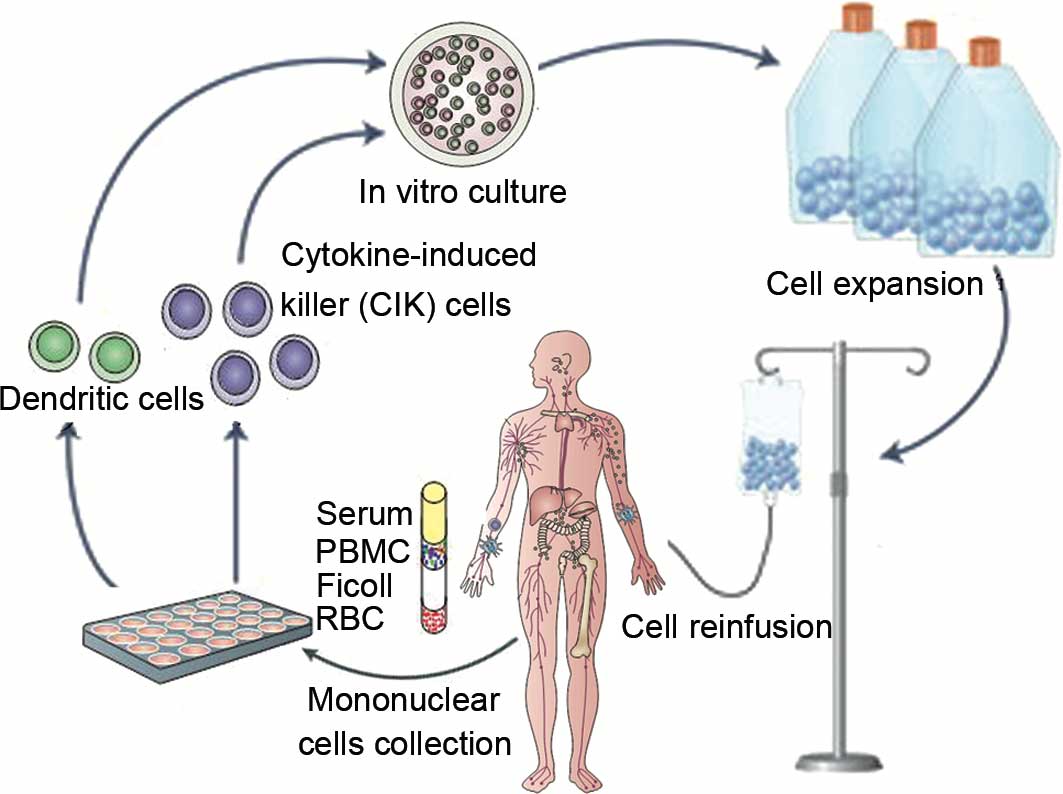
Introduction
In European countries, almost 1,000,000 cases of
cancer are diagnosed each year, and among them, >55% of cancers
occur in people who are aged >65 years (1). It is estimated that >60% of patients
with malignancies will be in this age group by the year 2020, if
the present demographic trends continue (2). Additionally, there are numerous
inadequacies in the treatment and care of older people (>65
years) with cancer compared to younger (≤50 years) patients, as
older cancer patients are usually diagnosed later in the disease
process, and in consideration of their physiological function, only
less aggressive treatment may be administered during the
therapeutic process, which leads to the comparatively lower
survival rates (3,4). Therefore, to choose a safe, effective,
and tolerable treatment for cancer patients aged >65 years is an
important and urgent challenge for clinicians.
