Introduction
Gastric cancer (GC) is one of the most prevalent
malignant tumors (1). A number of
cancer prevention studies have demonstrated that the most effective
way to reduce GC-associated mortality is through early diagnosis
and appropriate treatment (2–4). However, the diagnosis rate of early GC
remains extremely low (<10%) (5).
Patients with GC are usually diagnosed following identification of
tumors on B-mode ultrasound, computerized tomography, or magnetic
resonance imaging scans. However, early GC tumors are often not
large enough to be detected by conventional imaging techniques.
Furthermore, the sensitivity and specificity of traditional tumor
markers are not satisfactory (6).
Thus, investigations into new, more efficient tumor markers are
important to improve early diagnosis of this disease.
Long non-coding RNAs (lncRNAs) are a class of
regulatory non-coding RNAs (7).
Previous studies have investigated the roles of lncRNAs in
tumorigenesis and cancer development. For example, Zang et
al (8) reported that lncRNA-PEG10
expression was upregulated in esophageal cancer tissues, and that
it regulated the proliferation and invasion of cancer cells.
Furthermore, Shao et al (9)
demonstrated that lncRNA-AA174084 expression was downregulated in
gastric cancer tissues. A previous study determined that gastric
cancer associated transcript 2 (GACAT2) was not only significantly
downregulated in gastric cancer tissue, but was also aberrantly
expressed in gastric precancerous lesions and gastric cell lines
(10).
Blood is one of the most commonly used samples in
cancer screening, therefore in the present study, plasma GACAT2
expression was compared among groups of healthy individuals,
patients with GD, and patients with preoperative or postoperative
GC. The aim of the current study was to investigate whether plasma
GACAT2 levels may be used as a novel tumor marker to screen and
predict the prognosis of patients with GC.
Materials and methods
Plasma and clinical data
collection
A total of 343 plasma samples were collected from 80
healthy individuals, 29 patients with GD, 117 preoperative and 117
postoperative patients with GC at the Department of
Gastroenterology, Affiliated Hospital of Ningbo University School
of Medicine (Ningbo, China) between March 2013 and July 2014.
Postoperative plasma samples were collected 14 days following
surgery. No patients underwent chemotherapy or radiotherapy ahead
of sample collection. Tumors were classified using the
tumor-node-metastasis staging system (11). Histological grade was evaluated by the
National Comprehensive Cancer Network clinical practice guidelines
(V.1.2011) (12). Written informed
consent was provided by all patients, and all aspects of the study
were approved by the Human Research Ethics Committee of Ningbo
University (IRB No. 20120303).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from plasma using the
TRIzol® LS reagent (Ambion; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's protocol as
previously reported (13).
Complementary DNA was subsequently generated using the GoScript™
Reverse Transcription system (Promega Corporation, Madison, WI,
USA), following the manufacturer's protocol, as previously reported
(14). In order to detect plasma
GACAT2 levels, RT-qPCR was performed with GoTaq qPCR Master mix
(Promega Corporation) on a Mx3005P qPCR system (Stratagene; Agilent
Technologies, Inc., Santa Clara, CA, USA) following the
manufacturer's protocol. Glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) expression, which maintains a stable level in human plasma
(9), was used as a control for plasma
GACAT2 detection. Primers for GAPDH and GACAT2 were synthesized by
Sangon Biotech, Co., Ltd. (Shanghai, China). The primer sequences
were as follows: GACAT2 forward, 5′-TGGATGCTTACAAAGGACTGG-3′ and
reverse, 5′-CTGCAATTACGGAAAGAGCTG-3′; GAPDH forward,
5′-ACCCACTCCTCCACCTTTGAC-3′ and reverse,
5′-TGTTGCTGTAGCCAAATTCGTT-3′. The conditions of thermal cycling
were as follows: 95°C at 5 min for a hot-start, followed by 45
cycles at 94°C for 15 sec, 55°C for 30 sec and 72°C for 30 sec. The
level of GACAT2 was calculated using the 2−ΔΔCq method
(9). A lower quantification cycle
(Cq) indicates a higher level of plasma GACAT2. The ΔΔCq method
(ΔCqpost-op-ΔCqpre-op) was used to evaluate
individual relative changes of plasma GACAT2 following surgery, and
all results were expressed as the mean ± standard deviation of
three independent experiments.
