Introduction
Thyroid cancer is the most common endocrine
malignancy and papillary thyroid carcinoma (PTC) accounts for
80–90% of all thyroid malignancies (1,2). The
incidence of PTC has increased in most countries in the last three
decades (3,4). Although the majority of patients with
PTC have a favorable prognosis following appropriate treatments,
10–30% of patients with PTC progress to metastasis or recurrence,
and mortality in 5% of cases (5,6). The
mechanisms underlying the progression of PTC have yet to be
elucidated.
For the majority of patients with PTC, metastasis
remains the main cause of mortality; >90% of cancer-associated
mortalities are caused by tumor invasion and metastasis, which
emphasizes the importance of elucidating the mechanisms underlying
metastasis (7–9). The contribution of angiogenesis to tumor
progression is well established. In human solid cancer, the growth
and survival of tumor cells are angiogenesis-dependent. Following
the inhibition of angiogenesis, metastasis of the primary tumor is
affected (10,11). Therefore, it is necessary to
investigate novel factors that may inhibit metastasis by affecting
angiogenesis in human PTC.
Pigment epithelium-derived factor (PEDF) is a 50-kDa
secreted glycoprotein that was initially identified in cultured
retinal pigment epithelial cells (12). PEDF has numerous biological functions,
including differentiating activity, neurite outgrowth, survival
activity, anti-apoptosis and anti-angiogenic activities and the
induction of cell death (13–19). The anticancer role of PEDF has yet to
be elucidated; however, previous studies have suggested various
roles for PEDF in inhibiting cancer progression. For instance, PEDF
may cause tumors to differentiate to a less malignant phenotype,
PEDF may block angiogenesis-mediated activities and
neovascularization, and PEDF may suppress tumor cell invasion and
metastasis (14,15).
PEDF is one of the most promising anti-angiogenic
factors; a number of studies have demonstrated its anti-angiogenic
effects in various tumor models, including retinoblastoma,
neuroblastoma, prostate cancer, melanoma, Wilms' tumor, pancreatic
adenocarcinoma, hepatoblastoma, osteosarcoma, chondrosarcoma, human
cervical carcinoma, gastric carcinoma, nasopharyngeal carcinoma,
Lewis lung carcinoma, colorectal peritoneal carcinoma, glioma and
breast cancer xenografts (20–24). The
anti-angiogenic effect of PEDF is performed primarily through the
disruption of microvascular network distribution (25–28).
Vascular endothelial growth factor (VEGF) is an established
pro-angiogenic factor and numerous studies have reported an inverse
correlation between PEDF and VEGF expression levels in certain
tumor models (21,25–28).
The degree of oxygenation is crucial to
neovascularization (29). The
transcription factor hypoxia-inducible factor 1α (HIF1α) is a
critical protein involved in the response to hypoxia, and is able
to activate several downstream factors, including VEGF and glucose
transporter 1; its expression is associated with tumor progression
in various carcinomas, including pancreatic cancer, breast cancer,
cervical carcinoma and thyroid cancer (29–31).
Considering the associations between PEDF and tumor progression,
PEDF expression levels in patients with PTC were evaluated in the
current study. To the best of our knowledge, the present study is
the first to investigate the association between PEDF expression
levels and aggressive clinicopathological features in PTC, and to
determine whether PEDF affects the lymph node metastasis (LNM)
process in PTC by altering the HIF1α-VEGF signaling pathway.
Materials and methods
Patients
Patients with PTC who underwent thyroid excision
surgery during the period 2011–2013 at The Second Affiliated
Hospital of Harbin Medical University (Harbin, China) were
recruited for the present study. The standard pathological
diagnosis of PTC was based on the World Health Organization
criteria (32) and two pathologists
independently reviewed histological specimens in a blinded manner.
A total of 271 patients with PTC (24 males and 247 females) were
diagnosed and included in the current study. All patients met the
inclusion criteria, which were: i) Underwent thyroid excision
surgery; ii) confirmed as PTC by intraoperative rapid pathology and
postoperative pathological detection; and iii) no history of
thyroid disease and thyroid-related medication use. Informed
consent was obtained from all participants and the study was
performed according to the guidelines of the Ethics Committee of
the Harbin Medical University.
