Introduction
Human cytochrome P450 (CYP) 2A6 and mouse CYP2A5
share 82% of their amino acid sequences, and belong to the
cytochrome 2A family of enzymes (1,2). The two
enzymes are mainly expressed in the liver and are involved in the
metabolization of a series of xenobiotics including nicotine and
methoxyflurane, and in activating a variety of precarcinogens such
as afltoxin B1, tobacco-specific nitrosamines,
N-nitrosodiethylamine,
4-(methylnitrosamino)-1(3-pyridyl)-1-butanone and
N-nitrosonornicotine (3–5). Human CYP2A6 has received more attention,
as it is a key enzyme with respect to the biotransformation of
nicotine into inactive cotinine and the activation of
tobacco-specific nitrosamines (5).
Thus, the deliberate inhibition of CYP2A6 activity is a potential
strategy in chemoprevention, since CYP2A6 inhibition has been found
to prolong the biological lifetime of nicotine, therefore
decreasing cigarette consumption (6).
CYP2A6 has also been found to reduce the number of active
carcinogens transferred from tobacco (7).
Celery (Apium graveolens) belongs to the
umbelliferae family and is widely used in food and for medicinal
purposes. Studies have found that celery extracts can exert
beneficial effects, including antioxidant, hypoglycemic,
hypolipidemic and anti-platelet aggregation effects (8,9). A
previous study demonstrated that the juice extracted from Apium
graveolen and Petroselinum sativum extended the
analgesic effect of aminopyrine and paracetamol, suggesting that
the juice inhibited CYP activity in the liver, including that of
aminopyrine N-demethylase (10).
Whether or not other CYP enzymes are inhibited remains unclear.
Coumarin 7-hydroxylation is catalyzed by CYP2A enzymes and is thus
considered to be a specific indicator for the presence of CYP2A
enzymes, such as CYP2A5 in mice and CYP2A6 and CYP2A13 in humans
(11). Notably, celery extract
contains a number of coumarin derivatives including furocoumarins
and pyranocoumarins. The present study therefore postulates that
celery extract may inhibit CYP2A5/6-mediated coumarin 7-hydrolase
activity according to a structure-activity association. To the best
of our knowledge, there have been few studies on the inhibitory
effects and mechanisms of celery extract on CYP2A5/6. Therefore,
the objectives of the present study are to assess the inhibition
potency of celery extract on CYP2A5/6 activity using mouse and
human liver microsomes, and to analyze the inhibition mechanisms of
celery extract using time- and nicotinamide adenine dinucleotide
phosphate (NADPH)-dependent, ultracentrifugation tests. Finally,
the present study will clarify whether or not furocoumarin
5-methoxypsoralen (bergapten), present in celery extract, is the
predominant inhibitor of CYP2A5/6.
Materials and methods
Chemicals and reagents
Coumarin, 4-methylumbelliferyl and NADPH were
purchased from Sigma-Aldrich (EMD Millipore, Billerica, MA, USA).
The 7-hydroxycoumarin was purchased from Aladdin Shanghai
Biochemical Tech Co. Ltd. (Shanghai, China). Human liver microsomes
were purchased from the Research Institute for Liver Diseases Co.
Ltd. (Shanghai, China). Ultra-pure water was prepared with Milli-Q
(EMD Millipore). High performance liquid chromatography
(HPLC)-grade acetonitrile was obtained from Tedia (Fairfield, OH,
USA). All other reagents were of analytical grade or above and
commercially available.
Celery extract preparation
In total, 15 g fresh celery was used to make celery
powder by pulverizeration. Celery powder (4 g) was extracted with
60 ml petroleum ether at 40°C following saturation for three times.
The extracts were filtered and evaporated resulting in a volume of
~5 ml, and finally metered to 10 ml with petroleum ether. The final
liquid extract was stored at 4°C prior to use.
Animal experiments
All animal care and experimental protocols were
approved by the Animal Center of Chongqing Medical University
(Chongqing, China). Male Swiss mice (15–25 g, age 7 weeks) were
purchased from the Animal Center of Chongqing Medical University.
The mice were housed in a temperature-controlled room, with free
access to rodent chow and water and a 12:12 h light-dark cycle.
Subsequent to a one-week acclimation period, celery liquid extract
(0.2 mg/l; n=5) or solvent (n=5) was orally administered twice to
the mice, as previously described by Jakovljevic et al
(10). The mice were sacrificed 2 h
after the last administration, and their livers were harvested. The
liver microsomes were then prepared and the CYP2A5 activity was
determined as described below.
Mouse liver microsomes
preparation
Mouse liver microsomes were prepared from male Swiss
mice (15–25 g, age 6–8 weeks) as described by Pinto et al
(12). The protein concentrations of
microsomal samples were determined by the Lowry method (13).
