Introduction
Lung cancer is the primary cause of
cancer-associated mortality worldwide (1) and non-small cell lung cancer (NSCLC)
accounts for ~85% of all lung cancer cases. NSCLC is divided into
two major types, squamous cell carcinoma and non-squamous cell
carcinoma. Non-squamous cell carcinoma is comprised of a number of
subtypes, including adenocarcinoma and large-cell carcinoma.
Adenocarcinoma is the most frequent non-squamous cell carcinoma and
its prevalence is increasing (2).
For patients with advanced NSCLC that exhibit a
negative or unknown EGFR mutational status or ALK rearrangements,
platinum-based double chemotherapy is the typical first-line
therapy (3,4). In the treatment of non-squamous cell
carcinoma, pemetrexed combined with platinum demonstrates a
significantly improved toxicity profile and OS, compared with
taxane- or gemcitabine-based regimes (3,5,6). Nevertheless, a number of patients do not
benefit from pemetrexed and platinum-based therapy, due to
insensitivity or high toxicity to the combination (7–9).
Therefore, it is necessary to identify patients unlikely to benefit
from this treatment in order to avoid unnecessary treatment.
Previous studies have demonstrated that excision repair
cross-complementation group 1 (ERCC-1), breast cancer type 1
susceptibility protein (BRCA-1), ribonucleotide reductase catalytic
subunit M1 (RRM-1) and thymidylate synthase (TS) are predictive
markers of the chemotherapeutic effect of platinum agents, taxanes,
gemcitabine and pemetrexed (10–14).
However, the predictiveness of ERCC-1, RRM-1 and BRCA-1 has not
been reproducible (15–18). There is a particular need for further
phase III studies of TS to validate the reproducibility of the
applied immunohistochemistry and scoring systems for its use as a
predictive marker (18).
Consequently, it is important to identify novel biomarkers for the
prediction of chemotherapeutic benefit.
MicroRNAs (miRs) are small noncoding RNAs, of
between 19 and 25 nucleotides in length, which bind to the
3′-untranslated region of mRNAs to regulate their translation. miRs
are involved in numerous essential biological processes and are
associated with a number of types of cancer and disease (19). Numerous studies have demonstrated that
miRs are dysregulated in cancer, such as that of the lung (20–23). As
circulating miRs are stable in blood plasma, they have potential
applications as diagnostic markers (24,25).
Increasing evidence has shown circulating miR profile analysis can
aid in early diagnosis, staging, tracking and prognosis of
different types of cancer (26–32). In
addition, circulating miR profile analysis is useful in predicting
the response to chemotherapy (33,34).
The present study aimed to determine which plasma
miRs may be used to predict the clinical outcome of pemetrexed and
platinum treatment for advanced lung adenocarcinoma (LAC). In
contrast with previous research, patient benefit to the treatment
was examined instead of tumor response. Seidman et al
(35) observed that the overall
survival (OS) of patients with stable metastatic breast cancer
resembled that of patients with complete remission (CR) or partial
remission (PR), indicating that similar benefits to remission may
come from stable disease (SD). Therefore, benefit (CR+PR+SD) may be
deemed a more accurate indicator of treatment efficacy than tumor
response (36). A miR microarray and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) were used to identify and verify potential markers in
training and validation sets following screening. It was
demonstrated that high plasma expression levels of miR-25, miR-21,
miR-27b and miR-326 may predict non-benefit from chemotherapy, and
that increased levels of these miRs was inversely correlated with
progression-free survival (PFS).
Materials and methods
Study participants
A total of 129 participants (Table I) diagnosed with stage IIIB-IV LAC
were recruited from the Jiangsu Cancer Institute and Hospital
(Nanjing, China) between September 2010 and January 2013. All
patients had histological or cytological confirmation of their
tumor diagnosis. Tumors were staged based on the Seventh Edition
Tumor-Node-Metastasis Staging System of the American Joint
Committee on Cancer (37). All
patients received first-line chemotherapy, pemetrexed (500 mg/m2)
on day 1 with either cisplatin (75 mg/m2) or car-boplatin [area
under the curve (AUC)=5] on day 2 of a 21-day treatment cycle. All
patients experienced ≥2 cycles of chemotherapy. Therapeutic
response was evaluated by computed tomography following 2 cycles of
treatment, according to Response Evaluation Criteria in Solid
Tumors 1.1 (38). Response was
classified as PR, CR, SD or progressive disease (PD). Patients
classified as CR, PR or SD for ≥4 weeks were assigned to the
benefit group. Conversely, patients classified as PD were assigned
to the non-benefit group.
 | Table I.Demographic and clinical
characteristics of the non-benefit and benefit groups. |
Table I.
Demographic and clinical
characteristics of the non-benefit and benefit groups.
|
| Profiling set | Testing set | Validation set |
|---|
|
|
|
|
|
|---|
| Characteristic | Non-benefit | Benefit | Non-benefit | Benefit | P-value | Non-benefit | Benefit | P-value |
|---|
| Number of
patients | 4 | 4 | 15 | 29 |
| 26 | 51 |
|
| Mean age, (years ±
standard error) |
43±1.2 | 45±4.4 | 55±10.1 | 56±8.8 | 0.717a | 60±10.1 | 55±11.2 | 0.159a |
| Gender (no.) |
|
|
Male | 3 | 3 | 10 | 18 | 0.764b | 18 | 31 | 0.466b |
|
Female | 1 | 1 | 5 | 11 |
| 8 | 20 |
|
| Stage of LAC |
|
|
IIIB | 0 | 0 | 2 | 3 | 1.000c | 3 | 7 | 0.617c |
| IV | 4 | 4 | 13 | 26 |
| 23 | 44 |
|
| Smoking status |
|
|
Yes | 0 | 0 | 0 | 0 |
| 10 | 21 | 0.818b |
| No | 4 | 4 | 15 | 29 |
| 16 | 30 |
|
| EGFR genotype |
|
|
Wild-type | 1 | 0 | 1 | 3 | 1.000c | 4 | 5 | 0.477c |
|
Unknown | 3 | 4 | 14 | 26 |
| 22 | 46 |
|
| Prior serious or
chronic disease history |
|
|
Yes | 0 | 0 | 0 | 0 |
| 11 | 22 | 0.945b |
| No | 4 | 4 | 15 | 29 |
| 15 | 29 |
|
| KPS score |
|
|
≥80 | 4 | 4 | 15 | 29 |
| 26 | 51 |
|
<80 | 0 | 0 | 0 | 0 |
| 0 | 0 |
|
| Relative dose
intensity (%) |
|
|
Pemetrexed | 100.0 | 97.0 | 98.0 | 97.7 |
| 98.5 | 97.6 |
|
|
Carboplatin | 71.5 | 62.5 | 91.0 | 78.7 |
| 87.2 | 86.1 |
|
|
Cisplatin | 87.0 | 100.0 | 93.2 | 92.6 |
| 94.1 | 91.6 |
|
| Maintenance
therapy |
|
|
Yes | 0 | 2 | 0 | 19 |
| 0 | 24 |
|
| No | 4 | 2 | 15 | 10 |
| 26 | 27 |
|
Plasma samples from all patients were collected
prior to chemotherapy, between September 2010 and January 2013. A
miR microarray was used to screen the plasma miR expression
profiles of a screening set of eight patients prior to and
following treatment. Specifically, plasma miR-25, miR-21, miR-27b,
miR-326, miR-483-5p and miR-920 were selected for analysis in a
training set (n=44) prior to treatment. The screening and training
set patients were all non-smokers with no prior history of serious
or chronic disease. Consequently, the ∆∆Cq values of miR-25,
miR-21, miR-27b and miR-326 were further determined in a validation
set (n=77), which included smokers and patients with a prior
history of serious or chronic disease. Patients were observed until
December 2014. The present study was approved by the Medical Ethics
Committee of Jiangsu Cancer Institute and Hospital and all
participants provided informed consent.