Relevant patient data were collected, including
demographic features (gender and age), clinical features
(thyroid-stimulating hormone levels, tumor size and Hashimoto's
disease) and pathological features (multifocality, T stage,
extrathyroid invasion, intact capsule, prophylactic central
compartment lymph-node (neck) dissection and node metastases).
Tumor-node-metastasis (TNM) classification was performed according
to the American Joint Committee on Cancer (33). T1 was defined as a tumor ≤2 cm in
diameter and limited to the thyroid gland. T2 was defined as a
tumor that was >2 and ≤4 cm, and limited to the thyroid gland.
T3 was defined as a tumor >4 cm and limited to the thyroid
gland, or any tumor with minimal glandular infiltration, including
infiltration of the sternothyroid muscle or perithyroid soft
tissues.
Immunohistochemical analysis
PTC tissue specimens were sectioned (4-µm),
deparaffinized in xylene and rehydrated in a graded series of
ethanol. The slides were then incubated in 3% hydrogen peroxide in
distilled water at room temperature for 10 min to inactivate
endogenous peroxidase activity, and subsequently underwent antigen
retrieval in a sodium citrate solution in a microwave oven. The
tissue sections were blocked with 5% bovine serum albumin (Boster
Bio-Engineering, Wuhan, China) for 30 min at room temperature, and
then incubated overnight at 4°C with a rabbit anti-human PEDF
primary polyclonal antibody at 1:100 dilution (#BA1348-1; Boster
Bio-Engineering) in a humidified chamber. The tissue sections were
then incubated with a ready-to-use biotinylated
horseradish-peroxidase-conjugated secondary antibody (#SA1022;
Boster Bio-Engineering) and a streptavidin biotin complex (#SA1022;
Boster Bio-Engineering) at 37°C for 30 min. The staining procedures
were performed according to the manufacturer's instructions:
Visualization with a 3,3′-diaminobenzidine solution and
counterstaining with hematoxylin. The distribution and expression
levels of PEDF were examined from the images obtained using the
Olympus Imaging system (DP73: Olympus Corporation, Tokyo,
Japan).
PEDF expression levels were semi quantitatively
categorized into three groups, as follows: Negative (0 points), ≤5%
positive cells; weakly positive (1 point), 6–30% positive cells;
moderately positive (2 points), 31–60% positive cells.
Laser-capture microdissection
The frozen tissues specimens obtained from PTC
patients with LNM (n=15) and without LNM (n=10) were used for laser
capture microdissection (ArcturusXT™; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) to obtain target thyroid epithelial cells,
as described previously (34). The
age and gender of the participants were matched for each group.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the microdissected
cells using RNAiso Plus (9108; Takara Biotechnology Co., Ltd.,
Dalian, China). The extracted RNA was subsequently incubated with
Recombinant DNase I (D2270; Takara Biotechnology Co., Ltd.)
to erase the genomic DNA. cDNA was then obtained from mRNA using
the PrimeScript™ RT reagent kit (DRR025A; Takara Biotechnology Co.,
Ltd.), which was then amplified using PCR on the ABI 7500 Real-Time
PCR system (Thermo Fisher Scientific, Inc.) with SYBR Green I dye
(DRR041S; Takara Biotechnology Co., Ltd.), in accordance with the
manufacturer's protocol. The cycling conditions were as follows: 1
cycle as an initial denaturation at 95°C for 10 min; 40 cycles of
95°C for 5 sec, 60°C for 30 sec and 72°C for 15 sec; and a final
extension step at 72°C for 5 min. The relative expression levels of
PEDF, VEGF and HIF1α were determined using the
comparative Cq method (35),
following normalization to the endogenous GAPDH control gene. The
primer sequences used in the RT-qPCR are presented in Table I.
 | Table I.Information for the primers used in
the quantitative polymerase chain reaction. |
Table I.
Information for the primers used in
the quantitative polymerase chain reaction.
