,2). The estimated 5-year survival rate is
less than 5%, and the overall median survival time is less than 1
year following diagnosis (3,4). Gemcitabine monotherapy was initially
approved by the US Food and Drug Administration (FDA) in 1996, and
it has been the standard of care for patients with metastatic
pancreatic cancer for several decades (5). Although various combination therapies
have been developed, most demonstrate a minimal or no significant
change in overall survival compared with treatment of gemcitabine
alone (6), highlighting the
requirement for further mechanistic studies. Numerous factors have
been reported to be involved in gemcitabine's effects, including
various genes, proteins, signalling pathways and microRNAs
(7–10). Apoptosis is a core signalling pathway
in human pancreatic cancer, and a detailed understanding of
apoptosis is essential for the development of more effective or
‘targeted’ therapies (11–13).
The transcription factor activator protein 1 (AP-1)
is a dimeric complex comprised of the Jun, Fos, activating
transcription factor and musculoaponeurotic fibrosarcoma protein
families (14), and it is involved in
cellular proliferation, transformation and death (15). The AP-1 complex forms various
combinations of heterodimers and homodimers, and this combination
determines the genes that are regulated by AP-1 (16). c-Jun and c-Fos function differentially
modulates their target genes. It has been reported that
c-Jun−/− fibroblasts are resistant to alkylating
agent-induced apoptosis, which may be mediated by Fas ligand
induction (17). Additionally,
rhomboid domain-containing 1 inhibits UV-induced cell apoptosis by
activating and upregulating c-Jun and its downstream target B-cell
lymphoma 3 (Bcl-3) (18). c-Fos
downregulation in MCF-7/ADR cells resulted in enhanced apoptosis,
and altered expression of apoptosis-associated proteins, including
Bax, Bcl-2, p53 and PUMA (19).
In this study, we investigated the biological
effects of AP-1 on gemcitabine-induced apoptosis in pancreatic
cancer cells. Our results indicate that endogenous c-Jun
expression, but not c-Fos expression, increased following
gemcitabine treatment. Furthermore, c-Jun functioned as a
pro-apoptotic protein by regulating the downstream AP-1 target Bim.
These results are likely to provide further insight into the
molecular mechanisms of chemotherapy in pancreatic cancer.
Materials and methods
Cell culture, plasmids and
transfection
Cell lines were obtained from the Cell Resource
Centre at Peking Union Medical College (PUMC), China. Panc-1 cells
were cultured in Dulbecco's modified Eagle's medium (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) with 10% foetal bovine
serum (FBS), and SW1990 cells were cultured in RPMI-1640 (HyClone)
with 10% FBS in 5% CO2 at 37°C. Adherent cells were
passaged every 2–3 days with 0.5 mg/ml trypsin (1:250) and 0.53 mM
ethylenediaminetetraacetic acid. c-Jun expression plasmids were
cloned into pIRES-puro2 with a C-terminal Myc tag. siRNA
oligonucleotides were designed against c-Jun as follows: GAU GGA
AAC GAC CUU CUAU. The plasmids were constructed according to
standard cloning techniques. Cells were transfected using
Lipofectamine™ 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA).
Antibodies
The Bcl-3 and Bim antibodies were purchased from
Santa Cruz Biotechnology. The antibody against c-Jun was purchased
from BD Pharmingen (San Diego, CA, USA). Antibodies against
phospho-c-Jun (Ser73), Bax, PARP, caspase-7 and GAPDH were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Horseradish peroxidase (HRP)-conjugated secondary antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, MA,
USA).
Western blot analysis
Proteins were extracted with sodium dodecyl sulphate
(SDS) lysis buffer [50 mM Tris-HCl (pH 6.8), 10% glycerol and 2%
SDS] and quantified using the bicinchoninic acid protein assay
reagent (Thermo Fisher Scientific, Waltham, MA, USA). Extracts were
separated on a 12% SDS-polyacrylamide gel and electrophoretically
transferred to polyvinylidene fluoride membrane (GE Healthcare Life
Sciences). The membrane was blocked in 5% skimmed milk for 1 h at
room temperature and then incubated overnight with the indicated
antibodies at 4°C. The membrane was incubated with an anti-rabbit
or an anti-mouse IgG-HRP (Santa Cruz Biotechnology, Inc.) for 1 h
at room temperature. Chemiluminescence was detected using an
enhanced chemiluminescence blot detection system (Santa Cruz
Biotechnology, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from cells was extracted with TRIzol
reagent (Invitrogen Life Technologies) and 1 µg isolated total RNA
was converted to cDNA using a First-Strand cDNA synthesis kit
(Takara Biotechnology Co., Ltd., Dalian, China). Power SYBR-Green
master mix (Applied Biosystems, Foster City, CA, USA) was added to
cDNA samples that were then subjected to RT-qPCR using the StepOne
Real-Time PCR system (Applied Biosystems). Relative mRNA levels
were normalised against the housekeeping gene GAPDH. The primers
for RT-qPCR were as follows: c-Jun sense,
5′-TCCAAGTGCCGAAAAAGGAAG-3′ and antisense,
5′-CGAGTTCTGAGCTTTCAAGGT-3′; c-Fos sense,
5′-GGGGCAAGGTGGAACAGTTAT-3′ and antisense,
5′-CCGCTTGGAGTGTATCAGTCA-3′; GAPDH sense,
5′-TGAGTACGTCGTGGAGTCCA-3′, and antisense,
5′-TAGACTCCACGACATACTCA-3′.
Cell proliferation assay
Cell proliferation was assessed by the Cell Counting
Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.,
Shanghai, China). Cells were seeded at a density of 2,000
cells/well in 96-well plates. A total of 10 µl CCK-8 solution was
added to each well containing 100 µl culture medium and incubated
for 2 h at 37°C. Absorbance was measured at 450 nm using a
multiwell spectrophotometer (BioTek, Winooski, VT, USA).