Assay of coumarin 7-hydroxylation in
liver microsomes
A coumarin 7-hydroxylation assay was performed as
described by Aitio (14) with certain
modifications. The procedure was as follows. The incubation mixture
contained mouse (0.4 mg/l) or human liver microsomes (0.1 mg/l; a
kind gift from Dr Guo of Chongqing Medical University), coumarin
(100 mmol/l) and 100 mM potassium phosphate buffer (pH 7.4)
creating a total volume of 180 µl. Subsequent to 3 min
pre-incubation, the reaction was initiated by the addition of 20 µl
of 10 mM NADPH and performed for either 20 min (mouse liver
microsomes) or 15 min (human liver microsomes) at 37°C, and
terminated by the addition of 200 µl of 100 nM
4-methylumbelliferyl, an internal standard, in ice-cold
acetonitrile. The resulting samples were then centrifuged at 10,000
× g for 15 min. The product formed, 7-hydroxycoumarin
(umbelliferone), was quantified by HPLC, as previously described by
Farooq et al (15). Protein
concentration and incubation time were optimized and product
formation was linear under the aforementioned conditions.
Microsomal CYP2A5/6 inactivation
assay
To examine the concentration-dependence and the
value of the concentration of the inhibitor where binding is
reduced by half (IC50) of CYP2A5/6 inactivation, celery
extracts of increasing concentrations were pre-incubated with the
incubation mixture at 37°C for 3 min, and the same pre-incubation
mixture was added to the NADPH solution to start the reaction. The
reaction was performed as aforementioned to determine the level of
residual coumarin 7-hydroxylase activity.
To test the time-dependence of CYP2A5 inactivation,
celery extracts of increasing concentrations (0, 200, 400, 600, 800
and 1,000 µg/ml) were pre-incubated with the liver microsomes at
37°C for the selected time points. At selected time points (0, 3, 6
and 9 min), 20 µl of mixture was added to the NADPH solution to
start the reaction. The reaction was performed as aforementioned to
determine the residual coumarin 7-hydroxylase activity.
To investigate the dependence of CYP2A5/6
inactivation on NADPH, celery extract was pre-incubated with the
mouse or human liver microsomes and 100 mM of potassium phosphate
buffer (pH 7.4) at 37°C for 30 min in the presence or absence of
NADPH. The reaction was then initiated via the addition of coumarin
and NADPH and performed as aforementioned to determine the level of
residual coumarin 7-hydroxylase activity.
IC50 shift assay
The IC50 shift assay was performed as
described by Perloff et al (16) with certain modifications. Celery
extract of increasing concentration was pre-incubated with the
human liver microsomes, NADPH and potassium phosphate buffer for 0
or 30 min. Subsequent to pre-incubation, the pre-incubation
mixtures were then transferred to the secondary incubations
containing coumarin and NADPH to initiate the reaction. This
transferal took place at approximately the Km, which is
the concentration of substrate required to produce 50% of the
Vmax (maximum velocity or rate at which the enzyme
catalyzed the reaction). The reaction was performed as
aforementioned to determine the level of residual coumarin
7-hydroxylase activity and the value of IC50.
Ultracentrifugation test
The ultracentrifugation test was performed as
described by Lee et al (17)
with certain modifications. To evaluate the reversibility of the
drug-enzyme interaction, the celery extract was incubated with the
mouse liver microsomes and a potassium phosphate buffer (pH 7.4) at
37°C for 30 min. The microsomes were re-isolated by
ultracentrifugation of the pre-incubation mixtures at 80,000 ×
g for 60 min at 4°C. The microsomes were washed twice with
0.1 M potassium phosphate buffer (pH 7.4). The residual CYP2A5
activity was then determined as aforementioned.
Data analysis
Data is expressed as the mean ± standard error of
the mean. All tests were performed at least 3 times. The enzyme
kinetic and the Lineweaver-Burk plot analyses were performed with
GraphPad Prism 5.0 (GraphPad software, Inc., La Jolla, CA, USA).
Statistical significance was calculated using a one-tailed Student
t-test, or a one-way analysis of variance and Dunnet's test as a
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Celery extract inhibits CYP2A5/6
activity
The potential inhibitory effect of celery extract on
CYP2A5/6 activity was investigated in the mouse and human liver
microsomes, using coumarin as a specific probe. The Km
and Vmax values for the coumarin 7-hydroxylation of
CYP2A5 were 1.4 µM and 15.3 pM umbelliferone/min/mg microsomal
protein. The Km and Vmax values for CYP2A6
were 1.9 µM and 48.3 pM umbelliferone/min/mg microsomal protein.