| Gene | Sequence of primers
(5′-3′) | Length of target
fragment (bp) |
|---|
| GAPDH | Forward:
CCACATCGCTCAGACACCAT | 142 |
|
| Reward:
AGTTGAGGTCAATGAAGGGGT |
|
| PEDF | Forward:
CTCGCCATGAGATCAGCATTC | 168 |
|
| Reward:
AGCCATAGCGTAAAACAGCCT |
|
| VEGF | Forward:
CTCGCCATGAGATCAGCATTC | 154 |
|
| Reward:
AGCCATAGCGTAAAACAGCCT |
|
| HIF1α | Forward:
TGTCGGAGTTTGGAAAACAA | 198 |
|
| Reward:
AAGTGGCAACTGATGAGCAA |
|
Statistical analysis
All statistical analyses in the current study were
performed with SPSS software, version 13.01S (SPSS, Inc., Chicago,
IL, USA). An independent-sample t-test was used to compare the
means between two groups, and a χ2 or Fisher's exact
test was used to compare frequencies between the groups. The data
are presented as the mean ± standard deviation, or as percentages
where appropriate. A Pearson correlation analysis was used to
analyze the associations among the expression levels of
PEDF, VEGF and HIF1α in PTC cells. All t-tests
were two-tailed; P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinicopathological characteristics of
patients with PTC
The clinicopathological characteristics of the
patients are shown in Table II.
Between 2011 and 2013, 271 patients with PTC, including 24 male and
247 female patients, were enrolled in the present study. The mean
age of the patients was 43.1±10.6 years (age range, 19–73 years)
and the mean size of the tumor was 10.9±7.8 mm (range, 2–50 mm).
Multifocal tumors were identified in 12 patients; 14 patients
(5.2%) had extrathyroid invasion and 86 patients (31.7%) had LNM.
Hashimoto's disease was observed in 45 patients. A total of 245
patients had T1 tumors, 26 had T3 tumors and none had tumors in
another stage. The BRAFV600E mutation was present in
70.1% of patients. Immunohistochemistry with an anti-PEDF antibody
detected PEDF expression in 74.5% of the PTC tissues. PEDF
expression was determined to be negative in 69 patients, weakly
positive in 131 patients and moderately positive in 71
patients.
 | Table II.Clinicopathological characteristics