Flow cytometric analysis
Cells were harvested by trypsinisation and collected
by centrifugation, and then a fluorescein isothiocyanate
(FITC)-Annexin V kit (NeoBioscience, Shenzhen, China) was used to
stain cells according to the manufacturer's instructions. Apoptotic
cells were analysed with a BD Accuri® C6 flow cytometer
and corresponding CellFIT software (both from BD Biosciences, San
Diego, CA, USA).
Terminal deoxynucleotidyl transferase
dUTP nick end labelling (TUNEL)
Cells were fixed with 4% paraformaldehyde solution
for 30 min at room temperature. After rinsing with
phosphate-buffered saline (PBS), the samples were incubated with a
TUNEL reaction mixture containing terminal deoxynucleotidyl
transferase and FITC-dUTP (Roche Applied Science, Indianapolis, IN,
USA) for 1 h at 37 °C using an apoptosis detection kit (Roche
Applied Science). These cells were then stained with
4,6-diamidino-2-phenylindole (DAPI) to detect the cell nucleus.
Statistical analyses
Statistical analyses were performed using Student's
t-test in Microsoft Excel software (Redmont, WA, USA). The results
were presented as the means ± standard deviation of triplicates of
each experiment. All experiments were performed three times, unless
stated otherwise.
Results
Gemcitabine induces apoptosis in human
pancreatic cancer cells
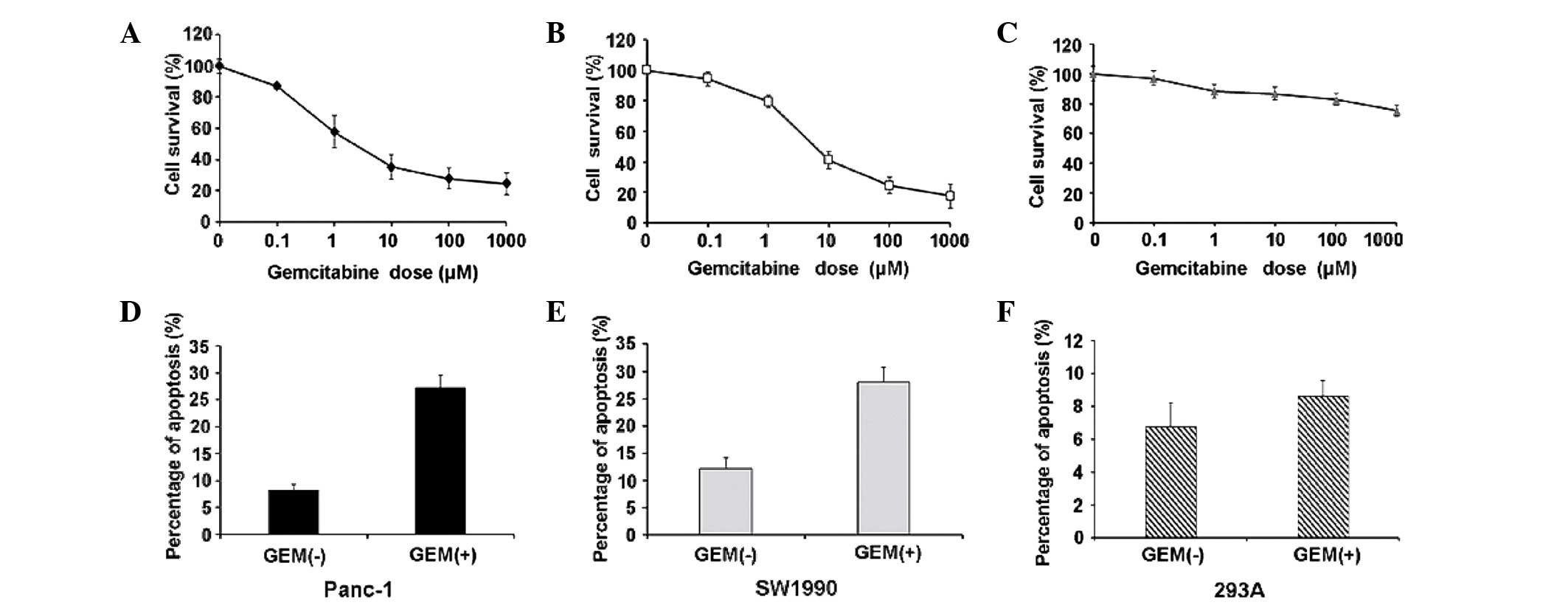
To determine the physiologically relevant dose of
gemcitabine that induces apoptosis in Panc-1 and SW1990 cells, we
first determined gemcitabine's cytotoxic effects and used 293A
cells as a control. We treated cells with increasing doses of
gemcitabine (from 0.1 to 1 mM) or PBS for control cells. The
results identified the 50% inhibitory concentration
(IC50) value in Panc-1 and SW1990 cells (Fig. 1A and B) while 293A cells did not
respond to gemcitabine (Fig. 1C).
Based on the data and previous studies reporting that micromolar
gemcitabine concentrations may be clinically achieved (20), we used 10 µM gemcitabine for all
subsequent experiments. Flow cytometric analysis revealed that 10
µM gemcitabine significantly increased apoptosis in Panc-1 and
SW1990 cells (Fig. 1D and E), but not
in 293A cells (Fig. 1F).