Dendritic cells (DCs) are major antigen-presenting
cells. They are capable of capturing and processing tumor antigens,
expressing lymphocyte co-stimulatory molecules, and secreting
cytokines to initiate immune responses (5). Cytokine-induced killer cells (CIKs) are
a heterogeneous population of effector CD8 T cells with diverse T
cell receptor specificities, possessing non-major
histocompatibility complex (MHC)-restricted cytolytic activities
against tumor cells (6). Therefore,
CIKs can lyse tumor cells in a non-MHC-restricted manner and serve
as an alternative cellular immunotherapy (7). Immunotherapy has become the fourth major
treatment option for malignant tumors, following surgery,
chemotherapy and radiotherapy (8). A
number of adoptive immunotherapies mediated by various killer cells
have been reported, including tumor-infiltrating lymphocytes (TIL)
(9), lymphokine-activated killer
cells (LAK) (10), and anti-CD3
monoclonal antibody-induced killer cells (11). However, the therapeutic efficacy is
not as good as expected; it is hypothesized that this maybe
associated with the low anti-tumor activities of the immunocyte
(12). At present, CIKs have been
recognized as a new type of anti-tumor effector cell, which are
predominantly CD3+CD56+ type II natural
killer T-cells, and can be expanded from peripheral blood
mononuclear cells (PBMCs) and proliferate rapidly with the timed
addition of cytokines, such as interleukin-2 (IL-2), interferon-γ
(IFN-γ) and anti-CD3 monoclonal antibody (mAb) in vitro
(13). CIKs have stronger anti-tumor
activity and broader spectrum of tumor targets than other reported
anti-tumor effector cells (8). In
addition, CIKs can regulate and generally enhance the immune
functions in patients with several types of cancer (7). In previous years, a large number of
studies (5,14,15) have
shown that DCs could activate CIKs by co-culture on changing the
surface molecule expression, increasing cytokine secretion and
causing a higher cytolytic capacity (16). The present retrospective study was
performed to evaluate the effects and safety of DC-CIK treatment on
different types of cancers in patients aged >65 years.
Patients and methods
Patient selection
Between September 2014 and January 2015, 58 patients
with cancer, including solid tumors and hematological malignancies,
were treated using immunotherapy (Table
I). Criteria for inclusion in the present study were:
Histopathologically confirmed cancers; expected survival duration
>3 months; Karnofsky performance status (KPS) >40%; aged
>65 years; free of congestive heart failure, severe coronary
artery disease, cardiac arrhythmias, organ transplant, serious
infection, severe autoimmune disease or central nervous system
disease; and without chemotherapy or immunomodulatory treatment
during the previous 4 weeks. Informed consent was obtained from all
patients prior to therapy.
 | Table I.Demographic and clinical
characteristics of enrolled patients. |
Table I.
Demographic and clinical
characteristics of enrolled patients.
| Demographic and
clinical characteristics | Number of
patients |
|---|
| Age, years |
|
|
Range | 65–84 |
|
Median | 72.7 |
| Gender |
|
|
Male | 49 |
|
Female | 9 |
| Tumor types |
|
|
Gallbladder carcinoma | 4 |
|
Colorectal adenoma | 18 |
| Lung
cancer | 6 |
|
Leukemia | 2 |
| Gastric
carcinoma | 14 |
|
Esophagus cancer | 7 |
| Pelvic
cancer | 3 |
|
Multiple myeloma | 1 |
| Liver
cancer | 3 |
| Stage |
|
| II | 19 |
|
III–IV | 39 |
| Prior therapy |
|
|
Surgery | 14 |
|
Radiotherapy | 26 |
|
Chemotherapy | 48 |
| KPS |
|
|
40–60 | 11 |
|
60–80 | 40 |
|
>80 | 7 |
DCs and CIKs preparation
The DCs and CIKs were generated following Good
Manufacturing Practice guidelines (17). Recombinant human
granulocyte-macrophage colony-stimulating factor (rhGM-CSF;
Peprotech, London, UK) was injected (150 µg) 24 h prior to blood
collection to mobilize white blood cells. PBMCs were collected from
patients or healthy donors using a COBE Spectra continuous flow
blood cell separator (Caridian BCT, Lakewood, CO, USA). The
concentrated PBMCs were suspended immediately in CIK medium [X–VIVO
20 serum-free medium (Lonza, Cologne, Germany), 50 ng/ml anti-CD3
antibody (BD Biosciences, San Jose, CA, USA), 1,000 U/ml
recombinant human IL-2 (rhIL-2; Peprotech) and 1,000 U/ml
recombinant human IFN-γ (Peprotech)], at 37°C with 5%
CO2 and fed every 5 days in fresh complete medium with
various types of cytokines, as aforementioned.
For DC culture, PBMCs were cultured in X-VIVO 20
medium containing 1,000 U/ml IL-4 and 500 U/ml rhGM-CSF. Autologous
tumor lysate (100 µg/ml) was added at day 6 and co-cultured with
DCs for 24 h. For CIK activation, CIKs were co-cultured with DCs
loaded with tumor antigen for 7 days. The DC-CIK cells were
harvested and analyzed for phenotype, then suspended in 100 ml
saline for intravenous injection. The final cell products were
assessed for viability by the dye-exclusion test and checked twice
for possible contamination by bacteria, fungi and endotoxins.