Total RNA isolation
Whole blood samples (5 ml per patient) were
collected in anticoagulant tubes and centrifuged at 1,811 × g for 5
min at 4°C. The supernatant was subsequently transferred into an
RNase/DNase-free 1 ml microfuge tube and immediately stored at
−80°C until required. As there is no established endogenous miR
control for blood plasma, prior to the isolation process,
cel-miR-238 (UUUGUACUCCGAUGCCAUUCAGA; Takara Biotechnology Co.,
Ltd., Dalian, China), a synthetic Caenorhabditis elegans miR, was
mixed with every sample as an internal control, at a final
concentration of 25 fmol. Separation of total RNA from the plasma
was performed using the NucleoSpin miRNA Plasma kit (Machery-Nagel
GmbH, Düren, Germany), according to the manufacturer's
instructions. RNA purity and quantity was determined using a
NanoDrop spectrophotometer (ND-1000; Thermo Fisher Scientific,
Inc., Waltham, MA, USA).
miR microarray analysis
The isolated RNAs were labeled using the miRCURY
Hy3/Hy5 Power Labeling kit (cat. no. 208520), and the Hy3TM-labeled
samples were then hybridized, following the manufacturer's
protocol, to the miRCURYTM LNA Array (cat. no. 208031-A) (both
Exiqon A/S, Vedbaek, Denmark). This array covered miRs annotated in
miRBase version 18.0 (www.mirbase.org), comprising of 3,100 capture probes,
including all human, rat and mouse miR genes. Subsequently, the
Axon GenePix 4000B Microarray Scanner (Molecular Devices, LLC,
Sunnyvale, CA, USA) was used to scan the slides at a wavelength of
532 nm. Image files were then transferred to GenePix Pro version
6.0 software (Molecular Devices, LLC) for further analysis.
Replicated miR readings were averaged and miRs with intensities ≥50
were selected for normalization using the median normalization
method (39). Subsequently, fold
change filtering identified differentially expressed miRs. All miR
microarray analysis was performed by KangChen Bio-tech (Shanghai,
China).
Quantification and confirmation of
candidate miRs using RT-qPCR
Plasma sample miRs were quantified using RT-qPCR.
Briefly, 1.67 µl RNA (50 ng) was included in a 5 µl reaction
mixture using the TaqMan MicroRNA Reverse Transcription kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and a RT
primer (TaqMan® MicroRNA Assay; Applied Biosystems;
Thermo Fisher Scientific, Inc.) to convert miRNA to cDNA. The RT
thermocycling conditions were as follows: 30 min at 16°C; 30 min at
42°C; 5 min at 85°C; and 4°C until required. Subsequently, qPCR was
carried out in a volume of 10 µl containing 4.5 µl diluted cDNA
(1:15), 5 µl TaqMan Universal PCR Master Mix (No AmpErase UNG),
AmpliTaq Gold® DNA Polymerase and 0.5 µl PCR probes
(TaqMan® MicroRNA Assay; Applied Biosystems; Thermo
Fisher Scientific, Inc.). qPCR was performed using a 7900 Fast
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). qPCR thermocycling conditions were as follows: 10 min at
95°C; 40 cycles of 95°C for 15 sec; and 60°C for 1 min. Each sample
was run in duplicate and the mean Cq value was determined. The
relative miRs expression level (Log2 relative expression level) was
calculated using the 2-∆∆Cq method (40), where ∆Cq was calculated as follows: Cq
(target miR)-Cq (cel-miR-238).
Statistical analysis
Fisher's exact test, a two-sided χ2 test and
Pearson's χ2 test were used to evaluate differences in the
clinicopathological factors between groups. To compare the
expression levels of different miRs between the benefit and
non-benefit groups, the Mann-Whitney U test was used. To estimate
the diagnostic potential of plasma miRs, receiver-operating
characteristics (ROC) curves were produced. The predictive power
was estimated by calculating the AUC of the ROCs, and the maximum
value of the Youden's index was used as a criterion for selecting
the optimum cut-off point (41). The
Kaplan-Meier estimator was used to evaluate PFS and compare log
rank statistics. SPSS software (version 19.0; IBM SPSS, Armonk, NY,
USA) was used for statistical analysis. Results are presented as
the mean ± standard deviation. P<0.05 was considered to indicate
a statistically significant difference.