Gemcitabine treatment induces c-Jun
expression in a dose-dependent manner
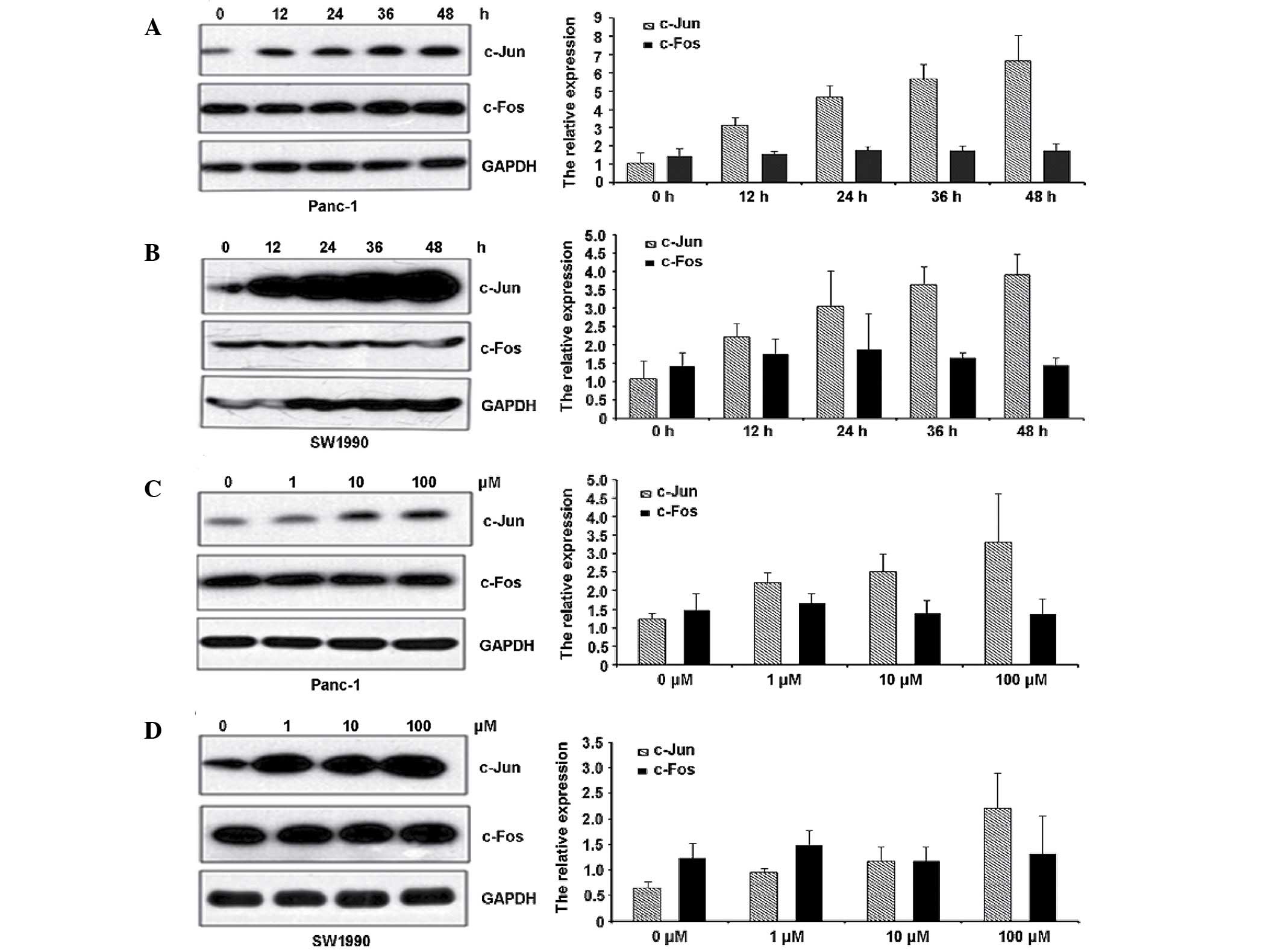
c-Jun and c-Fos are extensively studied components
of the AP-1 complex, which is involved in numerous cell activities,
including proliferation, apoptosis, survival, tumourigenesis and
tissue morphogenesis (21). To
further define the AP-1 mechanism in gemcitabine-induced pancreatic
cancer cell apoptosis, we examined the expression of c-Jun and
c-Fos following exposure to 10 µM gemcitabine at the indicated time
points through western blot analysis and RT-qPCR. As shown in
Fig. 2A and B, gemcitabine had little
effect on c-Fos expression but significantly increased c-Jun
expression. These results suggest that gemcitabine specifically
activates the AP-1 pathway through c-Jun but not c-Fos. In
addition, gemcitabine increased c-Jun expression in a
concentration-dependent manner (Fig. 2C
and D).
c-Jun promotes gemcitabine-induced
apoptosis by upregulating Bim
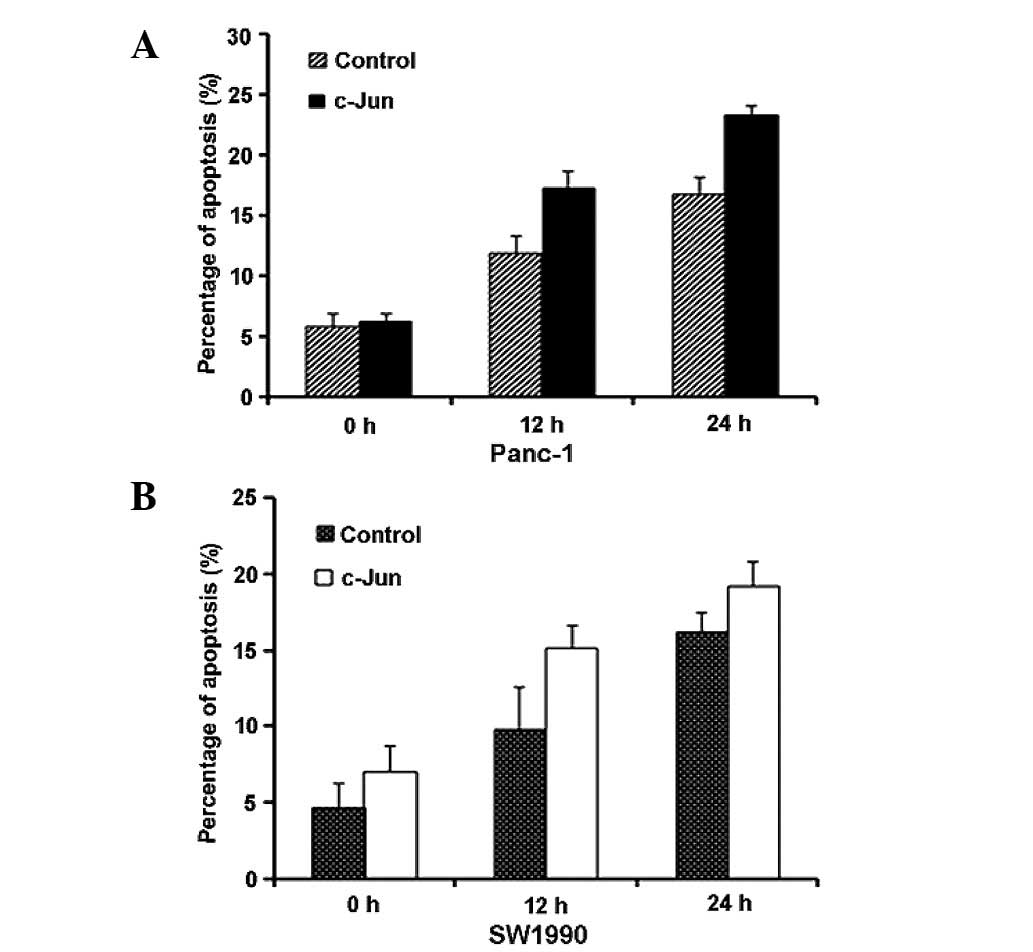
Based on the experimental data above, we