CIKs phenotype detection
Approximately 5×105 CIKs, with or without
activation by DCs, were resuspended in 20 µl 2% fetal bovine serum
and 1% sodium azide in phosphate buffered saline (PBS) and
incubated with 10 µl antibody against CD3-fluorescein
isothiocyanate (FITC; clone, UCHT1; dilution, 1:2.5; catalog no.,
11-0038-80; eBioscience, San Diego, CA, USA), CD4/FITC and CD8-RPE
(clones, MT310 and DK25; dilution, 1:50; catalog no., FR86850), and
CD3-FITC/CD56-RPE (clones, UCHT1 and C5.9; dilution, 1:50; catalog
no., FR91250; Dako, Carpentaria, CA, USA) for 30 min at 4°C.
Following incubation, cells were washed twice with PBS and
resuspended in 1 ml staining buffer (BD Pharmingen, San Diego, CA,
USA). The cell population was analyzed using flow cytometry (FCM;
BD Biosciences).
Treatments
Patients received 1–4 cycles of DC-activated CIK
treatment at intervals of 1 month after chemotherapy/radiotherapy.
For each cycle, patients were treated with a median of
(8.3±0.61)x109 DC-activated CIKs (range,
8.0–12.6×109) at 1-day intervals for two intravenous
infusions (Fig. 1). Additional care
was provided whenever required. Clinical examinations of these
patients were performed by oncology specialists weekly or biweekly,
including a complete blood count, liver and renal function tests,
computed tomography (CT) or magnetic resonance imaging (MRI)
scan.
Efficacy evaluation
Efficacy evaluation was performed prior to and
subsequent to 2 cycles of DC-CIK treatment. The evaluation indexes
of therapeutic efficiency included the objective remission rate of
measurable focus region, which was performed according to the World
Health Organization criterion, and comparing the tumor size prior
to and subsequent to 2 cycles of treatment by CT/MRI. The curative
effect was further divided into complete remission (CR), partial
remission (PR), stable disease (SD) and progressive disease (PD).
The ratio of CR and PR was analyzed. The clinical benefit rate
(CBR) was also analyzed. The quality of life and physical
improvement were measured by KPS, and the assessment of pain grade
was evaluated by numeric rating scale (NRS). The tumor markers
carcinoembryonic antigen (CEA), α fetoprotein (AFP), carbohydrate
antigen-125 (CA125), carbohydrate antigen-199 (CA199), carbohydrate
antigen-724 (CA724), prostate special antigen (PSA) and
neuron-specific enolase (NSE) were also detected by
Immunofluorescence Analysis system (Zeiss AG, Thornwood, NY, USA).
The lymphocyte subpopulation, consisting of the CD3+,
CD3+CD4+ and CD3+CD8+
populations of T lymphocytes, was measured by FCM, and the ratio of
CD4:CD8 cells was also analyzed.
Statistical analysis
The measurement data was compared using Student's
t-test and analyzed by SPSS 17.0 software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Amplification of DC-CIK in vitro
The median count of untreated PBMCs of all patients
was 3.16×109 (range,
2.67×109-3.59×109) per cycle. The median
count of CIK cells after 14 days of amplification could reach
9.64×109 (range,
8.57×109-12.41×109) per cycle. On the basis
of trypan blue staining, the cellular vitality was >95%.