Results
Patient characteristics
Patient characteristics are presented in Table I. The demographics and clinical
characteristics of patients in the benefit and non-benefit groups
were similar in regards to age, gender, smoking status, tumor
stage, EGFR/ALK state, prior serious/chronic disease history and
Karnofsky performance status score. The relative dose intensity of
each drug was the real dose (actual dose used): Ideal dose (the
dose planned) ratio. PFS time was calculated from the date of
initiation of chemotherapy to the date of the last follow-up, to
the date of detected progression or date of death owing to any
cause. Among the patients, 13 could not be evaluated for PFS due to
requiring further radiotherapy or being untraceable for
follow-ups.
miR expression profiles in the plasma
of patients with LAC prior to and following chemotherapy
miR expression profiles were assessed in 8 patients
(benefit group, n=4; non-benefit group, n=4), prior to and
following treatment, in duplicate, to give a total of 16 plasma
samples. The results showed that, prior to treatment, there were
312 upregulated miRs (fold change ≥2.0) in the benefit group and
233 upregulated miRs in the non-benefit group. Following treatment,
there were 213 upregulated and 186 downregulated miRs in the
benefit group, and 188 upregulated and 157 downregulated miRs in
the non-benefit group. The miRs selected for further confirmation
by RT-qPCR displayed the following: A 10-fold change in expression
between the benefit and non-benefit groups; >2-fold change in
expression in the same group between pre- and post-treatment; and
an association with cancer in published literature. A total of 6 of
the differentially expressed miRs (miR-483-5p, miR-920, upregulated
in the benefit group; miR-21, miR-27b, miR-326, miR-25, upregulated
in the non-benefit group) were further analyzed. Relative
expressions of these 6 miRs are listed in Table II.
 | Table II.Relative plasma expression of miRNAs
from microarray analysis. |
Table II.
Relative plasma expression of miRNAs
from microarray analysis.
|
| Relative plasma
expression (fold change) |
|---|
|
|
|
|---|
| miR type | B prior to
treatment: NB prior to treatment | B prior to
treatment: B following treatment | NB prior to
treament: NB following treatment |
|---|
| miR-21 | 0.018830 | 16.524080 | 0.474396 |
| miR-27b | 0.013600 | 40.662910 | <2.000000 |
| miR-326 | 0.030405 | <2.000000 | 0.483120 |
| miR-25 | 0.093175 | 12.106610 | <2.000000 |
| miR-483-5p | 17.768710 | 0.109332 | 4.362240 |
| miR-920 | 22.678370 | 0.027606 | 2.048308 |
miR expression in the training
set
RT-qPCR was used to confirm the expression levels of
6 candidate miRs in a training set (benefit, n=29; non-benefit,
n=15). To avoid the effect of compounding factors, all patients
recruited were non-smokers with no prior history of serious or
chronic disease. The ∆∆Cq values and relative expression levels of
the candidate miRs was calculated (Fig.
1A), confirming the screening results of miR-25, miR-27b,
miR-21 and miR-326. However, the relative expression levels of
miR-483-5p and miR-920 were inconsistent with the results of the
microarray (data not shown). As shown in Fig. 1A, the ∆∆Cq values of miR-25, miR-27b,
miR-21 and miR-326 in patient plasma prior to treatment were
significantly increased in the benefit group compared with the
non-benefit group (P<0.001). Therefore, miR-25, miR-27b, miR-21
and miR-326 were selected for further validation.
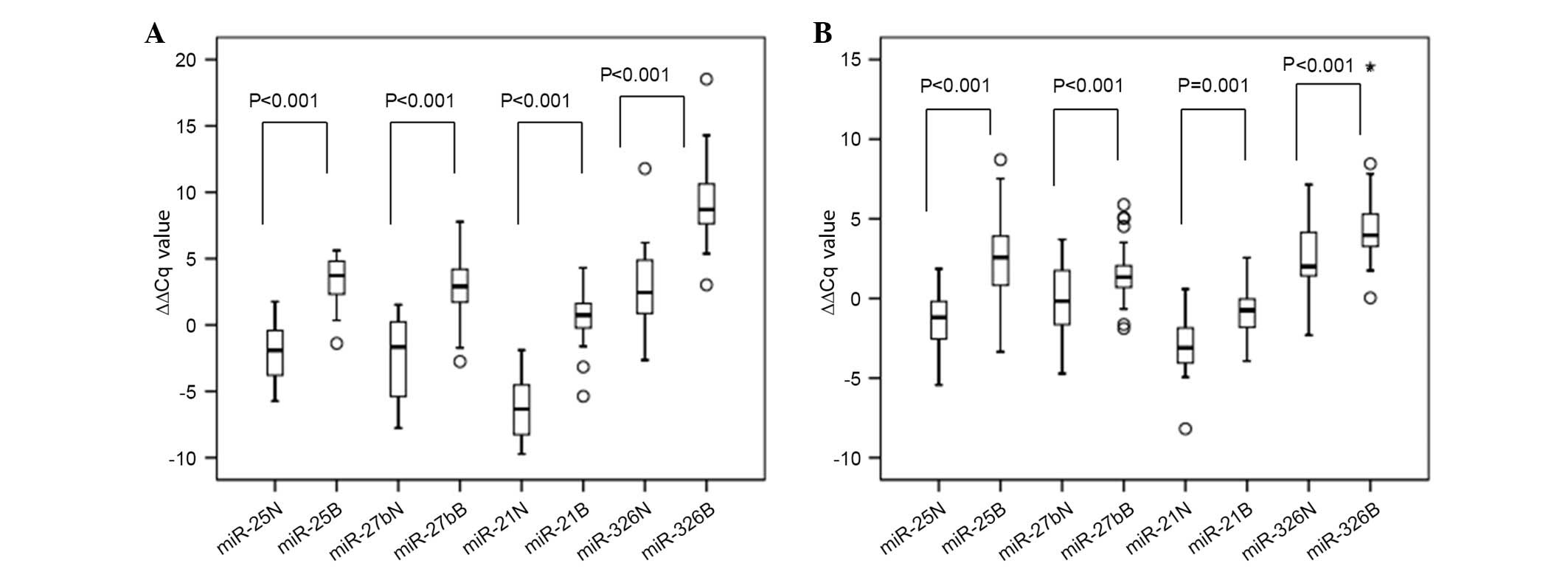 | Figure 1.Box-plots of plasma miR-25, miR-27b,
miR-21 and miR-326 expression ∆∆Cq values of the non-benefit and
benefit groups in the (A) training set (n=44), and (B) validation
set (n=77). Plasma miR expression levels were analyzed by reverse
transcription-quantitative polymerase chain reaction analysis and
∆∆Cq values were compared using the Mann-Whitney U test. ∆∆Cq
values for the 4 plasma miRs, prior to treatment, were
significantly upregulated in the benefit group compared with the
non-benefit group, in the training set and validation sets (ο,
outlier; *, abnormal value). Outliers: the deviation between the
measured value and the average value is less than two times the
standard deviation; abnormal: the deviation between the measured
value and the average value is more than two times the standard
deviation. miR, microRNA; N, non-benefit group; B, benefit
group. |
Confirmation of miR expression in the
validation set
As shown in Fig. 1B,
the ∆∆Cq values of plasma miR-25, miR-27b, miR-21 and miR-326 prior
to treatment were compared between the benefit and non-benefit
groups in a validation set (benefit, n=51; non-benefit, n=26). The
∆∆Cq values of these 4 miRs were significantly upregulated in the
benefit group compared with the non-benefit group (P≤0.001;
Fig. 1B). In addition, the trend in
the alteration of relative expression levels was similar to that
seen in the training set.