of patients with papillary thyroid carcinoma, recruited between
2011 and 2013. |
Table II.
Clinicopathological characteristics
of patients with papillary thyroid carcinoma, recruited between
2011 and 2013.
| Characteristic | Value |
|---|
| Gender |
|
|
Male | 24 (8.9) |
|
Female | 247 (91.1) |
| Age, years | 43.1±10.6
(19–73) |
|
<45 | 154 (56.8) |
|
≥45 | 117 (43.2) |
| Tumor size, mm | 10.9±7.8
(2–50) |
| Multifocality |
|
|
Single | 259 (95.6) |
|
Multiple (≥2) | 12 (4.4) |
| Extrathyroid
invasion |
|
|
Negative | 257 (94.8) |
|
Positive | 14 (5.2) |
| Lymph node
metastases |
|
|
Negative | 185 (68.3) |
|
Positive | 86 (31.7) |
| Hashimoto's
disease |
|
|
Negative | 226 (83.4) |
|
Positive | 45 (16.6) |
| TNM stage |
|
| T1 | 245 (90.4) |
| T3 | 26 (9.6) |
|
BRAFV600E mutation |
|
|
Negative | 81 (29.9) |
|
Positive | 190 (70.1) |
| PEDF |
|
|
Negative | 69 (25.5) |
| Weakly
positive | 131 (48.3) |
|
Moderately positive | 71 (26.2) |
Decreased PEDF levels correlate with
the progression of human PTC
In order to determine whether PEDF contributes to
the progression of PTC, the associations between PEDF and
aggressive clinicopathological features were investigated. As a
result of the importance of metastasis to the progression of PTC,
the association between PEDF expression levels and LNM was
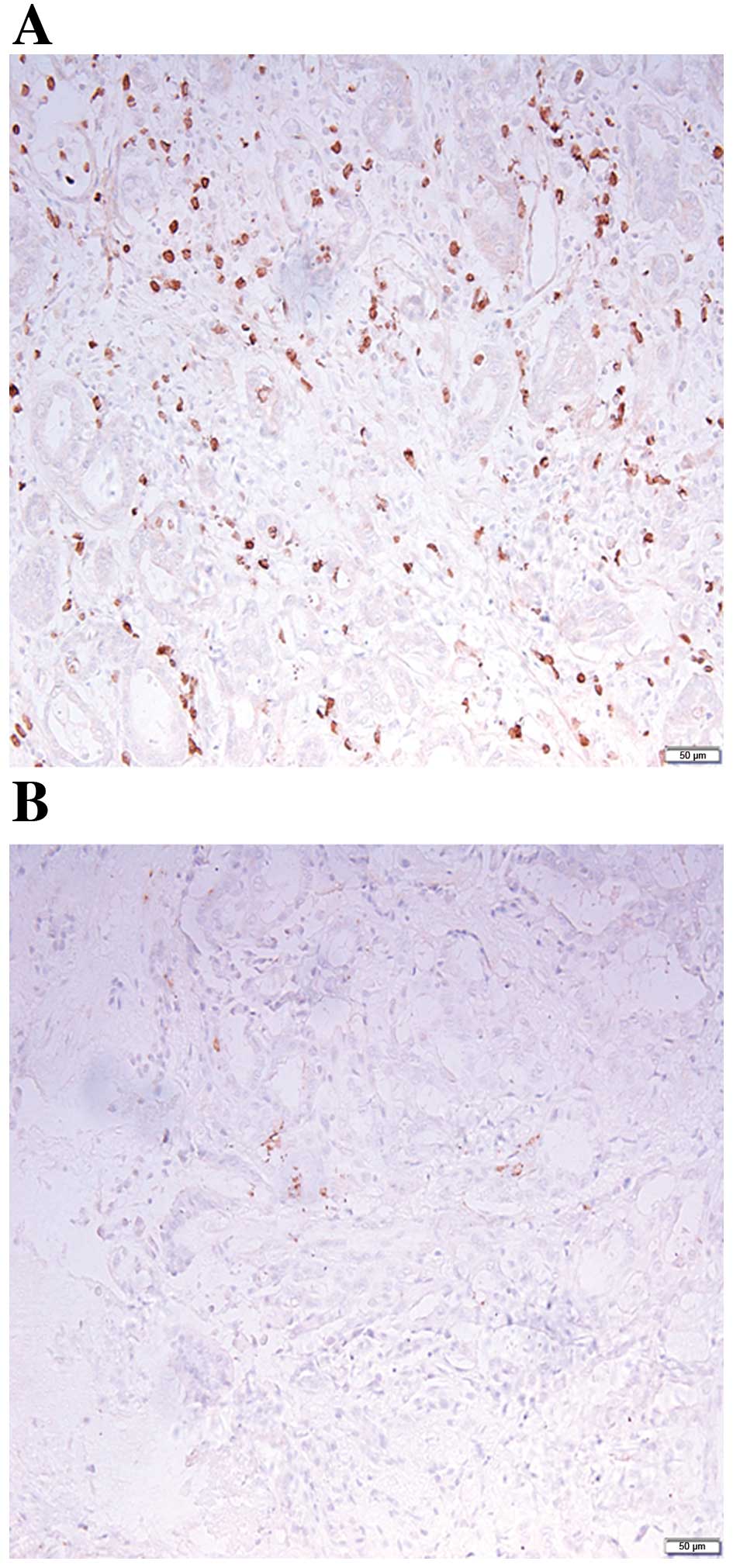
initially evaluated. As presented in Fig.
1 and Table III, PEDF
expression levels were significantly decreased in PTC tissues with
LNM, as compared with PTC tissues without LNM (P=0.006). Similarly,
PEDF expression levels were significantly decreased in PTC tissues
with extrathyroid invasion (P=0.035), a high TNM stage (P=0.013),
BRAFV600E mutation positivity (P<0.001) and a tumor
size of >10 mm (P=0.018). However, PEDF expression levels were
not significantly associated with multifocality (P=0.152; Table III).
 | Table III.Correlation between patient
clinicopathological features and PEDF expression levels. |
Table III.
Correlation between patient
clinicopathological features and PEDF expression levels.