hypothesised that c-Jun regulates gemcitabine-induced apoptosis. To
confirm this hypothesis, we transfected c-Jun (c-Jun-Myc) or a
control vector into Panc-1 and SW1990 cells for 24 h. We then
exposed the cells to gemcitabine and performed
fluorescence-activated cell sorting (FACS) analysis to measure
apoptosis at the indicated time points. The results revealed that
gemcitabine-induced apoptosis increased upon c-Jun overexpression
(Fig. 3A and B). Western blot
analysis also suggested that PARP and cleaved caspase 7 levels
increased, indicative of the increased apoptosis. AP-1 likely
affects apoptosis through the differential regulation of
pro-apoptotic and anti-apoptotic downstream factors (22,23).
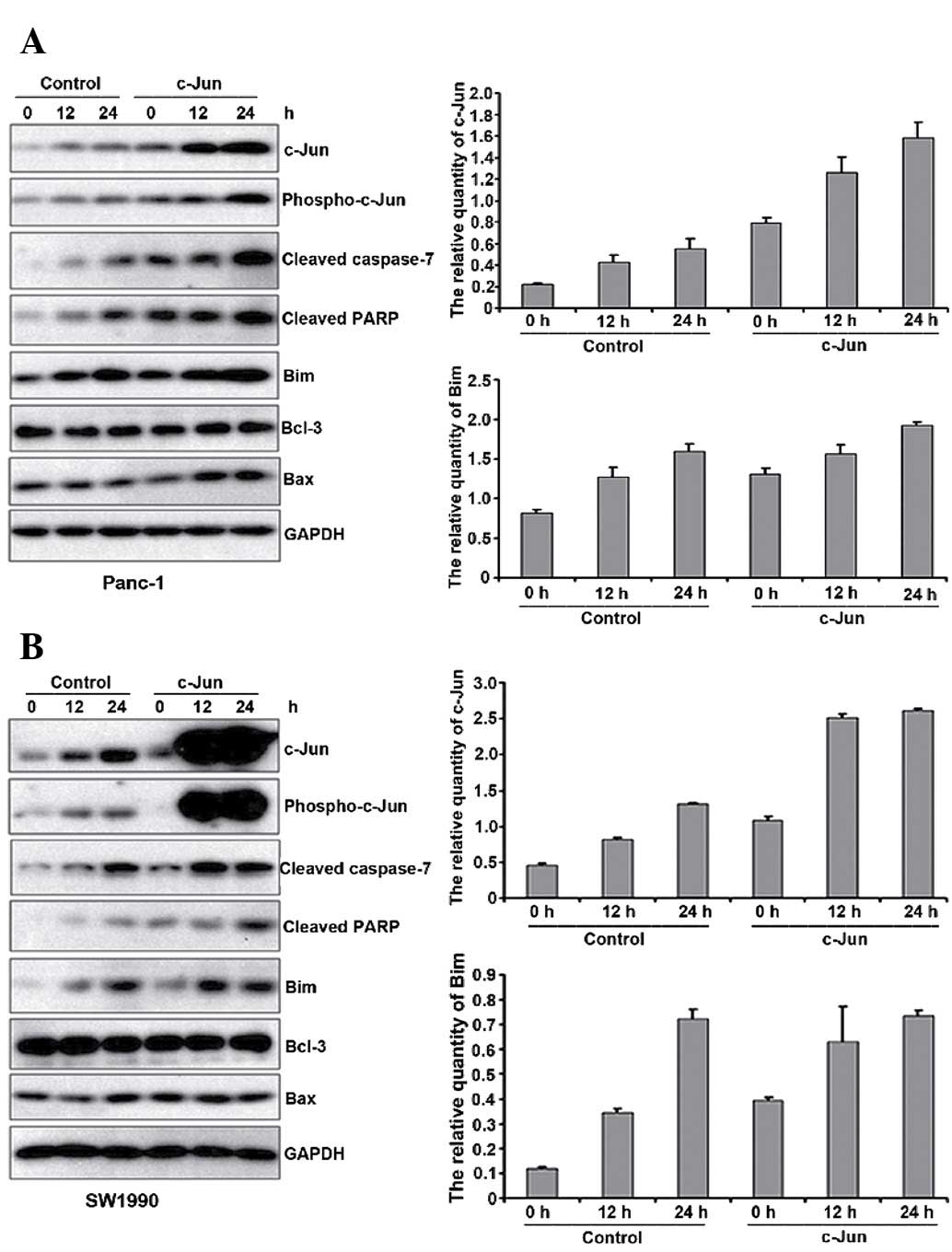
Therefore, we analysed Bcl-3, Bax and Bim expression. As shown in
Fig. 4A and B, Bcl-3 and Bax
expression did not change upon c-Jun overexpression, whereas Bim
expression increased. Therefore, c-Jun overexpression promotes
gemcitabine-induced apoptosis though Bim upregulation.