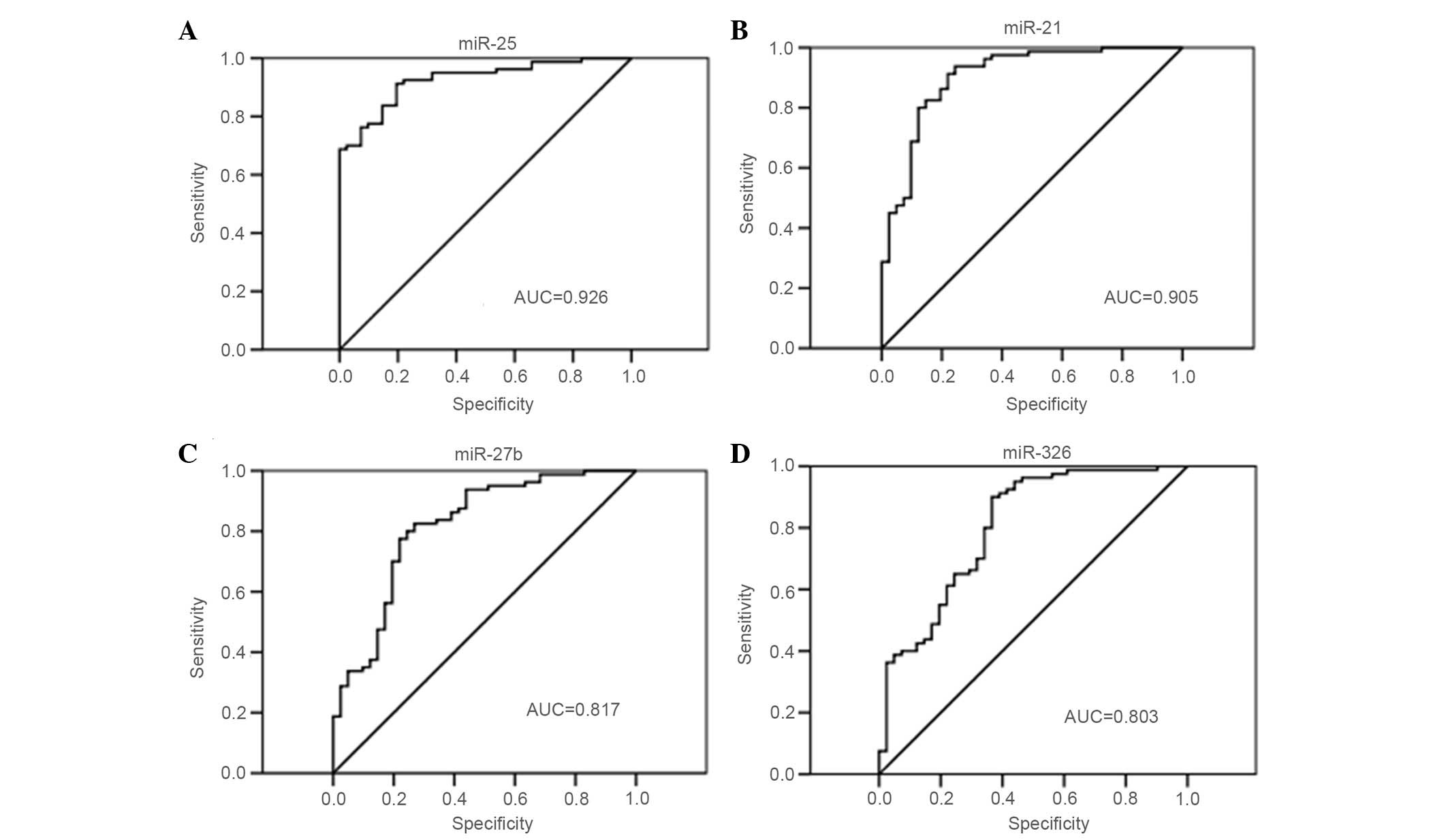
Evaluation of the diagnostic potential
of the candidate miRs
ROC curve analysis was performed on miR-25, miR-27b,
miR-21 and miR-326 in 121 patients to evaluate their suitability as
predictive biomarkers of pemetrexed and platinum insensitivity
(Fig. 2). The AUC of miR-25 was 0.926
[95% confidence interval (CI), 0.881–0.971], which was the highest
of the four miRs tested. The AUCs of miR-21, miR-27b and miR-326
were 0.905 (95% CI, 0.845–0.964), 0.817 (95% CI, 0.733–0.900) and
0.803 (95% CI, 0.717–0.890), respectively. The optimal cut-off
points were-0.171 (sensitivity, 91.3%; specificity, 80.5%) for
miR-25, 0.568 (sensitivity, 82.5%; specificity, 73.2%) for miR-27b,
−1.85 (sensitivity, 82.5%; specificity, 85.4%) for mir-21 and 3.05
(sensitivity, 90.0%; specificity, 63.4%) for mir-326. miR-25
exhibited the most accurate predictive power.