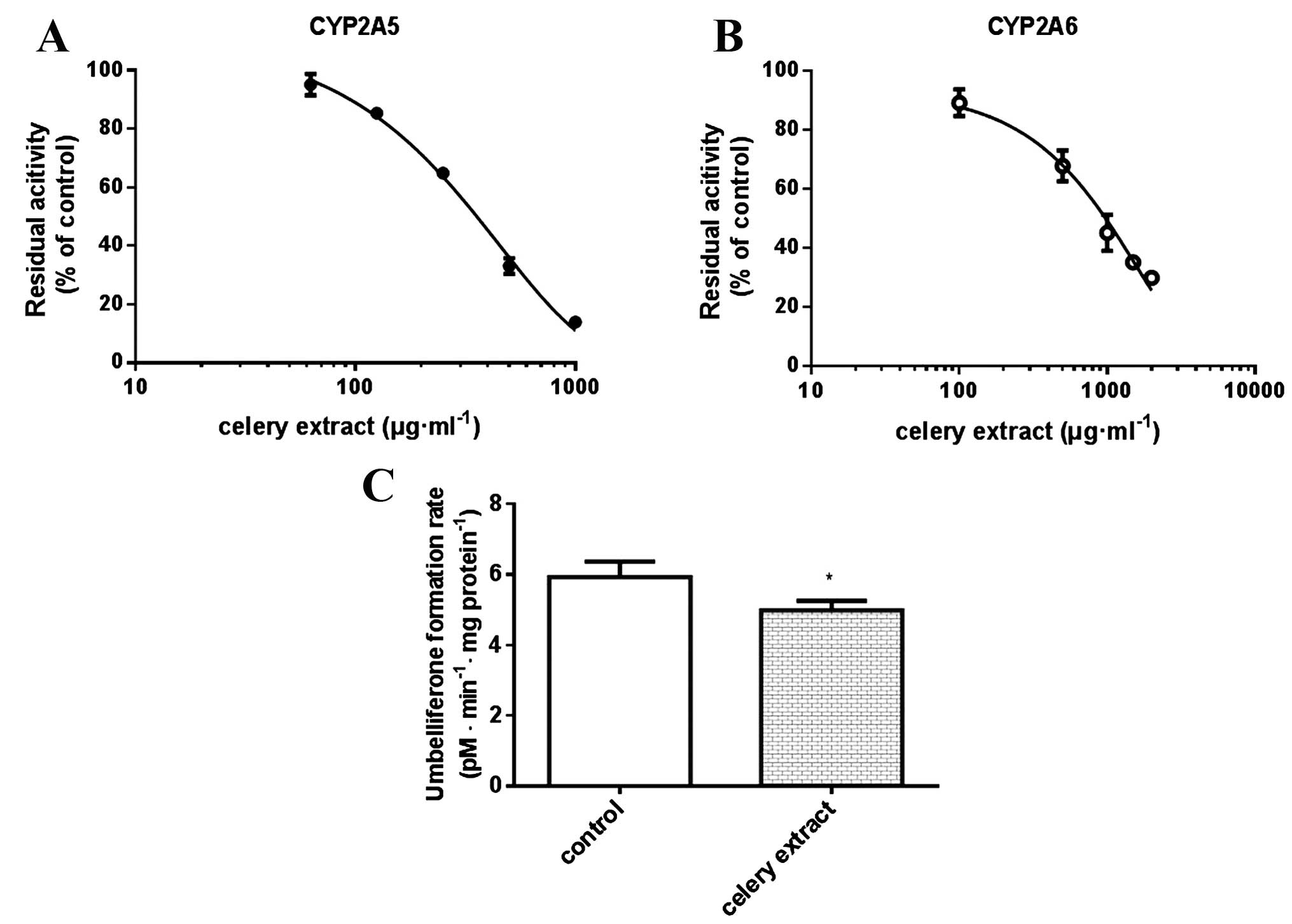
When celery extract was added to the incubation mixture at varying
concentrations, coumarin 7-hydroxylation was
concentration-dependently inhibited with IC50 values of
345.1 and 888.7 µg/ml, respectively, for CYP2A5 and CYP2A6
(Fig. 1A and B). The animal
experiment verified that celery extract significantly decreased the
CYP2A5 activity by 16% (P=0.039) (Fig.
1C). Therefore, celery extract was found to be an inhibitor of
CYP2A5/6.
Celery extract reversibly inhibits
mouse CYP2A5 but irreversibly inhibits human CYP2A6
The present study used kinetic inhibition studies to
investigate the inhibition modes of the celery extract on CYP2A5/6.