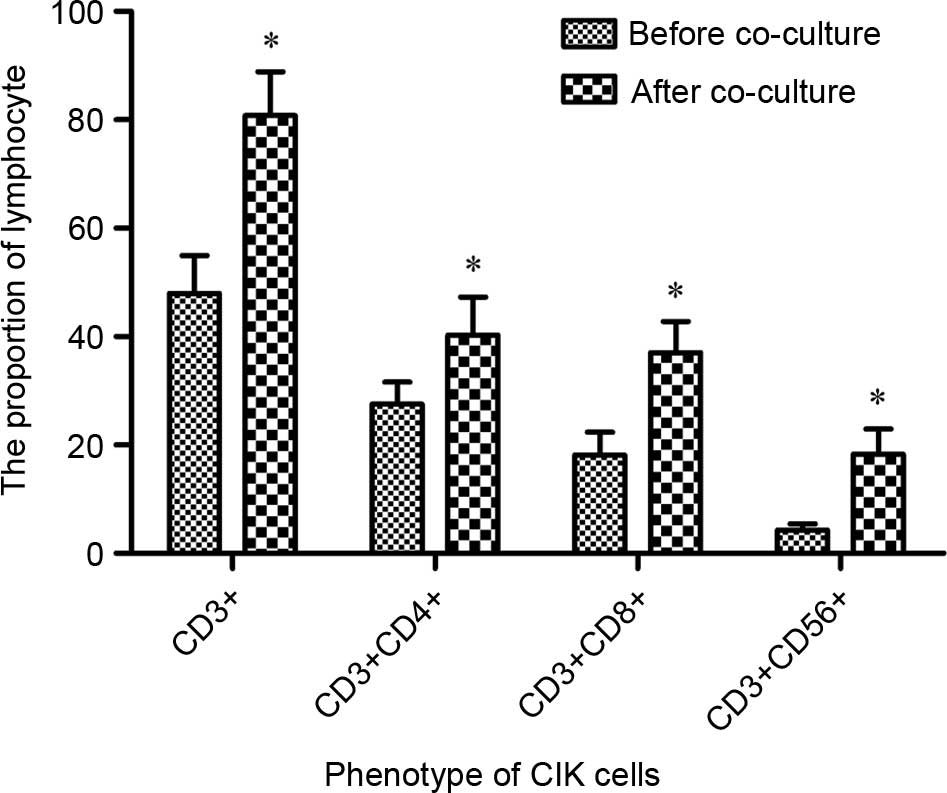
Phenotype detection of DC-activated
CIKs
To determine the immunological activation of DCs on
CIKs, the phenotype of CIKs prior to and subsequent to induction by
DCs was analyzed using FCM. Fig. 2
demonstrated that the population of CD3+,
CD3+CD4+, CD3+CD8+ and
CD3+CD56+CIK cells were significantly
increased between 47.94±7.02, 27.56±4.05, 18.13±4.26 and 4.28±1.18
(prior to co-culture) to 80.78±8.11, 40.31±6.94, 37.02±5.78 and
18.33±4.60% subsequent to co-culture, respectively (P=0.044).
Treatment response
In total, 26 out of 58 patients had a measurable
focus region: 1 achieved CR and 25 achieved PR, with an objective
remission rate of 44.83%. Among the remaining patients, 30 achieved
SD (51.72%) and 2 demonstrated PD (3.45%). There were 42 of 58
patients who underwent CBR evaluation: 36 had both KPS that was
increased >20% and pain relief of >50%, with an overall CBR
of 85.71% (36/42). Additionally, following treatment with
DC-activated CIKs, the KPS was 87.28±5.46, which was significantly
higher than prior to treatment (69.02±7.45). Subsequently, the
level of tumor markers including CEA, AFP, CA125, CA199, CA724, PSA