| Characteristic | Negative | Weakly
positive | Moderately
positive | P-value |
|---|
| Gender |
|
|
| 0.939 |
|
Male | 63 (91.3) | 120 (91.6) | 64 (90.1) |
|
|
Female | 6 (8.7) | 11 (8.4) | 7 (9.9) |
|
| Age, years |
|
|
| 0.878 |
|
<45 | 41 (59.4) | 73 (55.7) | 40 (56.3) |
|
|
≥45 | 28 (40.6) | 58 (44.3) | 31 (43.7) |
|
| Tumor size, mm |
|
|
| 0.018 |
|
≤10 | 33 (47.8) | 75 (57.3) | 48 (67.6) |
|
|
>10 | 36 (52.2) | 56 (42.7) | 23 (32.4) |
|
| Multifocality |
|
|
| 0.152 |
|
Single | 62 (91.2) | 127 (96.9) | 70 (97.2) |
|
|
Multiple | 6 (8.8) | 4 (3.1) | 2 (2.8) |
|
| Extrathyroid
invasion |
|
|
| 0.035 |
|
Negative | 61 (88.4) | 127 (96.9) | 69 (97.2) |
|
|
Positive | 8 (11.6) | 4 (3.1) | 2 (2.8) |
|
| Lymph node
metastasis |
|
|
| 0.006 |
|
Negative | 41 (59.4) | 85 (64.9) | 59 (83.1) |
|
|
Positive | 28 (40.6) | 46 (35.1) | 12 (16.9) |
|
| TNM stage |
|
|
| 0.013 |
| T1 | 57 (82.6) | 119 (90.8) | 69 (97.2) |
|
| T3 | 12 (17.4) | 12 (9.2) | 2 (2.8) |
|
|
BRAFV600E mutation |
|
|
| <0.001 |
|
Negative | 8 (11.6) | 33 (25.2) | 40 (56.3) |
|
|
Positive | 61 (88.4) | 98 (74.8) | 31 (43.7) |
|
Decreased expression levels of PEDF
are accompanied by an upregulation of the HIF1α-VEGF signaling
pathway in patients with PTC and LNM
To elucidate the potential mechanisms underlying the
role of PEDF in metastasis by affecting angiogenesis, the mRNA
expression profiles of PEDF, VEGF and HIF1α in
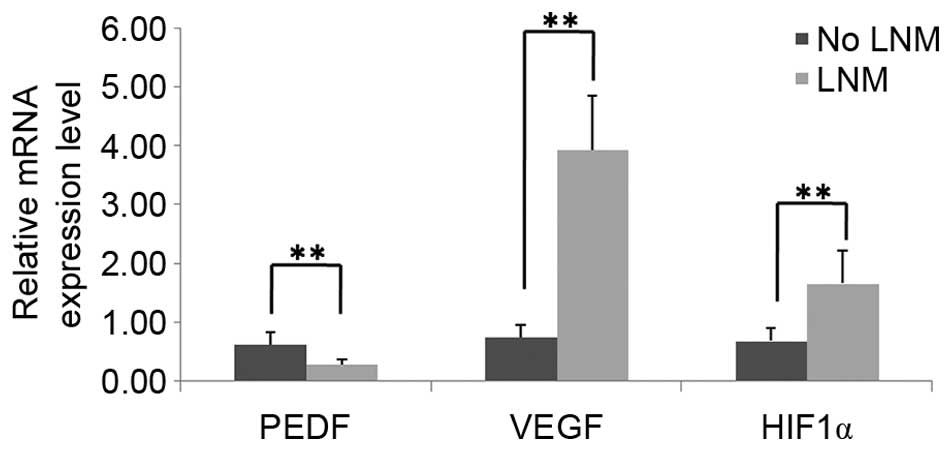
stored patient tissue samples were determined using RT-qPCR. As
indicated in Fig. 2, PEDF mRNA
expression levels were significantly decreased in PTC tissues with
LNM (0.2766±0.0910), as compared with PTC tissues without LNM
(0.6251±0.2034; P<0.01). By contrast, the mRNA expression levels
of HIF1α and VEGF were significantly increased in PTC
tissues with LNM (1.6646±0.5533 and 3.9321±0.9235, respectively),
as compared with PTC tissues without LNM (0.6847±0.2240 and
0.7537±0.1988, respectively; P<0.01). In addition, as shown in
Table IV, through the analysis of
the mRNA expression levels of PEDF, VEGF and
HIF1α in the thyroid tissues specimens obtained from PTC
patients with LNM (n=15) and without LNM (n=10), the present study
identified a significant inverse correlation between PEDF
and VEGF expression levels (r=−0.514; P=0.009), an inverse
association between PEDF and HIF1α expression levels
(r=−0.287; P=0.164) and a significant positive correlation between
VEGF and HIF1α expression levels (r=0.489;
P=0.013).
 | Table IV.Correlation analysis among the mRNA
expression levels of PEDF, VEGF and HIF1α. |
Table IV.
Correlation analysis among the mRNA
expression levels of PEDF, VEGF and HIF1α.
| Analysis | Correlation
coefficient (r) | P-value |
|---|
| PEDF vs.