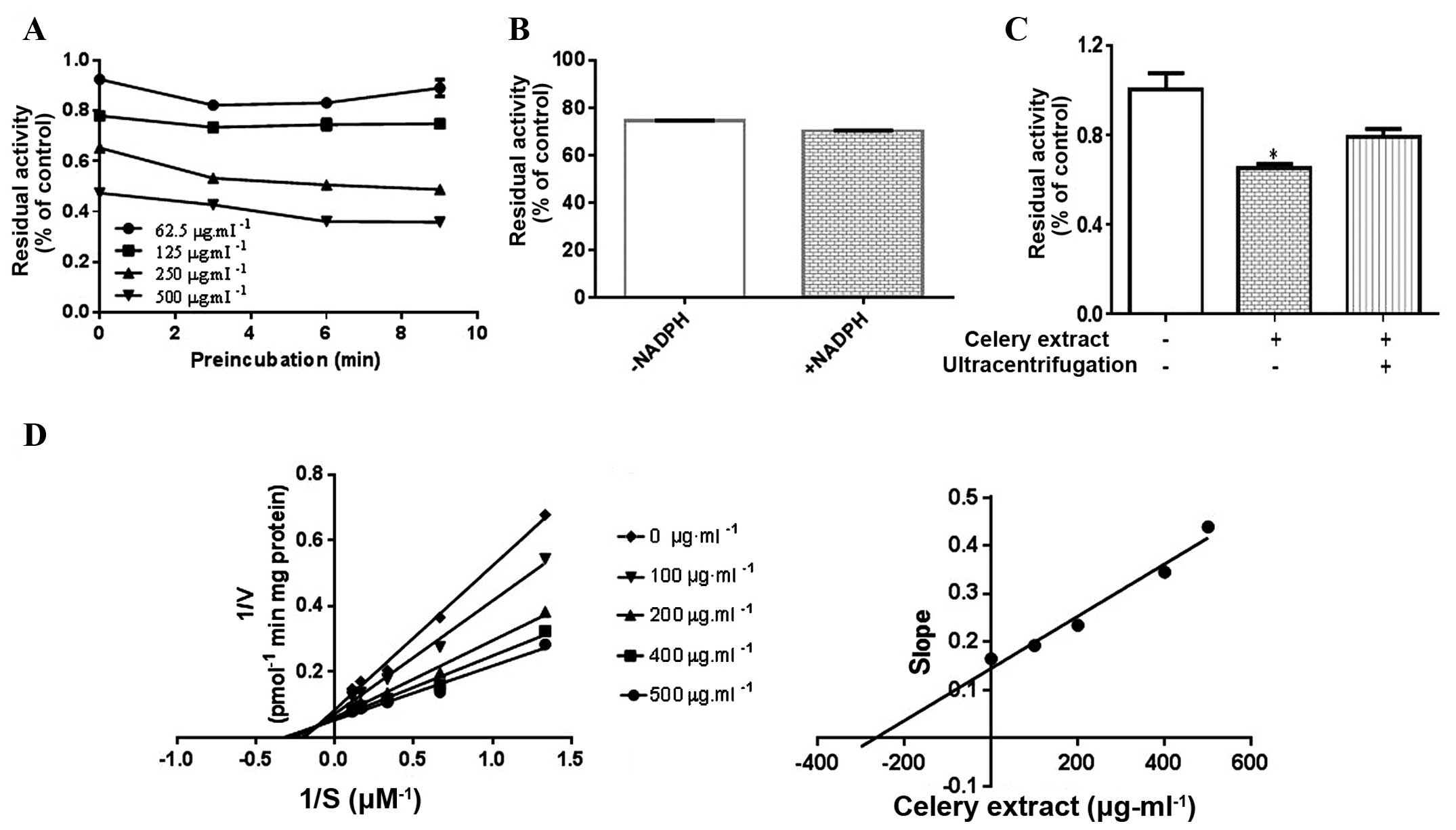
Time-dependent inhibition of CYP2A5 with celery extract was not
observed (Fig. 2A). Celery extract
samples that were pre-incubated in the presence and absence of
NADPH demonstrated the same levels of CYP2A5 activity (Fig. 2B), suggesting that CYP2A5 inactivation
occurs independently of NADPH. The ultracentrifugation test
demonstrated that CYP2A5 activity returned to a normal level if the
incubation mixture was ultracentrifuged at 80,000 × g for 60
min, indicating reversible non-covalent binding of the inhibitor to
CYP2A5, and the reversible inhibition of celery extract to CYP2A5
(Fig. 2C). The aforementioned results
suggest that celery extract is a reversible inhibitor for CYP2A5.
The Lineweaver-Burk plot demonstrates that the lines intersect on
the second quadrant, indicating that the inhibition of CYP2A5 was
mixed competitive/noncompetitive, with a Ki value of
266.4 µg/ml (Fig. 2D).