and NSE was monitored. The results demonstrated that there were 15,
8, 4, 2 and 2 patients with high CEA, CA199, NSE, CA724 and AFP
expression, respectively. Subsequent to DC-CIK mediated
immunotherapy for 2 cycles, 9 patients attained almost normal tumor
marker expression levels. The remaining patients did not
demonstrate a decline to the normal range, but CEA expression
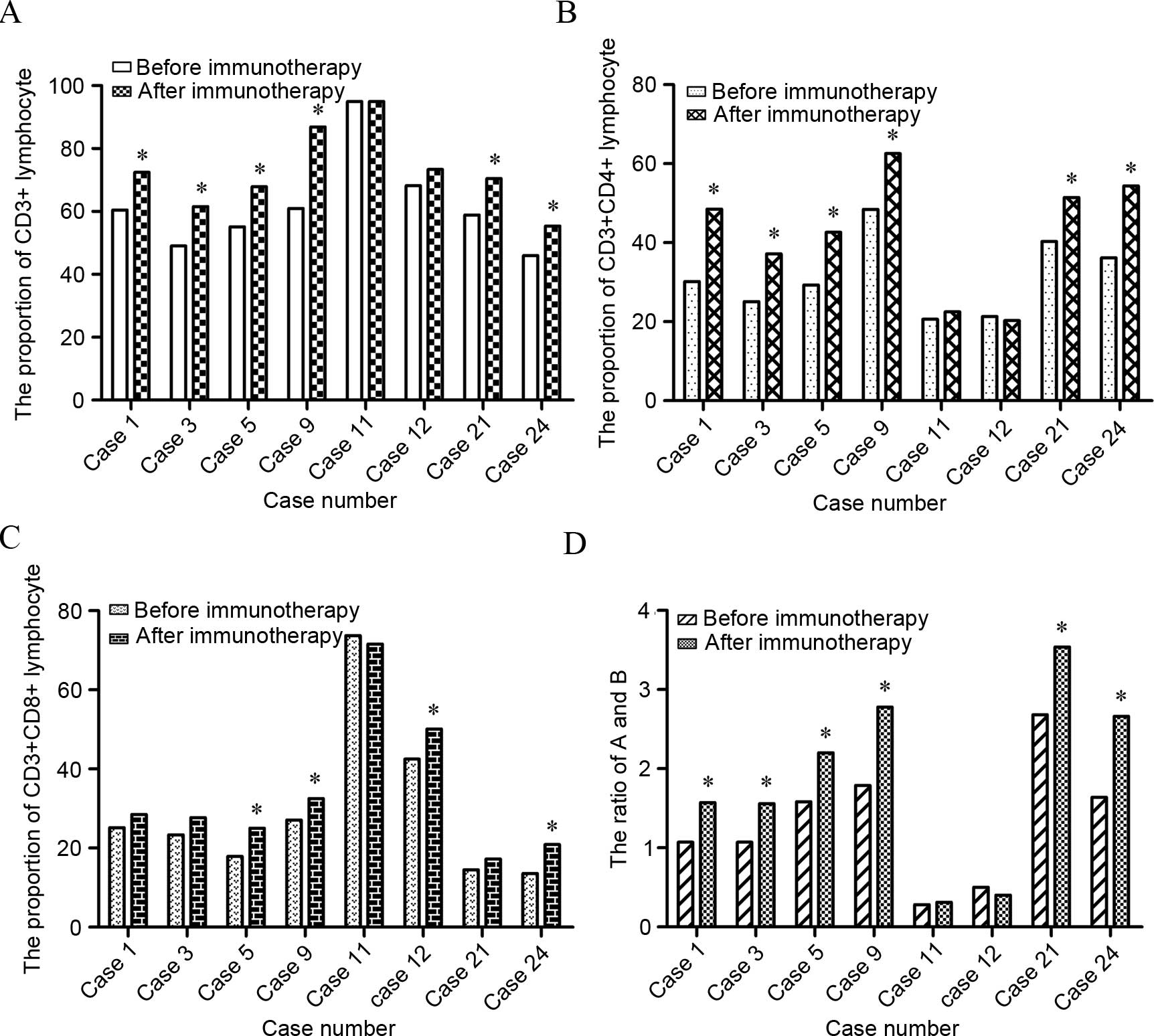
decreased (Fig. 3). Referring to the
lymphocyte subpopulation, the present test results indicated that
75% of patients showed an increase in
CD3+CD4+ lymphocytes (P=0.017) and 50% of
patients showed an increase in CD3+CD8+
lymphocytes (P=0.023). The population of CD4/CD8 cells was
significantly increased as well (P=0.024) (Fig. 4).
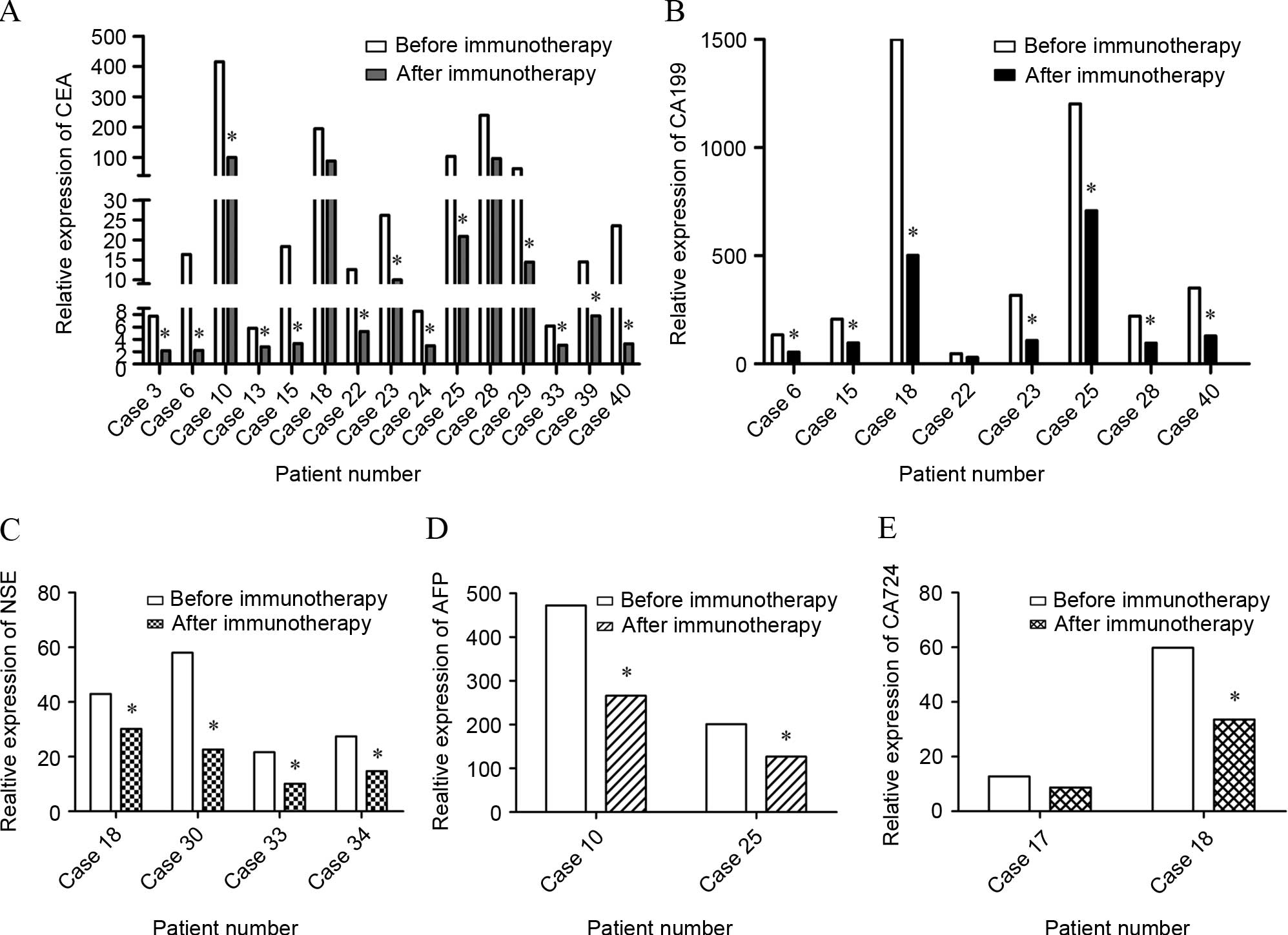 | Figure 3.Tumor marker level of patients. The
levels of tumor markers were monitored prior to and subsequent to 2
cycles of DC-CIK immunotherapy using FCM. (A) The expression of CEA
in 15 cases. Expression in 13 patients decreased significantly
compared with prior to DC-CIK infusion, and the expression in 7
patients reached the normal range. (B) The expression of CA199 in 8
patients. Expression in 7 patients decreased significantly compared
with prior to DC-CIK infusion, and the expression in 1 patient
reached the normal range. (C) The expression of NSE in 4 cases. The
expression of all 4 patients decreased compared with prior to
DC-CIK infusion, and 2 patients demonstrated a decrease to the
normal range. (D) The expression of AFP in 2 cases. AFP expression
in the 2 patients decreased compared with prior to DC-CIK infusion.
(E) The expression of CA-724 in 2 cases. The expression of CA-724
decreased significantly in the 2 patients compared with prior to
DC-CIK infusion, and the level in 1 patient reached the normal
range. *P<0.05. DC, dendritic cell; CIK, cytokine-induced killer
cell; FCM, flow cytometry; CEA, carcinoembryonic antigen; NSE,
neuron-specific enolase; AFP, α fetoprotein; CA, cancer antigen
724. |
Treatment toxicity
The distributions of side effects in the patients
are shown in Table II. No serious
side effects that may present a safety hazard to life were
observed. No patient failed to complete the DC-CIK immunotherapy.
There were no grade III–IV cell-associated toxicities, and common
grade I–II toxicities consisted of transient chills, fever,
fatigue, headache and anemia.
 | Table II.Distribution of toxicity. |
Table II.