VEGF | −0.514 | 0.009 |
| PEDF vs.
HIF1α | −0.287 | 0.164 |
| VEGF vs.
HIF1α |
0.489 | 0.013 |
Discussion
To the best of our knowledge, the present study is
the first to demonstrate that PEDF expression levels are correlated
with specific patient clinicopathological features, including LNM,
extrathyroid invasion, BRAFV600E mutation positivity,
tumor size and a high TNM stage in PTC tissues. The results also
indicated that PEDF may have an important role in metastasis by
affecting angiogenesis induced by HIF1α.
The actions of PEDF have been evaluated in various
types of tumor cells. The results of a number of previous studies
support the hypothesis that PEDF expression may promote tumor
growth, invasion and metastasis (19,21,22,25,26).
Reduced PEDF expression is a potent promoter of tumor growth and
angiogenesis in breast cancer (36),
and the loss of PEDF enables melanoma cells to acquire an invasive
phenotype (37). The results of the
present study were concordant with previous findings in the
majority of solid tumors (22,26,36,37),
which suggested that PEDF may have an important role in the
progression of PTC. However, certain observations of the current
study, including the association of PEDF expression levels with
tumor stage and its lack of association with multifocality, must be
investigated in further studies with a larger cohort, as only 26
cases of a T3 tumor and 12 cases of multifocality were recruited
for the present study.
Considering its potent function in angiogenesis, it
was hypothesized that PEDF exerts its role in metastasis mainly via
affecting angiogenesis in PTC tissues. Previous studies have
demonstrated that the HIF1α-VEGF signaling pathway is activated
during the progression of thyroid cancer (38,39). To
the best of our knowledge, the present study is the first to
identify an inverse association between PEDF expression levels and
the HIF1α-VEGF signaling pathway in human PTC tissues. Recent
studies supported these findings. For instance, PEDF was able to
suppress angiogenesis in a gastric carcinoma xenograft model by
down regulating HIF1α and VEGF expression (27). Furthermore, PEDF suppressed tumor
growth in a cervical cancer cell line by down regulating the
expression of VEGF and HIF1α, which demonstrated the
anti-angiogenic activity of PEDF (26). In addition, the HIF1α and VEGF/PEDF
signaling pathway is targeted in impacting nasopharyngeal carcinoma
cell proliferation and angiogenesis (28). PEDF may not be directly regulated by
HIF1α, as indicated by the inverse association between PEDF and
HIF1α identified in the current study. Therefore, HIF1α may also
regulate the expression of PEDF via other factors in human PTC,
which requires further analysis.
In conclusion, the results of the current study
suggested that PEDF expression levels were significantly associated
with certain aggressive clinicopathological features in human PTC,
and indicated that PEDF may exert an anti-angiogenesis role by
modulating the HIF1α-VEGF signaling pathway, which has an important
role in the metastasis of PTC. Additional studies should further
investigate these results using in vitro and in vivo
models, and elucidate the mechanism underlying the function of PEDF
in anti-angiogenesis, through the knock down of the PEDF gene.
Acknowledgements
This study was supported by the Natural Science
Foundation of Heilongjiang Province of China (grant no.
D201228).
References
|
1
|
Udelsman R and Chen H: The current
management of thyroid cancer. Adv Surg. 33:1–27. 1999.PubMed/NCBI
|
|
2
|
Paterson IC, Greenlee R and Jones D Adams:
Thyroid cancer in Wales, 1985–1996: A cancer registry-based study.
Clin Oncol (R Coll Radiol). 44:245–251. 1999. View Article : Google Scholar
|
|
3
|
Davies L and Welch HG: Increasing
incidence of thyroid cancer in the United States, 1973–2002. JAMA.
295:2164–2167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Lellis R, Lloyd R, Heitz PU and Eng C:
Pathology and genetics of tumors of the endocrine organs. IARC;
Lyon: 2004
|
|
5
|
Mazzaferri EL and Jhiang SM: Long-term
impact of initial surgical and medical therapy on papillary and
follicular thyroid cancer. Am J Med. 97:418–428. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schlumberger MJ: Papillary and follicular
thyroid carcinoma. N Engl J Med. 338:297–306. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Desgrosellier JS and Cheresh DA: Integrins
in cancer: Biological implications and therapeutic opportunities.