Detection of serum carcinoembryonic
antigen (CEA) and serum carbohydrate antigen (CA)19-9
To measure levels of CA19-9 and CEA, an Elecsys 2010
machine (Roche Diagnostics, Basel, Switzerland) was used. The
cut-off values of CA19-9 and CEA were set at 35 U/ml and 5 ng/ml,
respectively (15).
Statistical analysis
Data were analyzed using SPSS software v19.0 (IBM
SPSS, Armonk, NY, USA). The difference in plasma GACAT2 levels
among healthy controls, patients with GD, and preoperative and
postoperative patients with GC were analyzed by one-way analysis of
variance (ANOVA). Associations between plasma GACAT2 levels and
clinicopathological factors were analyzed by ANOVA and Student's
t-test. Graphs were plotted using SigmaPlot v12.1.0 (Systat
Software Inc., San Jose, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Plasma GACAT2 levels are significantly
decreased following surgery
GACAT2 levels in preoperative and postoperative
patients with GC were detected by RT-qPCR. As presented in Fig. 1, the level of plasma GACAT2 in the
postoperative gastric cancer patients was significantly lower than
that in the preoperative group (P=0.031), indicating that GACAT2
expression significantly decreased following surgery.
Plasma GACAT2 levels are significantly
increased in patients with GD and GC
Plasma GACAT2 levels were measured at two stages of
gastric carcinogenesis to gain further information regarding their
effect on gastric mucosal damage. The data indicated that plasma
GACAT2 levels in patients with GD (P<0.001) and patients with
preoperative GC (P=0.040) were significantly higher than those in
the healthy controls (Fig. 2).
However, there was no significant difference in GACAT2 expression