Distribution of toxicity.
| Side effects (WHO
criteria) | Grade I–II, n
(%) | Grade III–IV,
n |
|---|
| Chills | 4 (6.90) | 0 |
| Fever | 7
(12.07) | 0 |
| Hematological
symptoms | 5 (8.62) | 0 |
|
Anemia | 2 (3.45) | 0 |
|
Leucopenia | 2 (3.45) | 0 |
|
Thrombocytopenia | 1 (1.72) | 0 |
| Gastrointestinal
symptom | 3 (5.17) | 0 |
| Nausea and
vomiting | 2 (3.45) | 0 |
| Diarrhea | 1 (1.72) | 0 |
| Respiratory symptom
(dyspnea) | 1 (1.72) | 0 |
| Skin symptom
(allergy) | 3 (5.17) | 0 |
| Circulatory system
(arrhythmias) | 2 (3.45) | 0 |
| Neurological
symptoms | 8
(13.79) | 0 |
| Fatigue
and headaches | 6
(10.34) | 0 |
| Paresthesia | 2 (3.45) | 0 |
Discussion
Although systemic chemotherapy, radiotherapy and
surgery are the principle treatments for cancers, the prognosis
remains poor, particularly in older cancer patients, due to their
poor tolerance and weaker immunity (18). Increasing clinical studies have
suggested that immunotherapy may serve as an excellent strategy to
improve efficiency of cancer therapy (19). Adoptive immunotherapy using CIKs has
shown significant anti-tumor activity in pre-clinical experiments
and various animal tumor models (20). In a previous retrospective study, our
results showed that DCs could potentially increase the population
of the hallmark effector CD3+CD56+ cells,
which have powerful immune cytotoxicity effects (21). In the present study, DC-CIK
immunotherapy was performed subsequent to different prior therapy
in 58 patients; it was well tolerated, and could prevent tumor
progression to a certain extent and improve quality of life for
patients. As tumor markers may reflect tumorigenesis, progression
and response to treatment, determining the levels of markers prior
to and subsequent to immunotherapy in those patients may provide
information regarding the curative effect of DC-activated CIKs
action. The current study analyzed the expression changes of
several tumor markers, such as CEA, CA199 and NSE. The present
monitoring results indicated patients with DC-CIK mediated
immunotherapy had a lower level of those indexes compared with
prior to DC-CIK infusion.
DCs, known as the most powerful antigen presenting
cells, have been under intense investigation as components of
anti-tumor vaccines, specifically as a delivery mode for tumor
antigens (22). CIKs are a novel
population of immune effector cells and can be expanded in
vitro in the presence of rhIL-2, starting from PBMCs stimulated
by IFN-γ and anti-CD3 antibody (23).
It is well known that CIKs have become suitable candidates for cell
therapy regimens in both solid and hematopoietic tumor treatments
due to their easy and rapid production in vitro, stronger
anti-tumor activity, broader target tumor spectrum, and relatively
lower adverse effect compared to other reported anti-tumor effector
cells (24,25). In particular, CIKs can regulate and
enhance the immune function in patients with cancer, which is
particularly suitable for patients aged >65 years old.
In conclusion, the current results provide
information regarding the outcome of DC-CIK mediated immunotherapy
in cancer patients aged >65 years. Additional studies of the
expression of relevant cytokines, such as IFN-γ, interleukin, MIG,
MCP, TNF-α and TNF-β, and long-term survival time observation of a
larger sample size are required to further investigate the clinical
efficacy of this approach.
Acknowledgements
This study is supported by a research grant from
Shaanxi Province Science and Technology Development Fund (grant no.
2011KTCL03-10).
References
|
1
|
Chouliara Z, Kearney N, Stott D,
Molassiotis A and Miller M: Perceptions of older people with cancer
of information, decision making and treatment: A systematic review
of selected literature. Ann Oncol. 15:1596–1602. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soubeyran P, Fonck M, Blanc-Bisson C,
Blanc JF, Ceccaldi J, Mertens C, Imbert Y, Cany L, Vogt L, Dauba J,
et al: Predictors of early death risk in older patients treated
with first-line chemotherapy for cancer. J Clin Oncol.
30:1829–1834. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kenis C, Bron D, Libert Y, Decoster L, Van
Puyvelde K, Scalliet P, Cornette P, Pepersack T, Luce S,
Langenaeken C, et al: Relevance of a systematic geriatric screening
and assessment in older patients with cancer: Results of a
prospective multicentric study. Ann Oncol. 24:1306–1312. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aaldriks AA, Maartense E, le Cessie S,
Giltay EJ, Verlaan HA, van der Geest LG, Kloosterman-Boele WM,
Peters-Dijkshoorn MT, Blansjaar BA, van Schaick HW and Nortier JW:
Predictive value of geriatric assessment for patients older than 70
years, treated with chemotherapy. Crit Rev Oncol Hematol.