Nat Rev Cancer. 10:9–22. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kohn EC and Liotta LA: Molecular insights
into cancer invasion: Strategies for prevention and intervention.
Cancer Res. 55:1856–1862. 1995.PubMed/NCBI
|
|
10
|
Nucera C, Lawler J and Parangi S:
BRAF(V600E) and microenvironment in thyroid cancer: A functional
link to drive cancer progression. Cancer Res. 71:2417–2422. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Zhai Z, Liu D, Zhong X, Meng X, Yang
Q, Liu J and Li H: CD105 promotes hepatocarcinoma cell invasion and
metastasis through VEGF. Tumour Biol. 36:737–745. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tombran-Tink J, Chader GG and Johnson LV:
PEDF: A pigment epithelium-derived factor with potent neuronal
differentiative activity. Exp Eye Res. 53:411–414. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Becerra SP and Notario V: The effects of
PEDF on cancer biology: Mechanisms of action and therapeutic
potential. Nat Rev Cancer. 13:258–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Filleur S, Volz K, Nelius T, Mirochnik Y,
Huang H, Zaichuk TA, Aymerich MS, Becerra SP, Yap R, Veliceasa D,
et al: Two functional epitopes of pigment epithelial-derived factor
block angiogenesis and induce differentiation in prostate cancer.
Cancer Res. 65:5144–5152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Crawford SE, Stellmach V, Ranalli M, Huang
X, Huang L, Volpert O, De Vries GH, Abramson LP and Bouck N:
Pigment epithelium-derived factor (PEDF) in neuroblastoma: A
multifunctional mediator of Schwann cell antitumor activity. J Cell
Sci. 114:4421–4428. 2001.PubMed/NCBI
|
|
16
|
Tanimoto S, Kanamoto T, Mizukami M, Aoyama
H and Kiuchi Y: Pigment epithelium-derived factor promotes neurite
outgrowth of retinal cells. Hiroshima J Med Sci. 55:109–116.
2006.PubMed/NCBI
|
|
17
|
Unterlauft JD, Eichler W, Kuhne K, Yang
XM, Yafai Y, Wiedemann P, Reichenbach A and Claudepierre T: Pigment
epithelium-derived factor released by Muller glial cells exerts
neuroprotective effects on retinal ganglion cells. Neurochem Res.
37:1524–1533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao W, Tombran-Tink J, Chen W, Mrazek D,
Elias R and McGinnis JF: Pigment epithelium-derived factor protects
cultured retinal neurons against hydrogen peroxide -induced cell
death. J Neurosci Res. 57:789–800. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dawson DW, Volpert OV, Gillis P, Crawford
SE, Xu H, Benedict W and Bouck NP: Pigment epithelium-derived
factor: A potent inhibitor of angiogenesis. Science. 285:245–248.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stellmach V, Crawford SE, Zhou W and Bouck
N: Prevention of ischemia-induced retinopathy by the natural ocular
antiangiogenic agent pigment epithelium-derived factor. Proc Natl
Acad Sci USA. 98:2593–2597. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Browne M, Stellmach V, Cornwell M, Chung
C, Doll JA, Lee EJ, Jameson JL, Reynolds M, Superina RA, Abramson
LP and Crawford SE: Gene transfer of pigment epithelium-derived
factor suppresses tumor growth and angiogenesis in a hepatoblastoma
xenograft model. Pediatr Res. 60:282–287. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garcia M, Fernandez-Garcia NI, Rivas V,
Carretero M, Escamez MJ, Gonzalez-Martin A, Medrano EE, Volpert O,
Jorcano JL, Jimenez B, et al: Inhibition of xenografted human
melanoma growth and prevention of metastasis development by dual
antiangiogenic/antitumor activities of pigment epithelium-derived
factor. Cancer Res. 64:5632–5642. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu QJ, Gong CY, Luo ST, Zhang DM, Zhang S,
Shi HS, Lu L, Yan HX, He SS, Li DD, et al: AAV-mediated human PEDF
inhibits tumor growth and metastasis in murine colorectal
peritoneal carcinomatosis model. BMC Cancer. 12:1292012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maik-Rachline G, Shaltiel S and Seger R:
Extracellular phosphorylation converts pigment epithelium-derived
factor from a neurotrophic to an antiangiogenic factor. Blood.
105:670–678. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang H, Cheng R, Liu G, Zhong Q, Li C, Cai
W, Yang Z, Ma J, Yang X and Gao G: PEDF inhibits growth of
retinoblastoma by anti-angiogenic activity. Cancer Sci.