between the two patient groups.
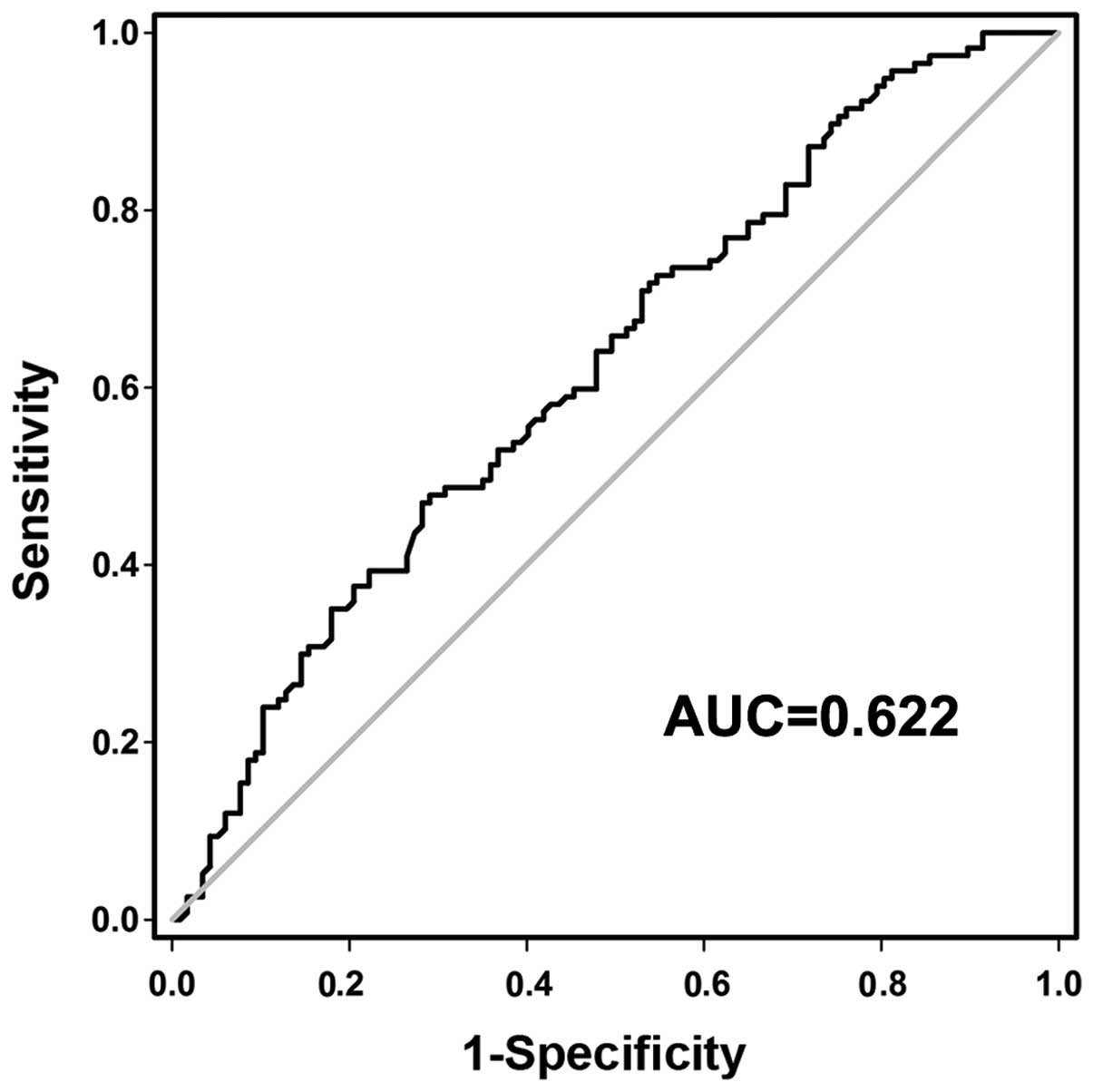
To investigate whether preoperative plasma GACAT2
may be used as a tumor biomarker for GC screening, a receiver
operating characteristic (ROC) curve was constructed to evaluate
its clinical value. The area under the curve (AUC) was 0.622 (95%
confidence interval, 0.551–0.694; P<0.05; Fig. 3), and the optimal cut-off value was
6.625, with which the sensitivity and specificity were 87.2 and
28.2%, respectively (Fig. 3). This
implies that plasma GACAT2 levels may be used as a reliable
biomarker in the screening of GC.
Identification of RT-qPCR products of
plasma GACAT2
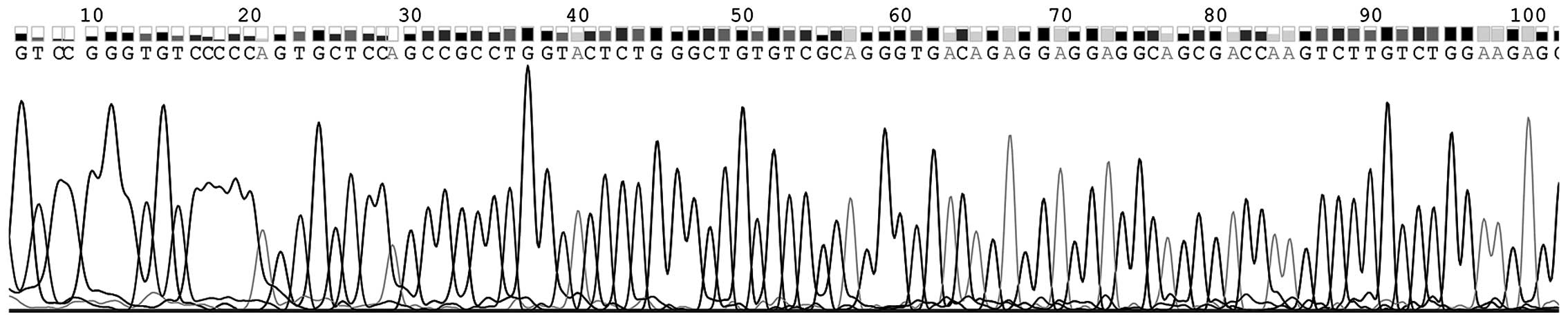
Blood is the primary material used in the diagnosis
of diseases; therefore, to confirm the results and prove the
existence of GACAT2 in plasma, the RT-qPCR products of plasma
GACAT2 were sequenced. Fig. 4
demonstrates that the sequence of plasma GACAT2 was completely
consistent with that from the genome database (http://www.ncbi.nlm.nih.gov/nuccore/NR_120598.1).
Association between plasma GACAT2
levels and clinicopathological factors of patients with GC
Based on the aforementioned results, associations
between plasma GACAT2 levels and the clinicopathological features
of patients with GC were investigated. The individual relative
changes of plasma GACAT2 following surgery were significantly
associated with lymphatic metastasis (P=0.034), distal metastasis
(P=0.035) and perineural invasion (P=0.039) (Table I), indicating a correlation between
GACAT2 and invasive GC.
 | Table I.Association of plasma GACAT2 level
changes (ΔΔCq) between postoperative and preoperative patients with
gastric cancer and clinicopathological factors. |
Table I.