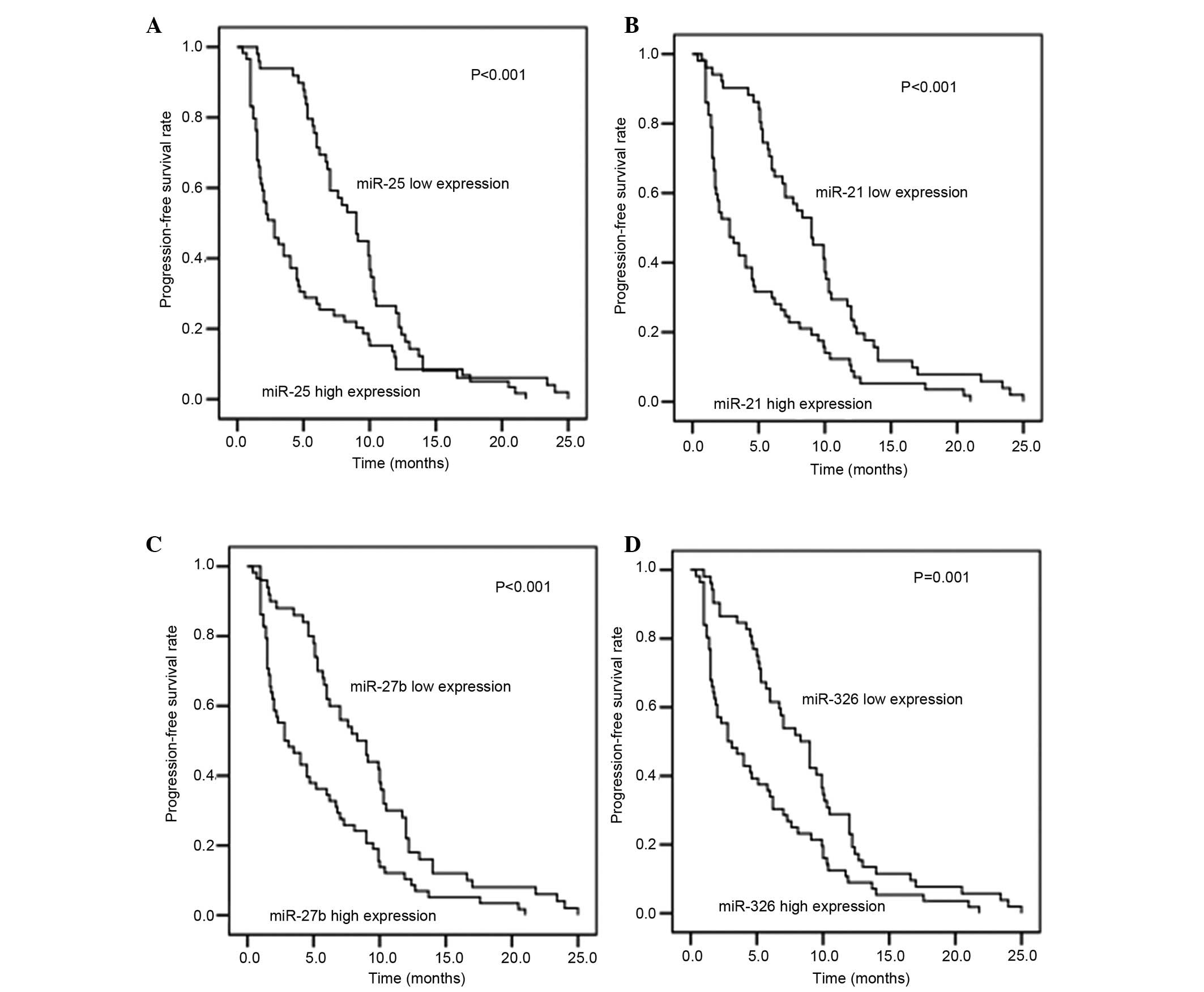
Prediction of PFS rate using plasma
miR expression levels
The association between plasma miR expression levels
and PFS rate in 108 patients (benefit group, n=67; non-benefit
group, n=41) was investigated. As shown in Fig. 3, the expression levels of miR-25,
miR-27b, miR-21 and miR-326 had a significant effect on the PFS
rate (all P≤0.001, high miR expression vs. low miR expression).
Plasma miR expression levels were inversely correlated with PFS,
indicating that increased expression of these miRs is associated
with decreased PFS.
Discussion
It is well established that miRs are stable in the
blood, and that circulating miRs may act as biomarkers for early
diagnosis and prognosis of human cancer (24–32).
Likewise, clinical studies have demonstrated that circulating miRs
may serve as predictors of resistance to anticancer agents
(33,34). In the present study, plasma miR
profiles were compared between benefit and non-benefit groups in
order to identify candidate circulating biomarkers that may be used
to predict non-benefit from first-line pemetrexed and platinum
therapy in patients with advanced LAC.
The expression profile of circulating miRs differs
markedly between individuals, disease states, types of cancer and
tissues. In the present study, to prevent the influence of this
diversity, patient plasma was compared prior to and following
chemotherapy using a microarray to measure target miR expression
levels. To avoid the compounding effect of nicotine, which may
alter the miR expression (42), and
comorbidities, including diabetes and hypertension, patients
exhibiting these characteristics were excluded from the screening
and the training sets. The results in the training set were
repeatable in the validation set.
From the results of the miR microarray screening, 6
miRs were selected for further analysis using RT-qPCR. It was
observed that in the training and validation sets, the plasma
expression levels of miR-25, miR-21, miR-27b and miR-326 prior to
treatment were significantly upregulated in the non-benefit group
compared with the benefit group. Furthermore, increased expression
of these four miRs was associated with poor PFS. The predictive
power of each miR was evaluated using ROC curves, in which miR-25
exhibited the highest degree of accuracy (AUC, 0.926; 95% CI,
0.881–0.971). Among these four miRs, miR-21 is recognized as a
marker of reduced therapeutic response and decreased survival in
patients with lung cancer (43–45). In
addition, miR-21 is associated with multidrug resistance (46), particularly to platinum in NSCLC
(47). In the present study, plasma
miR-21 expression levels were upregulated in the non-benefit group
compared with the benefit group, and high expression of miR-21 was
associated with poor PFS.
miR-25 is known to be dysregulated in various types
of cancer, and to have oncogenic and tumor suppressive functions
(48,49). In NSCLC cell lines and human tissue,
miR-25 expression is significantly increased compared with normal
lung cells or adjacent non-cancerous tissues, respectively
(50). Downregulation of miR-25 may
increase cisplatin sensitivity and suppress the growth of NSCLC
cells in vivo (51).
Furthermore, increased miR-25 expression has been associated with
poor OS in non-smoking females with LAC (52). The results of the present study
demonstrate that miR-25 is significantly upregulated in the blood
plasma of patients with LAC in the non-benefit group compared with
the benefit group, and that high plasma miR-25 expression is
associated with decreased PFS.
The expression level of miR-27b varies between
different types of cancer. miR-27b may be upregulated or
downregulated in chemoresistant cancer cells and tumor samples
(53–55). High expression levels of miR-27b in a
number of cancer samples have been reported to be associated with
good or bad prognoses (56,57). In previous studies miR-27b was found
to be downregulated in several NSCLC cell lines and lung cancer
tissues (58,59). In the present study, plasma miR-27b
was demonstrated to be significantly upregulated in patients with
LAC in the non-benefit group compared with the benefit group, and
high plasma expression of miR-27b was associated with decreased
PFS. Shen et al (60) reported
that a number of miRs did not exhibit similar expression patterns
in plasma and tumor tissue samples, suggesting that miR expression
may be altered by host-derived factors in response to the tumor and
metastases, in addition to by the tumor directly.
miR-326 is a suppressor of the Hedgehog, Notch and
mitogen-activated protein kinase signaling pathways that are
associated with brain tumors (61–63), and
may block expression of multidrug resistance-associated proteins in
breast cancer (64). Low expression
levels of miR-326 are correlated with poor OS in patients with
pathological grade III–IV glioma (65). In addition, miR-326 expression was
shown to be downregulated in metastatic compared with
non-metastatic primary loci in nude mouse NSCLC cells (66). Therefore, it may be possible to use
miR-326 expression levels to monitor bone metastasis in patients
with LAC (67). The results of the
present study are consistent with a previous study, which
demonstrated that high plasma expression of miR-27b, miR-148a and
miR-326 prior to treatment with 5-fluorouracil and oxaliplatin was
correlated with decreased PFS in patients with metastatic
colorectal cancer (68).