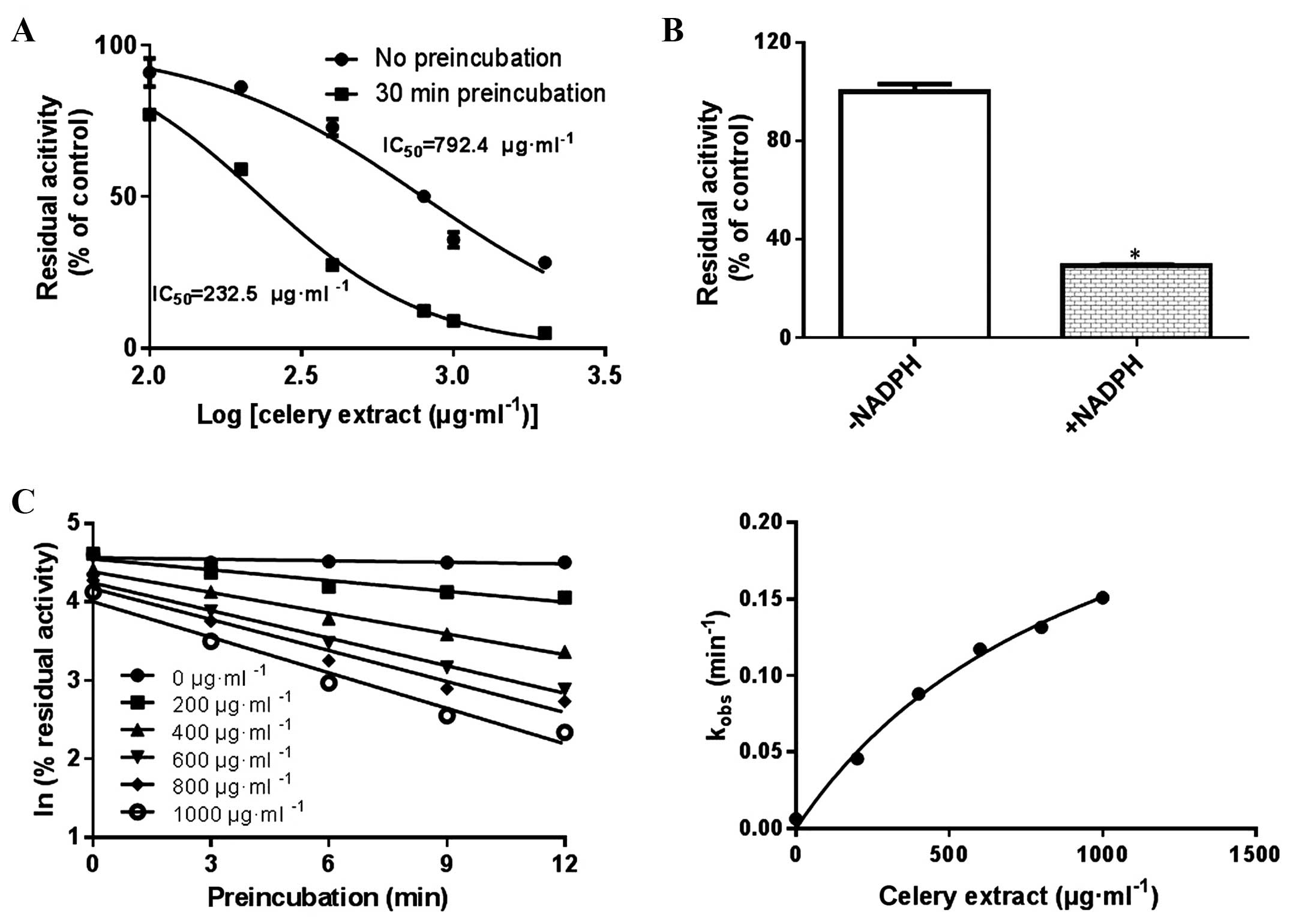
By contrast, time-dependent inhibition of CYP2A6 by
celery extract was observed, since a 3.4-fold shift for CYP2A6 was
observed in the IC50-shift experiments (Fig. 3A). Samples that were pre-incubated
with celery extract in the presence of NADPH exhibited less
activity compared with those incubated in the absence of NADPH
(P=0.019; Fig. 3B), suggesting the
dependence of CYP2A6 inactivation on NADPH. Therefore, the
aforementioned results revealed that the inactivation of CYP2A6
activity by celery extract is dependent on celery concentration,
time and NADPH, indicating that celery extract is an irreversible
inhibitor for CYP2A6. To characterize the irreversible inhibition
of CYP2A6 by celery extract, the Ki and
Kinact values were determined. The Ki and
Kinact values were 1,018 µg/ml and 0.3/min, respectively
(Fig. 3C).