Association of plasma GACAT2 level
changes (ΔΔCq) between postoperative and preoperative patients with
gastric cancer and clinicopathological factors.
| Characteristics | N | Mean ± SD | P-value |
|---|
| Age, years |
|
| 0.889 |
| ≥60 | 81 | 1.544±4.253 |
|
|
<60 | 36 | 1.340±3.996 |
| Gender |
|
| 0.449 |
| Male | 81 | 1.143±4.443 |
|
|
Female | 36 | 2.243±3.344 |
|
| Tumor location |
|
| 0.969 |
| Sinuses
ventriculi | 57 | 1.547±4.149 |
|
|
Cardia | 21 | 1.027±4.205 |
|
| Corpora
ventriculi | 21 | 1.206±3.103 |
|
|
Others | 18 | 2.123±5.797 |
|
| Diameter, cm |
|
| 0.252 |
| ≥5 | 60 | 0.735±4.393 |
|
|
<5 | 57 | 2.266±3.777 |
|
| Differentiation |
|
| 0.057 |
| Well | 6 | 5.070±1.725 |
|
|
Moderate | 57 | 2.608±4.290 |
|
| Poor | 54 | −0.108±3.571 |
|
| TNM stage |
|
| 0.326 |
|
Early | 24 | 2.775±3.990 |
|
|
Advanced | 93 | 1.147±4.155 |
|
| Borrmann type |
|
| 0.224 |
| I and
II | 33 | 2.386±3.091 |
|
| III and
IV | 60 | 0.466±4.567 |
|
| Pathological
diagnosis |
|
| 0.342 |
| Signet
ring cell cancer | 18 | −0.117±5.671 |
|
|
Adenocarcinoma | 99 | 1.752±3.831 |
|
| Invasion |
|
| 0.223 |
|
T1 and
T2 | 36 | 2.701±3.917 |
|
|
T3 and
T4 | 81 | 0.939±4.168 |
|
| Lymphatic
metastasis |
|
| 0.034a |
|
N0 | 45 | 3.232±3.805 |
|
|
N1–3 | 72 | 0.387±4.005 |
|
| Distal
metastasis |
|
| 0.035a |
|
M0 | 96 | 2.070±3.720 |
|
|
M1 | 21 | −1.210±5.093 |
|
| Venous
invasion |
|
| 0.246 |
|
Negative | 60 | 2.236±3.916 |
|
|
Positive | 57 | 0.687±4.292 |
|
| PNI |
|
| 0.039a |
|
Negative | 63 | 2.731±3.326 |
|
|
Positive | 54 | 0.022±4.563 |
|
| Blood CEA |
|
| 0.767 |
|
Positive | 51 | 1.722±5.144 |
|
|
Negative | 66 | 1.295±3.246 |
|
| Blood CA19-9 |
|
| 0.793 |
|
Positive | 39 | 1.232±5.011 |
|
|
Negative | 78 | 1.606±3.708 |
|
Discussion
Previous studies have identified the differential
expression of lncRNAs in various diseases and suggested their
possible associations with tumorigenesis (16). Advanced cancer genomic techniques have
demonstrated that lncRNAs are important in determining GC
occurrence and development (17).
Blood plasma is the most commonly used sample in
clinical diagnosis (18). A number of
changes in tumor cells may be detected by measuring the expression
of different proteins in plasma. RT-qPCR may provide a method to
detect changes in the expression of DNA or RNA associated with
tumorigenesis. The present study investigated whether plasma-based
lncRNAs could be used as biomarkers for the screening of GC and
prediction of prognosis in patients with the disease. Plasma GACAT2
levels between preoperative and postoperative patients with GC were
compared. The results demonstrated that plasma GACAT2 levels in
postoperative patients with GC were significantly lower than those
in the preoperative group (Fig. 1).
To obtain further information regarding the change in GACAT2
expression during gastric tumorigenesis, plasma GACAT2 levels in
two stages of gastric carcinogenesis were measured. Compared with
healthy controls, plasma GACAT2 levels were not only increased in
the gastric cancer group as a whole (P=0.040), they were also
significantly higher in patients with GD (P<0.001; Fig. 2). The AUC was 0.622 (Fig. 3). When the optimal cut-off value was
6.625, the sensitivity and specificity were 87.2 and 28.2%,
respectively. Taken together, the results of the current study
indicated that plasma GACAT2 has the potential value for the early
screening of GC.