In the current study, the EGFR genotype of the
majority of patients was unknown. Previous studies have identified
an interaction between EGFR and miRs (69). For instance, miR-21 is positively
regulated by EGFR in cancer cells (70) and the gene encoding EGFR is a
potential target of miR-23b/27b (71). Therefore, EGFR genotype status may
influence miR expression levels. In the present study, the EGFR
genotype status (known or unknown) was similar in the non-benefit
and benefit groups, which may have reduced this effect to a certain
extent.
In conclusion, the results of the present study
demonstrate that overexpression of plasma miR-25, miR-21, miR-27b
and miR-326 in patients with advanced LAC is predictive of
non-benefit to pemetrexed and platinum therapy, and that increased
expression of these miRs is associated with decreased PFS. Among
these miRs, miR-25 exhibited the highest degree of accuracy in
predicting non-benefit, indicating that it is the most promising
biomarker. The results of the current study suggest that plasma
miRs may be used as minimally invasive independent molecular
biomarkers to predict non-benefit from chemotherapy and PFS rates
in patients with advanced LAC.
Acknowledgements
The authors thank Professor Tingting Wang of the
Immunology and Reproduction Biology Laboratory of Nanjing
University for her advice.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naive patients with advanced-stage non-small-cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JK, Hahn S, Kim DW, Suh KJ, Keam B,
Kim TM, Lee SH and Heo DS: Epidermal growth factor receptor
tyrosine kinase inhibitors vs conventional chemotherapy in
non-small cell lung cancer harboring wild-type epidermal growth
factor receptor: A meta-analysis. JAMA. 311:1430–1437. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scagliotti G, Brodowicz T, Shepherd FA,
Zielinski C, Vansteenkiste J, Manegold C, Simms L, Fossella F,
Sugarman K and Belani CP: Treatment-by-histology interaction
analyses in three phase III trials show superiority of pemetrexed
in nonsquamous non-small cell lung cancer. J Thorac Oncol. 6:64–70.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pennell NA: Selection of chemotherapy for
patients with advanced non-small cell lung cancer. Cleve Clin J
Med. 79:Electronic (Suppl 1). eS46–eS50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim YH, Hirabayashi M, Togashi Y, Hirano
K, Tomii K, Masago K, Kaneda T, Yoshimatsu H, Otsuka K, Mio T, et
al: Phase II study of carboplatin and pemetrexed in advanced
non-squamous, non-small-cell lung cancer: Kyoto thoracic oncology
research group trial 0902. Cancer Chemother Pharmacol. 70:271–276.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hartmann JT and Lipp HP: Toxicity of
platinum compounds. Expert Opin Pharmacother. 4:889–901. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gota V, Kavathiya K, Doshi K, Gurjar M,
Damodaran SE, Noronha V, Joshi A and Prabhash K: High plasma
exposure to pemetrexed leads to severe hyponatremia in patients
with advanced non small cell lung cancer receiving
pemetrexed-platinum doublet chemotherapy. Cancer Manag Res.
6:261–265. 2014.PubMed/NCBI
|
|
10
|
Olaussen KA, Dunant A, Fouret P, Brambilla
E, André F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH,
et al: DNA repair by ERCC1 in non-small-cell lung cancer and
cisplatin-based adjuvant chemotherapy. N Engl J Med. 355:983–991.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cobo M, Isla D, Massuti B, Montes A,
Sanchez JM, Provencio M, Viñolas N, Paz-Ares L, Lopez-Vivanco G,
Muñoz MA, et al: Customizing cisplatin based on quantitative
excision repair cross-complementing 1 mRNA expression: A phase III
trial in non-small-cell lung cancer. J Clin Oncol. 25:2747–2754.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boukovinas I, Papadaki C, Mendez P, Taron
M, Mavroudis D, Koutsopoulos A, Sanchez-Ronco M, Sanchez JJ,
Trypaki M, Staphopoulos E, et al: Tumor BRCA1, RRM1 and RRM2 mRNA
expression levels and clinical response to first-line gemcitabine
plus docetaxel in non-small-cell lung cancer patients. PLoS One.
3:e36952008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carser JE, Quinn JE, Michie CO, O'Brien
EJ, McCluggage WG, Maxwell P, Lamers E, Lioe TF, Williams AR,
Kennedy RD, et al: BRCA1 is both a prognostic and predictive
biomarker of response to chemotherapy in sporadic epithelial
ovarian cancer. Gynecol Oncol. 123:492–498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nicolson MC, Fennell DA, Ferry D, O'Byrne
K, Shah R, Potter V, Skailes G, Upadhyay S, Taylor P, André V, et
al: Thymidylate synthase expression and outcome of patients
receiving pemetrexed for advanced nonsquamous non-small-cell lung
cancer in a prospective blinded assessment phase II clinical trial.
J Thorac Oncol. 8:930–939. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Friboulet L, Olaussen KA, Pignon JP,
Shepherd FA, Tsao MS, Graziano S, Kratzke R, Douillard JY, Seymour
L, Pirker R, et al: ERCC1 isoform expression and DNA repair in
non-small-cell lung cancer. N Engl J Med. 368:1101–1110. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bepler G, Williams C, Schell MJ, Chen W,
Zheng Z, Simon G, Gadgeel S, Zhao X, Schreiber F, Brahmer J, et al:
Randomized international phase III trial of ERCC1 and RRM1
expression-based chemotherapy versus gemcitabine/carboplatin in
advanced non-small-cell lung cancer. J Clin Oncol. 31:2404–2412.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moran T, Wei J, Cobo M, Qian X, Domine M,
Zou Z, Bover I, Wang L, Provencio M, Yu L, et al: Two
biomarker-directed randomized trials in European and Chinese
patients with nonsmall-cell lung cancer: The BRCA1-RAP80 Expression
Customization (BREC) studies. Ann Oncol. 25:2147–2155. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kerr KM, Bubendorf L, Edelman MJ,
Marchetti A, Mok T, Novello S, O'Byrne K, Stahel R, Peters S and
Felip E: Panel Members; Panel Members: Second ESMO consensus
conference on lung cancer: Pathology and molecular biomarkers for
non-small-cell lung cancer. Ann Oncol. 25:1681–1690. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Farazi TA, Spitzer JI, Morozov P and
Tuschl T: miRNAs in human cancer. J Pathol. 223:102–115. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Tian X, Han R, Zhang X, Wang X,
Shen H, Xue L, Liu Y, Yan X, Shen J, et al: Downregulation of
miR-486-5p contributes to tumor progression and metastasis by
targeting protumorigenic ARHGAP5 in lung cancer. Oncogene.