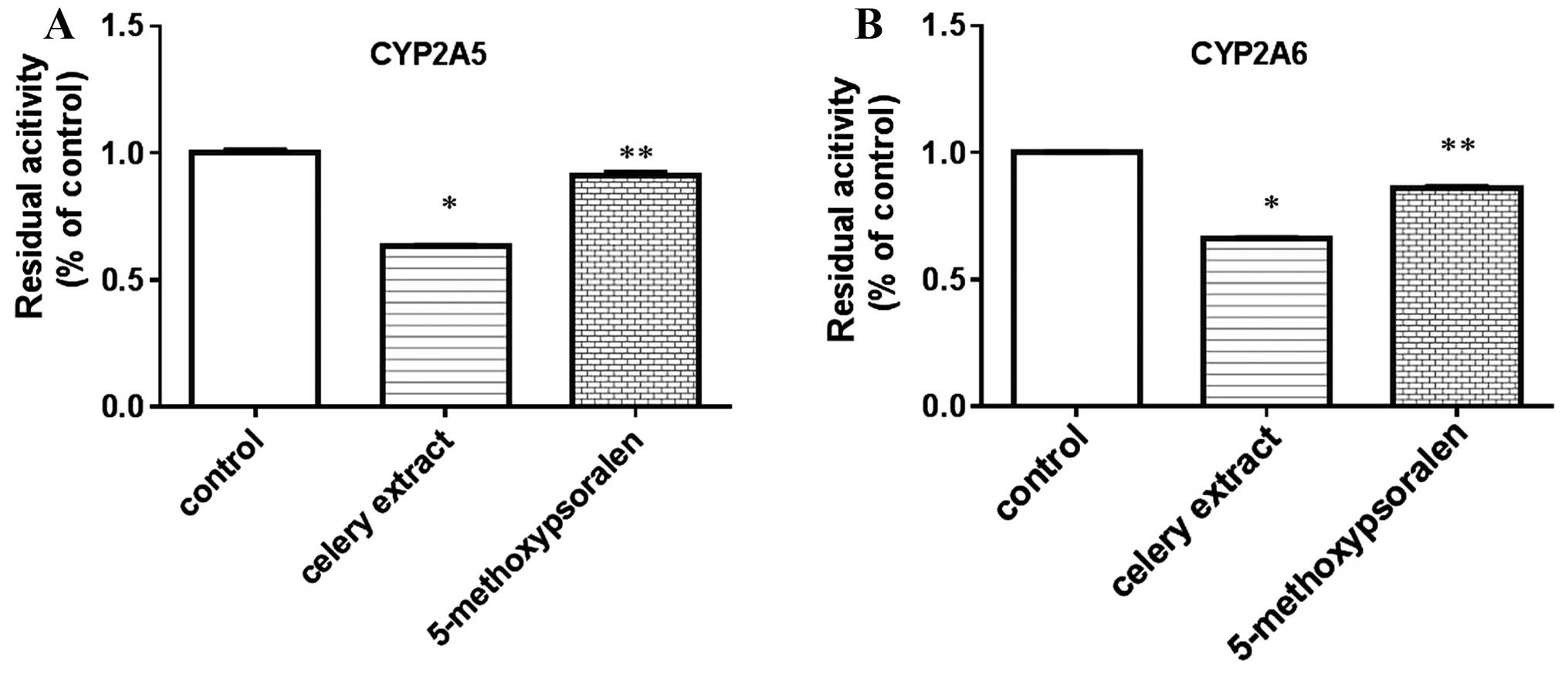
5-methoxypsoralen reduces less
CYP2A5/6 activity compared with celery extract
To explore whether 5-methoxypsoralen, the main
component present in celery extract, played a critical role in the
inhibition of CYP2A5/6 activity, the present study analyzed the
inhibitory effects of 5-methoxypsoralen. Fig. 4 demonstrates that 5-methoxypsoralen
(0.3 µM) significantly decreased CYP2A5/6 activity (P=0.037), but
to a lesser extent compared with celery extract (400 µg/ml) did,
compared with the control (P=0.031). The results suggest that other
coumarin derivatives, besides 5-methoxypsoralen, also contribute to
the inhibition of CYP2A5/6 activity.
Discussion
The present study revealed that celery extract
inhibited mouse CYP2A5- and human CYP2A6-mediated coumarin
7-hydroxylase activity via different mechanisms. To explore whether
celery extract played a concentration-dependent role on the level
of CYP2A5/6 activity, the present study used 3 different
concentrations of celery extract. The results demonstrate that
celery extract is an inhibitor of CYP2A5/6 (Fig. 1A). For CYP2A5, celery extract behaves
as a reversible (mixed competitive and noncompetitive) inhibitor,
but for CYP2A6, celery extract behaves as an irreversible
inhibitor. In addition, it appears that celery extract is a more
potent inhibitor of CYP2A5 compared with CYP2A6, since the
IC50 value for CYP2A5 is ~2-times smaller than that of
CYP2A6. A previous study also identified that 8-methoxypsoralen, a
furocoumarin present in celery extract, is a more potent
inactivator for CYP2A5 compared with for CYP2A6 in vitro,
with IC50 values 1.0 and 5.4 µM, respectively (18).