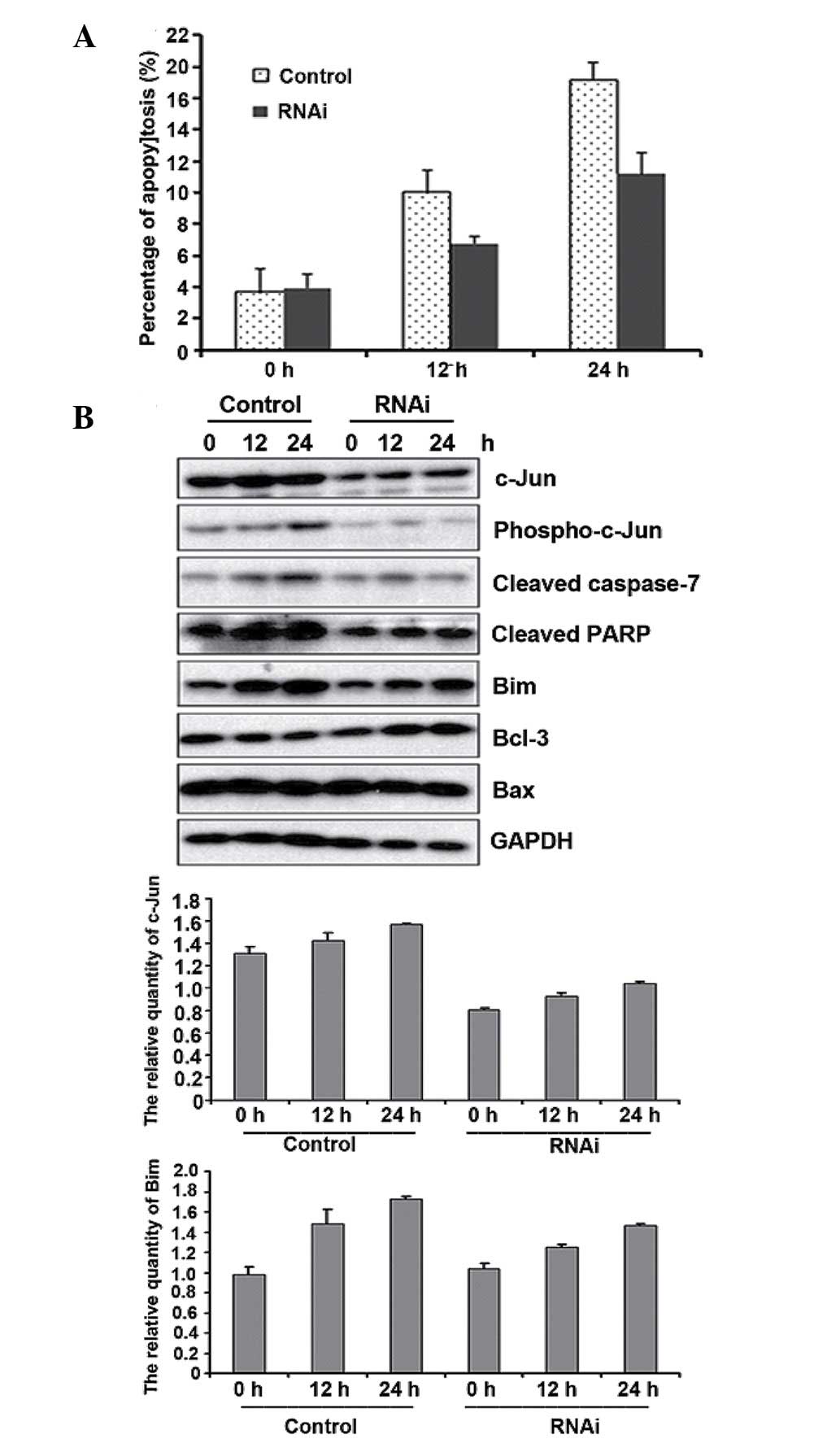
Endogenous c-Jun suppression inhibits
gemcitabine-induced apoptosis by reducing Bim
To further determine the pro-apoptotic properties of
c-Jun in gemcitabine resistance, we suppressed endogenous c-Jun
expression in Panc-1 cells by transfecting cells with siRNA against
c-Jun. FACS and western blot analysis indicated that c-Jun
knockdown inhibited gemcitabine-induced apoptosis (Fig. 5A). We also observed that Bim
expression decreased following gemcitabine treatment in c-Jun
knockdown cells. However, c-Jun did not affect Bax and Bcl-3
expression under the same conditions (Fig. 5B). Taken together, our findings
suggest that c-Jun exerts pro-apoptotic effects on
gemcitabine-treated cells by regulating its downstream target Bim
in pancreatic cancer cells.