A previous study demonstrated that GACAT2 expression
was significantly downregulated in gastric tissue and gastric
cancer cell lines (10). The reason
plasma GACAT2 expression increased in patients with GC and GD in
the current study remains unknown. A recent study compared
lncRNA-LINC00152 levels between plasma and exosomes extracted from
the blood and observed that no difference between the two (19). One hypothesis suggests that lncRNAs
that are stable in human plasma are protected by exosomes (19). It has been demonstrated that exosomes
serve an important role in the protection and secretion of
non-coding RNAs, including miRNAs and lncRNAs (20,21), and
cells affected by stimuli, such as oxidative stress, may increase
the secretion of exosomes (22,23). In
addition, GC cells are able to release related RNAs into the
extracellular environment via exosomes during tumorgenesis
(24). Therefore, GACAT2 may be
released in a similar manner, leading to elevated GACAT2 levels in
the blood plasma of patients with malignant GC.
Lymphatic metastasis, distal metastasis and
perineural invasion are important factors affecting GC prognosis
(25,26). The present study demonstrated that
individual relative changes of plasma GACAT2 following surgery were
significantly associated with lymphatic metastasis (P=0.034),
distal metastasis (P=0.035) and perineural invasion (P=0.039).
Higher GACAT2 levels in plasma therefore are correlated with a
worse pathological situation; therefore plasma-based GACAT2 may be
used as a tumor marker to predict the prognosis of patients with
GC.
In conclusion, our study demonstrated that plasma
GACAT2 levels were significantly increased in patients with GD and
preoperative patients with GC. In addition, the individual relative
changes of plasma GACAT2 expression following surgery were
significantly associated with lymphatic metastasis, distal
metastasis and perineural invasion. Our findings suggested that
plasma-based GACAT2 as a tumor marker has a potential diagnostic
value for the screening of GC and prediction of prognosis.
Acknowledgements
The present study was supported by the Zhejiang
Provincial Natural Science Foundation of China (grant no.
LY14C060003), the Applied Research Project on Nonprofit Technology
of Zhejiang Province (grant no. 2014C33222), the National Natural
Science Foundation of China (grant no. 81171660), the Medical
Scientific Research Project of The Affiliated Hospital of Ningbo
University School of Medicine (grant no. XYY14009) and the K.C.
Wong Magna Fund in Ningbo University (grant no. 2016001).
References
|
1
|
Chen W, Zheng R, Zeng H, Zhang S and He J:
Annual report on status of cancer in China, 2011. Chin J Cancer
Res. 27:2–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carcas LP: Gastric cancer review. J
Carcinog. 13:142014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Y, Shao Y, Zhu M, Li Q, Yang F, Lu X,
Xu C, Xiao B, Sun Y and Guo J: Using gastric juice
lncRNA-ABHD11-AS1 as a novel type of biomarker in the screening of
gastric cancer. Tumour Biol. 37:1183–1188. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li P, Chen S, Chen H, Mo X, Li T, Shao Y,
Xiao B and Guo J: Using circular RNA as a novel type of biomarker
in the screening of gastric cancer. Clin Chim Acta. 444:132–136.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zou WB, Yang F and Li ZS: How to improve
the diagnosis rate of early gastric cancer in China. Zhejiang Da
Xue Xue Bao Yi Xue Ban. 44:9–14. 2015.(In Chinese). PubMed/NCBI
|
|
6
|
Zhou H, Xiao B, Zhou F, Deng H, Zhang X,
Lou Y, Gong Z, Du C and Guo J: MiR-421 is a functional marker of
circulating tumor cells in gastric cancer patients. Biomarkers.
17:104–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li T, Mo X, Fu L, Xiao B and Guo J:
Molecular mechanisms of long noncoding RNAs on gastric cancer.
Oncotarget. 7:8601–8612. 2016.PubMed/NCBI
|
|
8
|
Zang W, Wang T, Huang J, Li M, Wang Y, Du
Y, Chen X and Zhao G: Long noncoding RNA PEG10 regulates
proliferation and invasion of esophageal cancer cells. Cancer Gene
Ther. 22:138–144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shao Y, Ye M, Jiang X, Sun W, Ding X, Liu
Z, Ye G, Zhang X, Xiao B and Guo J: Gastric juice long noncoding
RNA used as a tumor marker for screening gastric cancer. Cancer.