These differences may be associated with the size of
mouse CYP2A5 and human CYP2A6 active sites. The majority of
compounds studied, including furocoumarins, metyrapone, miconazole
and lactone derivatives, have been found to be stronger suppressors
of CYP2A5 activity than of CYP2A6 activity (18–20).
Previous studies demonstrated that larger compounds were more
powerful inhibitors of CYP2A5 activity than of CYP2A6 activity,
suggesting that CYP2A5 has a larger active site compared with
CYP2A6 (19,21). Therefore, the compounds in celery
extract are more likely to get into, and interact with, the active
site of CYP2A5. These differences may be due to amino acid changes
in the active sites of these enzymes. A single mutation, whereby
phenylalanine 209 is substituted by leucine in the
substrate-binding site, changes the specificity of CYP2A5 from
coumarin to steroid hydroxylation (19,22).
Therefore, similar amino acid sequences are shared in CYP2A5/6
enzymes, but small amino acid changes between active sites affect
their substrate-recognition ability. The present study supports
previous studies that revealed that CYP2A5 and CYP2A6 have
different substrates and inhibitor specificities (21,23).
In addition, the present study demonstrated that the
coumarin derivative 5-methoxypsoralen in celery extract markedly
inhibited CYP2A5/6 activity, but to a lesser extent than celery
extract did, suggesting that other coumarin derivatives besides
5-methoxypsoralen also contribute to the inactivation of CYP2A5/6
activity. A coumarin derivative that may be present in celery
extract was considered to be the furocoumarin 8-methoxypsoralen,
since it is the only well-characterized inhibitor for CYP2A enzymes
including CYP2A5 and CYP2A6 and as it also demonstrated distinct
inhibition modes for CYP2A5 and CYP2A6 (24–26).
Pilot studies suggested that inactive variations of
the CYP2A6 gene have been revealed to markedly increase the
bioavailability of nicotine and thus decrease cigarette
consumption, and reduce the risk of developing lung cancer due to
smoking (27–29). Therefore, CYP2A6 inhibitors may act as
promising adjunct drugs in smoking cessation therapy. Whether
celery extract, as a CYP2A6 inhibitor, could affect nicotine
kinetics in vivo and therefore that affects smoking
behaviors must be investigated in another study.
In conclusion, the present study suggests that
celery extract inhibits mouse CYP2A5 and human CYP2A6 via different
mechanisms, suggesting that the mouse model is not applicable for
future studies involving the inhibition of CYP2A enzymes by celery
extract. Future studies must be performed to identify which
components of celery extract play the main role in the inhibition
of CYP2A enzyme activity, and to investigate whether the deliberate
inhibition of CYP2A6 by celery extract can modulate smoking
behavior and therefore reduce the risk of lung cancer.
Acknowledgements
This study was supported by the National Nature
Science Foundation of China (grant no. 81370403) and Specialized
Research Fund for the Doctoral Program of Higher Education (grant
no. 20125503110008).
References
|
1
|
Raunio H and Rahnasto-Rilla M: CYP2A6:
Genetics, structure, regulation, and function. Drug Metabol Drug
Interact. 27:73–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raunio H, Rautio A and Pelkonen O: The
CYP2A subfamily: Function, expression and genetic polymorphism.
IARC Sci Publ. 148:197–207. 1999.
|
|
3
|
Pelkonen P, Lang MA, Wild CP, Negishi M
and Juvonen RO: Activation of aflatoxin B1 by mouse CYP2A enzymes
and cytotoxicity in recombinant yeast cells. Eur J Pharmacol.
292:67–73. 1994.PubMed/NCBI
|
|
4
|
Camus AM, Geneste O, Honkakoski P,
Béréziat JC, Henderson CJ, Wolf CR, Bartsch H and Lang MA: High
variability of nitrosamine metabolism among individuals: Role of
cytochromes P450 2A6 and 2E1 in the dealkylation of
N-nitrosodimethylamine and N-nitrosodiethylamine in mice and
humans. Mol Carcinog. 7:268–275. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oscarson M: Genetic polymorphisms in the
cytochrome P450 2A6 (CYP2A6) gene: Implications for interindividual
differences in nicotine metabolism. Drug Metab Dispos. 29:91–95.