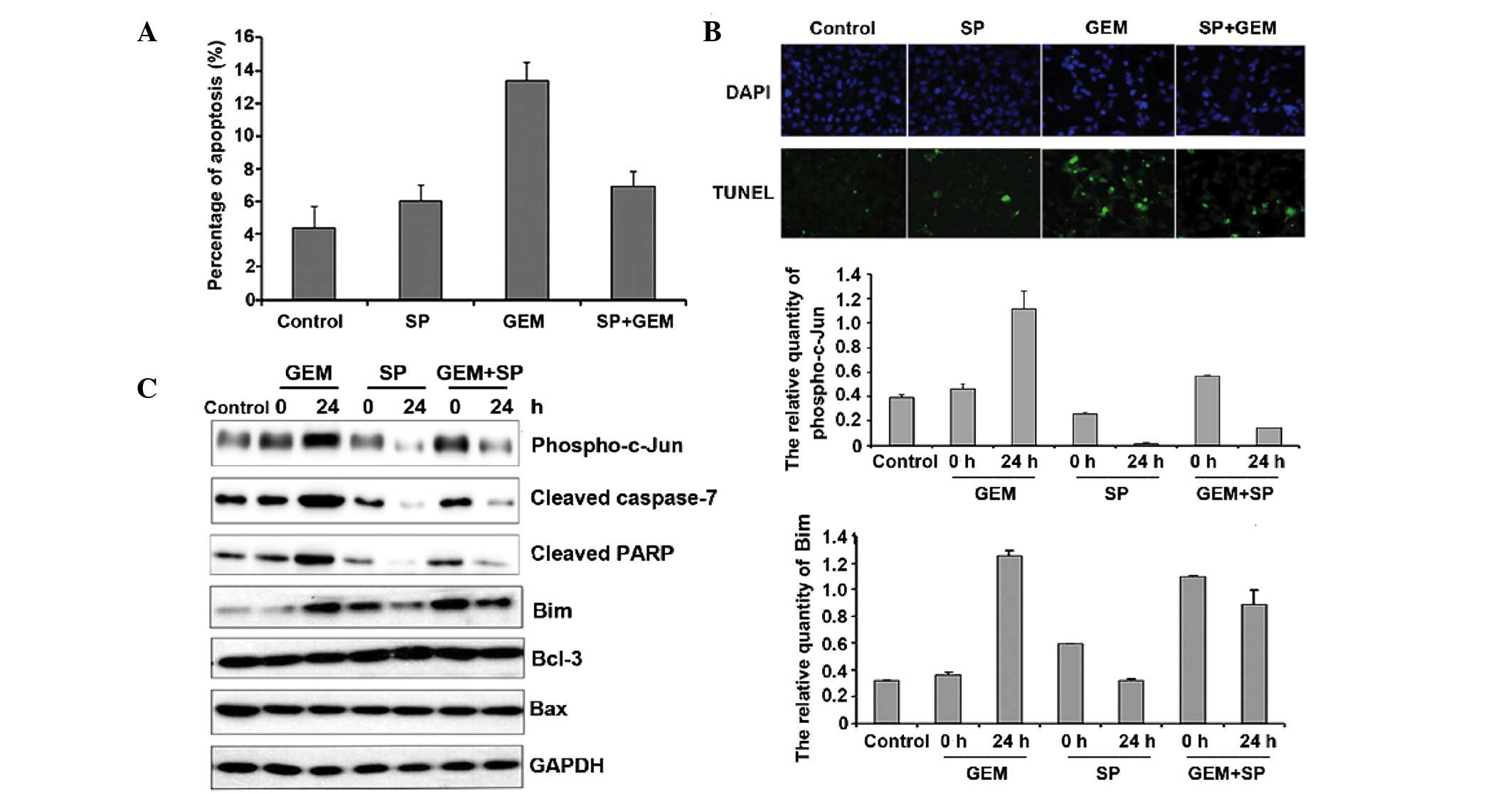
Reduced c-Jun activity inhibits
gemcitabine-induced apoptosis by decreasing Bim expression
In addition to the expression level, the activity of
c-Jun is also critical for its function. Therefore, we treated
cells with SP600125, which blocks c-Jun phosphorylation. We exposed
Panc-1 cells to gemcitabine for 24 h in the presence or absence of
20 µM (24) SP600125 and examined
apoptosis by FACS analysis. The results revealed that SP600125
alone had little effect on Panc-1 cells, but SP600125 pre-treatment
partially decreased gemcitabine-mediated apoptosis (Fig. 6A). TUNEL staining was used to further
confirm the apoptosis results and the data also indicated that
SP600125 pre-treatment partially decreased gemcitabine-mediated
apoptosis (Fig. 6B). SP600125
attenuated PARP and cleaved caspase 7 levels (Fig. 6C). These results suggest that c-Jun
activation is required for gemcitabine-induced apoptosis. Moreover,
compared with the uniform Bcl-3 and Bax expression, a
gemcitabine-induced increase in Bim expression was attenuated upon
SP600125 treatment.
Discussion
Pancreatic cancer is one of the most aggressive
diseases due to the difficulties in early detection and the low
resection rate (7,25). At present, ~40% of patients have
metastatic disease, and these patients are primarily treated with
palliative therapy (26). The current
chemotherapeutic agent of choice for pancreatic cancer is
gemcitabine, which was approved by the FDA in 1996. Gemcitabine
confers a median survival advantage of 6 months (27), and 5-fluorouracil contributes to an
improvement of only 1 month over gemcitabine. Erlotinib targets
epidermal growth factor receptor and adds only two additional weeks
to the average overall survival time (28). Since gemcitabine remains the first
line of chemotherapy, an understanding of its molecular mechanism
is essential in developing new therapeutic approaches.
Chemotherapy resistance occurs primarily due to
cellular evasion of apoptosis, one of the characteristics of
cancer. Apoptosis is a genetically controlled physiological process
characterised by its morphology and biochemical events, including
cellular shrinkage, chromatin condensation and apoptotic body
formation (29). In the development
and regulation of multicellular organisms, apoptosis is an active
and well-defined programmed cell death. However, the balance
between proliferation and apoptosis is disrupted at certain stages
in tumour development. The imbalance leads to deregulated cell
proliferation and subsequent tumour formation (30). Therefore, induction of tumour cell
apoptosis with limited or minimal toxicity to surrounding normal
cells has been recognised as an effective cancer chemotherapy
target. In the present study, we screened various gemcitabine
concentrations in two pancreatic cancer cell lines and calculated
the IC50 value. Considering the gemcitabine
concentrations used in the clinic, we used 10 µM gemcitabine in
subsequent experiments to induce various degrees of apoptosis
throughout the time course.
Gemcitabine sensitivity has been reported to
correlate with the activation of the p38 (31), c-Jun N-terminal kinase (32) and ERK (33) signalling pathways. Significantly, the
AP-1 transcription factor is at the hub of the signalling network.