2100:2419–2425. 2009. View Article : Google Scholar
|
|
26
|
Yang J, Chen S, Huang X, Han J, Wang Q,
Shi D, Cheng R, Gao G and Yang X: Growth suppression of cervical
carcinoma by pigment epithelium-derived factor via
anti-angiogenesis. Cancer Biol Ther. 9:967–974. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Han J, Yang X, Shao C, Xu Z,
Cheng R, Cai W, Ma J, Yang Z and Gao G: Pigment epithelium-derived
factor inhibits angiogenesis and growth of gastric carcinoma by
down-regulation of VEGF. Oncol Rep. 26:681–686. 2011.PubMed/NCBI
|
|
28
|
Xu Z, Fang S, Zuo Y, Zhang Y, Cheng R,
Wang Q, Yang Z, Cai W, Ma J, Yang X and Gao G: Combination of
pigment epithelium-derived factor with radiotherapy enhances the
Anti-tumor effects on nasopharyngeal carcinoma by down-regulating
vascular endothelial growth factor expression and angiogenesis.
Cancer Sci. 102:1789–1798. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Birner P, Schindl M, Obermair A, Plank C,
Breitenecker G and Oberhuber G: Overexpression of hypoxia-inducible
factor 1alpha is a marker for an unfavorable prognosis in
early-stage invasive cervical cancer. Cancer Res. 60:4693–4696.
2000.PubMed/NCBI
|
|
30
|
Schindl M, Schoppmann SF, Samonigg H,
Hausmaninger H, Kwasny W, Gnant M, Jakesz R, Kubista E, Birner P
and Oberhuber G: Austrian Breast and Colorectal Cancer Study Group:
Overexpression of hypoxia-inducible factor 1alpha is associated
with an unfavorable prognosis in lymph node-positive breast cancer.
Clin Cancer Res. 8:1831–1837. 2002.PubMed/NCBI
|
|
31
|
Koperek O, Akin E, Asari R, Niederle B and
Neuhold N: Expression of hypoxia-inducible factor 1 alpha in
papillary thyroid carcinoma is associated with desmoplastic stromal
reaction and lymph node metastasis. Virchows Arch. 463:795–802.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hedinger C, Williams ED and Sobin LH: The
WHO histological classification of thyroid tumors: A commentary on
the second edition. Cancer. 63:908–911. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stratmann M, Sekulla C, Dralle H and
Brauckhoff M: Current TNM system of the UICC/AJCC: The prognostic
significance for differentiated thyroid carcinoma. Chirurg.
83:646–651. 2012.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang L, Liu R, Li W, Chen C, Katoh H, Chen
GY, McNally B, Lin L, Zhou P, Zuo T, et al: Somatic single hits
inactivate the X-linked tumor suppressor FOXP3 in the prostate.
Cancer Cell. 16:336–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cai J, Parr C, Watkins G, Jiang WG and
Boulton M: Decreased pigment epithelium-derived factor expression
in human breast cancer progression. Clinical Cancer Res.
12:3510–3517. 2006. View Article : Google Scholar
|
|
37
|
Orgaz JL, Ladhani O, Hoek KS,
Fernández-Barral A, Mihic D, Aguilera O, Seftor EA, Bernad A,
Rodríguez-Peralto JL, Hendrix MJ, et al: Loss of pigment
epithelium-derived factor enables migration, invasion and
metastatic spread of human melanoma. Oncogene. 28:4147–4161. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lan L, Luo Y, Cui D, Shi BY, Deng W, Huo
LL, Chen HL, Zhang GY and Deng LL: Epithelial-mesenchymal
transition triggers cancer stem cell generation in human thyroid
cancer cells. Int J Oncol. 43:113–120. 2013.PubMed/NCBI
|
|
39
|
Koperek O, Bergner O, Pichlhöfer B,
Oberndorfer F, Hainfellner JA, Kaserer K, Horvat R, Harris AL,
Niederle B and Birner P: Expression of hypoxia-associated proteins
in sporadic medullary thyroid cancer is associated with
desmoplastic stroma reaction and lymph node metastasis and may
indicate somatic mutations in the VHL gene. J Pathol. 225:63–72.
2011. View Article : Google Scholar : PubMed/NCBI
|