2001.PubMed/NCBI
|
|
6
|
Tyndale RF and Sellers EM: Variable
CYP2A6-mediated nicotine metabolism alters smoking behavior and
risk. Drug Metab Dispos. 29:548–552. 2001.PubMed/NCBI
|
|
7
|
Miyamoto M, Umetsu Y, Dosaka-Akita H,
Sawamura Y, Yokota J, Kunitoh H, Nemoto N, Sato K, Ariyoshi N and
Kamataki T: CYP2A6 gene deletion reduces susceptibility to lung
cancer. Biochem Biophys Res Commun. 261:658–660. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sowbhagya HB: Chemistry, technology, and
nutraceutical functions of celery (Apium graveolens L.): An
overview. Crit Rev Food Sci Nutr. 54:389–398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Madkour NK: Beneficial role of celery oil
in lowering the di(2-ethylhexyl) phthalate-induced testicular
damage. Toxicol Ind Health. 30:861–872. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jakovljevic V, Raskovic A, Popovic M and
Sabo J: The effect of celery and parsley juices on pharmacodynamic
activity of drugs involving cytochrome P450 in their metabolism.
Eur J Drug Metab Pharmacokinet. 27:153–156. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. Biol
Chem. 193:265–274. 1951.
|
|
12
|
Pinto LF, Moraes E, Albano RM, Silva MC,
Godoy W, Glisovic T and Lang MA: Rat oesophageal cytochrome P450
(CYP) monooxygenase system: Comparison to the liver and relevance
in N-nitrosodiethylamine carcinogenesis. Carcinogenesis.
22:1877–1883. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
14
|
Aitio A: A simple and sensitive assay of
7-ethoxycoumarin deethylation. Anal Biochem. 85:488–491. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Farooq S, Shakeel-u-Rehman, Dangroo NA,
Priya D, Banday JA, Sangwan PL, Qurishi MA, Koul S and Saxena AK:
Isolation, cytotoxicity evaluation and HPLC-quantification of the
chemical constituents from Prangos pabularia. PLoS One.
9:e1087132014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Perloff ES, Mason AK, Dehal SS, Blanchard
AP, Morgan L, Ho T, Dandeneau A, Crocker RM, Chandler CM, Boily N,
et al: Validation of cytochrome P450 time-dependent inhibition
assays: A two-time point IC50 shift approach facilitates kinact
assay design. Xenobiotica. 39:99–112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JY, Lee SY, Oh SJ, Lee KH, Jung YS and
Kim SK: Assessment of drug-drug interactions caused by
metabolism-dependent cytochrome P450 inhibition. Chem Biol
Interact. 198:49–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mäenpää J, Sigusch H, Raunio H, Syngelmä
T, Vuorela P, Vuorela H and Pelkonen O: Differential inhibition of
coumarin 7-hydroxylase activity in mouse and human liver
microsomes. Biochem Pharmacol. 45:1035–1042. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Juvonen RO, Gynther J, Pasanen M, Alhava E
and Poso A: Pronounced differences in inhibition potency of lactone
and non-lactone compounds for mouse and human coumarin
7-hydroxylases (CYP2A5 and CYP2A6). Xenobiotica. 30:81–92. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wood AW: Genetic regulation of coumarin
hydroxylase activity in mice. Biochemical characterization of the
enzyme from two inbred strains and their F1 hybrid. J Biol Chem.
254:5641–5646. 1979.PubMed/NCBI
|
|
21
|
Rahnasto M, Raunio H, Poso A and Juvonen
RO: More potent inhibition of human CYP2A6 than mouse CYP2A5 enzyme
activities by derivatives of phenylethylamine and benzaldehyde.
Xenobiotica. 33:529–539. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lindberg RL and Negishi M: Alteration of
mouse cytochrome P450coh substrate specificity by mutation of a
single amino-acid residue. Nature. 339:632–634. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Negishi M, Iwasaki M, Juvonen RO and Aida
K: Alteration of the substrate specificity of mouse 2A P450s by the
identity of residue-209: Steroid-binding site and orientation. J
Steroid Biochem Mol Biol. 43:1031–1036. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Visoni S, Meireles N, Monteiro L, Rossini
A and Pinto LF: Different modes of inhibition of mouse Cyp2a5 and
rat CYP2A3 by the food-derived 8-methoxypsoralen. Food Chem
Toxicol. 46:1190–1195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Draper AJ, Madan A and Parkinson A:
Inhibition of coumarin 7-hydroxylase activity in human liver
microsomes. Arch Biochem Biophys. 341:47–61. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
von Weymarn LB, Zhang QY, Ding X and
Hollenberg PF: Effects of 8-methoxypsoralen on cytochrome P450
2A13. Carcinogenesis. 26:621–629. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu YL, Xu Y, Li F, Chen H and Guo SL:
CYP2A6 deletion polymorphism is associated with decreased
susceptibility of lung cancer in Asian smokers: A meta-analysis.
Tumour Biol. 34:2651–2657. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mwenifumbo JC and Tyndale RF: Genetic
variability in CYP2A6 and the pharmacokinetics of nicotine.
Pharmacogenomics. 8:1385–1402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakajima M: Smoking behavior and related
cancers: The role of CYP2A6 polymorphisms. Curr Opin Mol Ther.
9:538–544. 2007.PubMed/NCBI
|