120:3320–3328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shao Y, Chen H, Jiang X, Chen S, Li P, Ye
M, Li Q, Sun W and Guo J: Low expression of lncRNA-HMlincRNA717 in
human gastric cancer and its clinical significances. Tumour Biol.
35:9591–9595. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sobin LH and Compton CC: TNM seventh
edition: What's new, what's changed: Communication from the
International Union Against Cancer and the American Joint Committee
on Cancer. Cancer. 116:5336–5339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ajani JA, Bentrem DJ, Besh S, D'Amico TA,
Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE, et
al: Gastric cancer, version 2.2013: Featured updates to the NCCN
Guidelines. J Natl Compr Canc Netw. 11:531–546. 2013.PubMed/NCBI
|
|
13
|
Liu Z, Shao Y, Tan L, Shi H, Chen S and
Guo J: Clinical significance of the low expression of FER1L4 in
gastric cancer patients. Tumour Biol. 35:9613–9617. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia T, Chen S, Jiang Z, Shao Y, Jiang X,
Li P, Xiao B and Guo J: Long noncoding RNA FER1L4 suppresses cancer
cell growth by acting as a competing endogenous RNA and regulating
PTEN expression. Sci Rep. 5:134452015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu C, Shao Y, Xia T, Yang Y, Dai J, Luo L,
Zhang X, Sun W, Song H, Xiao B and Guo J: lncRNA-AC130710 targeting
by miR-129-5p is upregulated in gastric cancer and associates with
poor prognosis. Tumour Biol. 35:9701–9706. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li CH and Chen Y: Targeting long
non-coding RNAs in cancers: Progress and prospects. Int J Biochem
Cell Biol. 45:1895–1910. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song H, Sun W, Ye G, Ding X, Liu Z, Zhang
S, Xia T, Xiao B, Xi Y and Guo J: Long non-coding RNA expression
profile in human gastric cancer and its clinical significances. J
Transl Med. 11:2252013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang R, Wen H, Xu Y, Chen Q, Luo Y, Lin Y,
Luo Y and Xu A: Circulating microRNAs as a novel class of
diagnostic biomarkers in gastrointestinal tumors detection: A
meta-analysis based on 42 articles. PLoS One. 9:e1134012014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Q, Shao Y, Zhang X, Zheng T, Miao M,
Qin L, Wang B, Ye G, Xiao B and Guo J: Plasma long noncoding RNA
protected by exosomes as a potential stable biomarker for gastric
cancer. Tumour Biol. 36:2007–2012. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xin H, Li Y and Chopp M: Exosomes/miRNAs
as mediating cell-based therapy of stroke. Front Cell Neurosci.
8:3772014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gezer U, Özgür E, Cetinkaya M, Isin M and
Dalay N: Long non-coding RNAs with low expression levels in cells
are enriched in secreted exosomes. Cell Biol Int. 38:1076–1079.
2014.PubMed/NCBI
|
|
22
|
King HW, Michael MZ and Gleadle JM:
Hypoxic enhancement of exosome release by breast cancer cells. BMC
Cancer. 12:4212012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park JE, Tan HS, Datta A, Lai RC, Zhang H,
Meng W, Lim SK and Sze SK: Hypoxic tumor cell modulates its
microenvironment to enhance angiogenic and metastatic potential by
secretion of proteins and exosomes. Mol Cell Proteomics.
9:1085–1099. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ohshima K, Inoue K, Fujiwara A, Hatakeyama
K, Kanto K, Watanabe Y, Muramatsu K, Fukuda Y, Ogura S, Yamaguchi K
and Mochizuki T: Let-7 microRNA family is selectively secreted into
the extracellular environment via exosomes in a metastatic gastric
cancer cell line. PLoS One. 5:e132472010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sugita H, Kojima K, Inokuchi M and Kato K:
Long-term outcomes of laparoscopic gastrectomy for gastric cancer.
J Surg Res. 193:190–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tarsitano A, Tardio ML and Marchetti C:
Impact of perineural invasion as independent prognostic factor for
local and regional failure in oral squamous cell carcinoma. Oral
Surg Oral Med Oral Pathol Oral Radiol. 119:221–228. 2015.
View Article : Google Scholar : PubMed/NCBI
|