33:1181–1189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fuse M, Kojima S, Enokida H, Chiyomaru T,
Yoshino H, Nohata N, Kinoshita T, Sakamoto S, Naya Y, Nakagawa M,
et al: Tumor suppressive microRNAs (miR-222 and miR-31) regulate
molecular pathways based on microRNA expression signature in
prostate cancer. J Hum Genet. 57:691–699. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Selcuklu SD, Donoghue MT and Spillane C:
miR-21 as a key regulator of oncogenic processes. Biochem Soc
Trans. 37:918–925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K,
Guo J, Zhang Y, Chen J, Guo X, et al: Characterization of microRNAs
in serum: A novel class of biomarkers for diagnosis of cancer and
other diseases. Cell Res. 18:997–1006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rabinowits G, Gerçel-Taylor C, Day JM,
Taylor DD and Kloecker GH: Exosomal microRNA: A diagnostic marker
for lung cancer. Clin Lung Cancer. 10:42–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang Z, Huang D, Ni S, Peng Z, Sheng W
and Du X: Plasma microRNAs are promising novel biomarkers for early
detection of colorectal cancer. Int J Cancer. 127:118–126. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Toiyama Y, Hur K, Tanaka K, Inoue Y,
Kusunoki M, Boland CR and Goel A: Serum miR-200c is a novel
prognostic and metastasis-predictive biomarker in patients with
colorectal cancer. Ann Surg. 259:735–743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eichelser C, Flesch-Janys D, Chang-Claude
J, Pantel K and Schwarzenbach H: Deregulated serum concentrations
of circulating cell-free microRNAs miR-17, miR-34a, miR-155, and
miR-373 in human breast cancer development and progression. Clin
Chem. 59:1489–1496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu C, Ren C, Han J, Ding Y, Du J, Dai N,
Dai J, Ma H, Hu Z, Shen H, et al: A five-microRNA panel in plasma
was identified as potential biomarker for early detection of
gastric cancer. Br J Cancer. 110:2291–2299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang C, Wang C, Chen X, Chen S, Zhang Y,
Zhi F, Wang J, Li L, Zhou X, Li N, et al: Identification of seven
serum microRNAs from a genome-wide serum microRNA expression
profile as potential noninvasive biomarkers for malignant
astrocytomas. Int J Cancer. 132:116–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng H, Zhang L, Zhao Y, Yang D, Song F,
Wen Y, Hao Q, Hu Z, Zhang W and Chen K: Plasma miRNAs as diagnostic
and prognostic biomarkers for ovarian cancer. PLoS One.
8:e778532013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nolen BM, Marks JR, Ta'san S, Rand A,
Luong TM, Wang Y, Blackwell K and Lokshin AE: Serum biomarker
profiles and response to neoadjuvant chemotherapy for locally
advanced breast cancer. Breast Cancer Res. 10:R452008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hansen TF, Sørensen FB, Lindebjerg J and
Jakobsen A: The predictive value of microRNA-126 in relation to
first line treatment with capecitabine and oxaliplatin in patients
with metastatic colorectal cancer. BMC Cancer. 12:832012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Seidman AD, O'Shaughnessy J and Misset JL:
Single-agent capecitabine: A reference treatment for
taxane-pretreated metastatic breast cancer. Oncologist 7: (Suppl
6). 20–28. 2002. View Article : Google Scholar
|
|
36
|
Cecchin E, Innocenti F, D'Andrea M, Corona
G, De Mattia E, Biason P, Buonadonna A and Toffoli G: Predictive
role of the UGT1A1, UGT1A7, and UGT1A9 genetic variants and their
haplotypes on the outcome of metastatic colorectal cancer patients
treated with fluorouracil, leucovorin, and irinotecan. J Clin
Oncol. 27:2457–2465. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th.
Springer-Verlag; New York, NY: 2010
|
|
38
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Churchill GA: Fundamentals of experimental
design for cDNA microarrays. Nat Genet. 32:(Suppl). 490–495. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Youden WJ: Index for rating diagnostic
tests. Cancer. 3:32–35. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ng TK, Carballosa CM, Pelaez D, Wong HK,
Choy KW, Pang CP and Cheung HS: Nicotine alters MicroRNA expression
and hinders human adult stem cell regenerative potential. Stem
Cells Dev. 22:781–790. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu ZL, Wang H, Liu J and Wang ZX:
MicroRNA-21 (miR-21) expression promotes growth, metastasis, and
chemo- or radioresistance in non-small cell lung cancer cells by
targeting PTEN. Mol Cell Biochem. 372:35–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gao W, Lu X, Liu L, Xu J, Feng D and Shu
Y: MiRNA-21: A biomarker predictive for platinum-based adjuvant
chemotherapy response in patients with non-small cell lung cancer.
Cancer Biol Ther. 13:330–340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu XG, Zhu WY, Huang YY, Ma LN, Zhou SQ,
Wang YK, Zeng F, Zhou JH and Zhang YK: High expression of serum
miR-21 and tumor miR-200c associated with poor prognosis in
patients with lung cancer. Med Oncol. 29:618–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dong Z, Ren L, Lin L and Li J, Huang Y and
Li J: Effect of microRNA-21 on multidrug resistance reversal in
A549/DDP human lung cancer cells. Mol Med Rep. 11:682–690.
2015.PubMed/NCBI
|
|
47
|
Xu L, Huang Y, Chen D, He J, Zhu W, Zhang
Y and Liu X: Downregulation of miR-21 increases cisplatin
sensitivity of non-small-cell lung cancer. Cancer Genet.