Jun proteins preferentially regulate genes involved in
proliferation and apoptosis, including Bim (34), Bcl-3 (35) and cyclin D1 (36), whereas Fos proteins, including DNA
(cytosine-5)-methyltransferase 1 (37) and matrix metalloproteinase 1, are
often required for angiogenesis and invasion by malignant tumours
(38). To clarify the factors
involved in gemcitabine-induced apoptosis, we analysed c-Jun and
c-Fos. Western blot analysis demonstrated that gemcitabine
increased c-Jun expression, but had little effect on c-Fos. Flow
cytometric analysis and the alterations in cleaved PARP and caspase
7 expression revealed that c-Jun promotes gemcitabine-induced
apoptosis.
Bcl-2 protein family members regulate apoptotic
mitochondrial events (39). c-Jun has
been demonstrated to lead to induction of pro-apoptotic molecules,
including Bim. However, c-Jun functions as an anti-apoptotic or
pro-apoptotic factor depending on the downstream targets.
Immunoblotting assays indicated that c-Jun overexpression enhanced
Bim activity, and the increase in Bim was inhibited when c-Jun
expression decreased. However, other pro-apoptotic and
anti-apoptotic factors, including Bcl-3 and Bax, were not affected
by c-Jun (21). We also used SP600125
to reduce c-Jun activity and compared its effect with that of
gemcitabine treatment. The results demonstrated that SP600125
treatment inhibited gemcitabine-induced apoptosis and attenuated
the increase in Bim expression. Gemcitabine is a nucleoside
analogue that causes cytotoxicity by inducing DNA replication
blocks (40), and Bim suppression was
reported to reduce cyclins and cyclin-dependent kinases, which
would control DNA replication (41).
Taken together, c-Jun regulates gemcitabine-induced apoptosis in
pancreatic cancer by activating its downstream target Bim.
In summary, our results indicate that c-Jun is a
pro-apoptotic protein that promotes gemcitabine-induced cell
apoptosis by upregulating Bim activity and expression. Our study is
the first to implicate the AP-1 pathway in gemcitabine-induced
apoptosis, and we suggest that it plays the most significant role
since c-Jun is at the crossroads with several signalling pathways.
The effect of c-Jun in regulating gemcitabine-induced apoptosis is
recognised for its potential value in treating pancreatic
cancer.
Acknowledgements
This study was supported by a grant for the youth
from the National Natural Science Foundation of China (81201734),
the Research Special Fund for the Public Welfare Industry of Health
(201402001), and the Research Fund for the Doctoral Program of
Higher Education (20131106110008 and 20121106120048).
References
|
1
|
Jones OP, Melling JD and Ghaneh P:
Adjuvant therapy in pancreatic cancer. World J Gastroenterol.
20:14733–14746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silvestris N, Gnoni A, Brunetti AE,
Vincenti L, Santini D, Tonini G, Merchionne F, Maiello E, Lorusso
V, Nardulli P, et al: Target therapies in pancreatic carcinoma.
Curr Med Chem. 21:948–965. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thota R, Pauff JM and Berlin JD: Treatment
of metastatic pancreatic adenocarcinoma: a review. Oncology
(Williston Park). 28:70–74. 2014.PubMed/NCBI
|
|
6
|
Lima CM Rocha, Green MR, Rotche R, Miller
WH Jr, Jeffrey GM, Cisar LA, Morganti A, Orlando N, Gruia G and
Miller LL: Irinotecan plus gemcitabine results in no survival
advantage compared with gemcitabine monotherapy in patients with
locally advanced or metastatic pancreatic cancer despite increased
tumor response rate. J Clin Oncol. 22:3776–3783. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu J, Wang T, Cao Z, Huang H, Li J, Liu W,
Liu S, You L, Zhou L, Zhang T and Zhao Y: MiR-497 downregulation
contributes to the malignancy of pancreatic cancer and associates
with a poor prognosis. Oncotarget. 5:6983–6993. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Al-Hajeili M, Azmi AS and Choi M:
Nab-paclitaxel: potential for the treatment of advanced pancreatic
cancer. Onco Targets Ther. 7:187–192. 2014.PubMed/NCBI
|
|
9
|
Chiorean EG and Von Hoff DD: Taxanes:
impact on pancreatic cancer. Anticancer Drugs. 25:584–592. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sherman MH, Yu RT, Engle DD, Ding N,
Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S,
et al: Vitamin D receptor-mediated stromal reprogramming suppresses
pancreatitis and enhances pancreatic cancer therapy. Cell.
159:80–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Z, Li D, Zheng X, Wang E and Wang J:
Selective induction of apoptosis: promising therapy in pancreatic
cancer. Curr Pharm Des. 19:2259–2268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bianco R, Melisi D, Ciardiello F and
Tortora G: Key cancer cell signal transduction pathways as
therapeutic targets. Eur J Cancer. 42:290–294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nicholson DW: From bench to clinic with
apoptosis-based therapeutic agents. Nature. 407:810–816. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao C, Qiao Y, Jonsson P, Wang J, Xu L,
Rouhi P, Sinha I, Cao Y, Williams C and Dahlman-Wright K:
Genome-wide profiling of AP-1-regulated transcription provides
insights into the invasiveness of triple-negative breast cancer.
Cancer Res. 74:3983–3994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shaulian E: AP-1 - the Jun proteins:
oncogenes or tumor suppressors in disguise? Cell Signal.
22:894–899. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chinenov Y and Kerppol TK: Close
encounters of many kinds: Fos-Jun interactions that mediate
transcription regulatory specificity. Oncogene. 20:2438–2452. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kolbus A, Herr I, Schreiber M, Debatin KM,
Wagner EF and Angel P: c-Jun-dependent CD95-L expression is a
rate-limiting step in the induction of apoptosis by alkylating
agents. Mol Cell Biol. 20:575–582. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ren X, Song W, Liu W, Guan X, Miao F, Miao
S and Wang L: Rhomboid domain containing 1 inhibits cell apoptosis
by upregulating AP-1 activity and its downstream target Bcl-3. FEBS
Lett. 587:1793–1798. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi R, Peng H, Yuan X, Zhang X, Zhang Y,
Fan D, Liu X and Xiong D: Down-regulation of c-fos by shRNA
sensitizes adriamycin-resistant MCF-7/ADR cells to chemotherapeutic
agents via P-glycoprotein inhibition and apoptosis augmentation. J
Cell Biochem. 114:1890–1900. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leijen S, Veltkamp SA, Huitema AD, van
Werkhoven E, Beijnen JH and Schellens JH: Phase I dose-escalation
study and population pharmacokinetic analysis of fixed dose rate
gemcitabine plus carboplatin as second-line therapy in patients
with ovarian cancer. Gynecol Oncol. 130:511–517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meng Q and Xia Y: c-Jun, at the crossroad
of the signaling network. Protein Cell. 2:889–898. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Passegué E, Jochum W, Schorpp-Kistner M,
Möhle-Steinlein U and Wagner EF: Chronic myeloid leukemia with
increased granulocyte progenitors in mice lacking junB expression
in the myeloid lineage. Cell. 104:21–32. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kashatus D, Cogswell P and Baldwin AS:
Expression of the Bcl-3 proto-oncogene suppresses p53 activation.
Genes Dev. 20:225–235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li J, Liang X and Yang X: Ursolic acid
inhibits growth and induces apoptosis in gemcitabine-resistant
human pancreatic cancer via the JNK and PI3K/Akt/NF-κB pathways.
Oncol Rep. 28:501–510. 2012.PubMed/NCBI
|
|
25
|
Dai MH, Liu SL, Chen NG, Zhang TP, You L,
Q Zhang F, Chou TC, Szalay AA, Fong Y and Zhao YP: Oncolytic
vaccinia virus in combination with radiation shows synergistic
antitumor efficacy in pancreatic cancer. Cancer Lett. 344:282–290.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chugh R, Sangwan V, Patil SP, Dudeja V,
Dawra RK, Banerjee S, Schumacher RJ, Blazar BR, Georg GI, Vickers
SM and Saluja AK: A preclinical evaluation of Minnelide as a
therapeutic agent against pancreatic cancer. Sci Transl Med.
4:156ra1392012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Trouilloud I, Dubreuil O, Boussaha T,
Lepère C, Landi B, Zaanan A, Bachet JB and Taieb J: Medical
treatment of pancreatic cancer: new hopes after 10 years of
gemcitabine. Clin Res Hepatol Gastroenterol. 35:364–374. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee JG and Wu R: Erlotinib-cisplatin
combination inhibits growth and angiogenesis through c-MYC and
HIF-1α in EGFR-mutated lung cancer in vitro and in vivo. Neoplasia.
17:190–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Elmore S: Apoptosis: a review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Evans GL and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bu HQ, Luo J, Chen H, Zhang JH, Li HH, Guo
HC, Wang ZH and Lin SZ: Oridonin enhances antitumor activity of
gemcitabine in pancreatic cancer through MAPK-p38 signaling
pathway. Int J Oncol. 41:949–958. 2012.PubMed/NCBI
|
|
32
|
Teraishi F, Zhang L, Guo W, Dong F, Davis
JJ, Lin A and Fang B: Activation of c-Jun NH2-terminal kinase is
required for gemcitabine's cytotoxic effect in human lung cancer
H1299 cells. FEBS Lett. 579:6681–6687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang XL, Lin FJ, Guo YJ, Shao ZM and Ou
ZL: Gemcitabine resistance in breast cancer cells regulated by
PI3K/AKT-mediated cellular proliferation exerts negative feedback
via the MEK/MAPK and mTOR pathways. Onco Targets Ther. 7:1033–1042.
2014.PubMed/NCBI
|
|
34
|
Wang H, Yang YB, Shen HM, Gu J, Li T and
Li XM: ABT-737 induces Bim expression via JNK signaling pathway and
its effect on the radiation sensitivity of HeLa cells. PLoS One.
7:e524832012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rebollo A, Dumoutier L, Renauld JC,
Zaballos A, Ayllón V and Martínez-A C: Bcl-3 expression promotes
cell survival following interleukin-4 deprivation and is controlled
by AP1 and AP1-like transcription factors. Mol Cell Biol.
20:3407–3416. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang W, Du Z, Yan J, Ma D, Shi M, Zhang M,
Peng C and Li H: Mesenchymal stem cells promote liver regeneration
and prolong survival in small-for-size liver grafts: involvement of
C-Jun N-terminal kinase, cyclin D1, and NF-κB. PLoS One.
9:e1125322014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Niu Q, Liu H, Guan Z, Zeng Q, Guo S, He P,
Guo L, Gao P, Xu B, Xu Z, et al: The effect of c-Fos demethylation
on sodium fluoride-induced apoptosis in L-02 cells. Biol Trace Elem
Res. 149:102–109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Renaud SJ, Kubota K, Rumi MA and Soares
MJ: The FOS transcription factor family differentially controls
trophoblast migration and invasion. J Biol Chem. 289:5025–5039.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ganesan V and Colombini M: Regulation of
ceramide channels by Bcl-2 family proteins. FEBS Lett.
584:2128–2134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Smith SC, Petrova AV, Madden MZ, Wang H,
Pan Y, Warren MD, Hardy CW, Liang D, Liu EA, Robinson MH, et al: A
gemcitabine sensitivity screen identifies a role for NEK9 in the
replication stress response. Nucleic Acids Res. 42:11517–11527.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gautam S, Kirschnek S, Wiesmeier M, Vier J
and Häcker G: Roscovitine-induced apoptosis in neutrophils and
neutrophil progenitors is regulated by the Bcl-2-family members
Bim, Puma, Noxa and Mcl-1. PLoS One. 8:e793522013. View Article : Google Scholar : PubMed/NCBI
|