207:214–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang H, Zuo Z, Lu X, Wang L, Wang H and
Zhu Z: MiR-25 regulates apoptosis by targeting Bim in human ovarian
cancer. Oncol Rep. 27:594–598. 2012.PubMed/NCBI
|
|
49
|
Li Q, Zou C, Zou C, Han Z, Xiao H, Wei H,
Wang W, Zhang L, Zhang X, Tang Q, et al: MicroRNA-25 functions as a
potential tumor suppressor in colon cancer by targeting Smad7.
Cancer Lett. 335:168–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang T, Chen T, Li Y, Gao L, Zhang S, Wang
T and Chen M: Downregulation of miR-25 modulates non-small cell
lung cancer cells by targeting CDC42. Tumour Biol. 36:1903–1911.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang T, Chen T, Li Y, Gao L, Zhang S, Wang
T and Chen M: Downregulation of miR-25 modulates non-small cell
lung cancer cells by targeting CDC42. Tumour Biol. 36:1903–1911.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu FX, Su YL, Zhang H, Kong JY, Yu H and
Qian BY: Prognostic implications for high expression of MiR-25 in
lung adenocarcinomas of female non-smokers. Asian Pac J Cancer
Prev. 15:1197–1203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rasmussen MH, Jensen NF, Tarpgaard LS,
Qvortrup C, Rømer MU, Stenvang J, Hansen TP, Christensen LL,
Lindebjerg J, Hansen F, et al: High expression of microRNA-625-3p
is associated with poor response to first-line oxaliplatin based
treatment of metastatic colorectal cancer. Mol Oncol. 7:637–646.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Park YT, Jeong JY, Lee MJ, Kim KI, Kim TH,
Kwon YD, Lee C, Kim OJ and An HJ: MicroRNAs overexpressed in
ovarian ALDH1-positive cells are associated with chemoresistance. J
Ovarian Res. 6:182013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bera A, VenkataSubbaRao K, Manoharan MS,
Hill P and Freeman JW: A miRNA signature of chemoresistant
mesenchymal phenotype identifies novel molecular targets associated
with advanced pancreatic cancer. PLoS One. 9:e1063432014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Buffa FM, Camps C, Winchester L, Snell CE,
Gee HE, Sheldon H, Taylor M, Harris AL and Ragoussis J:
microRNA-associated progression pathways and potential therapeutic
targets identified by integrated mRNA and microRNA expression
profiling in breast cancer. Cancer Res. 71:5635–5645. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Goto Y, Kojima S, Nishikawa R, Enokida H,
Chiyomaru T, Kinoshita T, Nakagawa M, Naya Y, Ichikawa T and Seki
N: The microRNA-23b/27b/24-1 cluster is a disease progression
marker and tumor suppressor in prostate cancer. Oncotarget.
5:7748–7759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jiang J, Lv X, Fan L, Huang G, Zhan Y,
Wang M and Lu H: MicroRNA-27b suppresses growth and invasion of
NSCLC cells by targeting Sp1. Tumour Biol. 35:10019–10023. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yanaihara N, Caplen N, Bowman E, Seike M,
Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et
al: Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shen J, Todd NW, Zhang H, Yu L, Lingxiao
X, Mei Y, Guarnera M, Liao J, Chou A, Lu CL, et al: Plasma
microRNAs as potential biomarkers for non-small-cell lung cancer.
Lab Invest. 91:579–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kefas B, Comeau L, Floyd DH, Seleverstov
O, Godlewski J, Schmittgen T, Jiang J, diPierro CG, Li Y, Chiocca
EA, et al: The neuronal microRNA miR-326 acts in a feedback loop
with notch and has therapeutic potential against brain tumors. J
Neurosci. 29:15161–15168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ferretti E, De Smaele E, Miele E, Laneve
P, Po A, Pelloni M, Paganelli A, Di Marcotullio L, Caffarelli E,
Screpanti I, et al: Concerted microRNA control of Hedgehog
signalling in cerebellar neuronal progenitor and tumour cells. EMBO
J. 27:2616–2627. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhou J, Xu T, Yan Y, Qin R, Wang H, Zhang
X, Huang Y, Wang Y, Lu Y, Fu D and Chen J: MicroRNA-326 functions
as a tumor suppressor in glioma by targeting the Nin one binding
protein (NOB1). PLoS One. 8:e684692013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liang Z, Wu H, Xia J, Li Y, Zhang Y, Huang
K, Wagar N, Yoon Y, Cho HT, Scala S and Shim H: Involvement of
miR-326 in chemotherapy resistance of breast cancer through
modulating expression of multidrug resistance-associated protein 1.
Biochem Pharmacol. 79:817–824. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang S, Lu S, Geng S, Ma S, Liang Z and
Jiao B: Expression and clinical significance of microRNA-326 in
human glioma miR-326 expression in glioma. Med Oncol. 30:3732013.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang R, Chen XF and Shu YQ: Prediction of
non-small cell lung cancer metastasis-associated microRNAs using
bioinformatics. Am J Cancer Res. 5:32–51. 2014.eCollection 2015.
PubMed/NCBI
|
|
67
|
Valencia K, Martín-Fernández M, Zandueta
C, Ormazábal C, Martínez-Canarias S, Bandrés E, de la Piedra C and
Lecanda F: miR-326 associates with biochemical markers of bone
turnover in lung cancer bone metastasis. Bone. 52:532–539. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kjersem JB, Ikdahl T, Lingjaerde OC, Guren
T, Tveit KM and Kure EH: Plasma microRNAs predicting clinical
outcome in metastatic colorectal cancer patients receiving
first-line oxaliplatin-based treatment. Mol Oncol. 8:59–67. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gomez GG, Wykosky J, Zanca C, Furnari FB
and Cavenee WK: Therapeutic resistance in cancer: microRNA
regulation of EGFR signaling networks. Cancer Biol Med. 10:192–205.
2013.PubMed/NCBI
|
|
70
|
Seike M, Goto A, Okano T, Bowman ED,
Schetter AJ, Horikawa I, Mathe EA, Jen J, Yang P, Sugimura H, Gemma
A, Kudoh S, Croce CM and Harris CC: MiR-21 is an EGFR-regulated
anti-apoptotic factor in lung cancer in never-smokers. Proc Natl
Acad Sci USA. 106:12085–12090. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chiyomaru T, Seki N, Inoguchi S, Ishihara
T, Mataki H, Matsushita R, Goto Y, Nishikawa R, Tatarano S, Itesako
T, et al: Dual regulation of receptor tyrosine kinase genes EGFR
and c-Met by the tumor-suppressive microRNA-23b/27b cluster in
bladder cancer. Int J Oncol. 46:487–496. 2015.PubMed/NCBI
|