79:205–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhong R, Han B and Zhong H: A prospective
study of the efficacy of a combination of autologous dendritic
cells, cytokine-induced killer cells, and chemotherapy in advanced
non-small cell lung cancer patients. Tumour Biol. 35:987–994. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gammaitoni L, Giraudo L, Leuci V,
Todorovic M, Mesiano G, Picciotto F, Pisacane A, Zaccagna A, Volpe
MG, Gallo S, et al: Effective activity of cytokine-induced killer
cells against autologous metastatic melanoma including cells with
stemness features. Clin Cancer Res. 19:4347–4358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang J, Wu C and Lu B: Cytokine-induced
killer cells promote antitumor immunity. J Transl Med. 11:832013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hontscha C, Borck Y, Zhou H, Messmer D and
Schmidt-Wolf IG: Clinical trials on CIK cells: First report of the
international registry on CIK cells (IRCC). J Cancer Res Clin
Oncol. 137:305–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Turksma AW, Coupé VM, Shamier MC, Lam KL,
de Weger VA, Belien JA, van den Eertwegh AJ, Meijer GA, Meijer CJ
and Hooijberg E: Extent and Location of Tumor-Infiltrating
Lymphocytes in Microsatellite-Stable Colon Cancer Predict Outcome
to Adjuvant Active Specific Immunotherapy. Clin Cancer Res.
22:346–356. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saito H, Ando S, Morishita N, Lee KM,
Dator D, Dy D, Shigemura K, Adhim Z, Nibu K, Fujisawa M and
Shirakawa T: A combined lymphokine-activated killer (LAK) cell
immunotherapy and adenovirus-p53 gene therapy for head and neck
squamous cell carcinoma. Anticancer Res. 34:3365–3370.
2014.PubMed/NCBI
|
|
11
|
Huang J, Li C, Wang Y, Lv H, Guo Y, Dai H,
Wicha MS, Chang AE and Li Q: Cytokine-induced killer (CIK) cells
bound with anti-CD3/anti-CD133 bispecific antibodies target
CD133(high) cancer stem cells in vitro and in vivo. Clin Immunol.
149:156–168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Zhu L, Wei J, Liu L, Yin Y, Gu Y
and Shu Y: The effects of cytokine-induced killer cells for the
treatment of patients with solid tumors: A clinical retrospective
study. J Cancer Res Clin Oncol. 138:1057–1062. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu XC, Yang B, Yu RL, Chi XH, Tuo S, Tuo
CW, Zhu HL, Wang Y, Jiang CG, Fu XB, et al: Clinical study of
autologous cytokine-induced killer cells for the treatment of
elderly patients with diffuse large B-cell lymphoma. Cell Biochem
Biophys. 62:257–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao D, Li C, Xie X, Zhao P, Wei X, Sun W,
Liu HC, Alexandrou AT, Jones J, Zhao R and Li JJ: Autologous tumor
lysate-pulsed dendritic cell immunotherapy with cytokine-induced
killer cells improves survival in gastric and colorectal cancer
patients. PLoS One. 9:e938862014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao P, Bu X, Wei X, Sun W, Xie X, Li C,
Guo Q, Zhu D, Wei X and Gao D: Dendritic cell immunotherapy
combined with cytokine-induced killer cells promotes skewing toward
Th2 cytokine profile in patients with metastatic non-small cell
lung cancer. Int Immunopharmacol. 25:450–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang L, Ren B, Li H, Yu J, Cao S, Hao X
and Ren X: Enhanced antitumor effects of DC-activated CIKs to
chemotherapy treatment in a single cohort of advanced
non-small-cell lung cancer patients. Cancer Immunol Immunother.
62:65–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Castiglia S, Mareschi K, Labanca L,
Lucania G, Leone M, Sanavio F, Castello L, Rustichelli D, Signorino
E, Gunetti M, et al: Inactivated human platelet lysate with
psoralen: A new perspective for mesenchymal stromal cell production
in Good Manufacturing Practice conditions. Cytotherapy. 16:750–763.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Angelis R, Sant M, Coleman MP,
Francisci S, Baili P, Pierannunzio D, Trama A, Visser O, Brenner H,
Ardanaz E, et al: Cancer survival in Europe 1999–2007 by country
and age: Results of EUROCARE-5-a population-based study. Lancet
Oncol. 15:23–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Couzin-Frankel J: Breakthrough of the year
2013. Cancer immunotherapy. Science. 342:1432–1433. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li H, Wang C, Yu J, Cao S, Wei F, Zhang W,
Han Y and Ren XB: Dendritic cell-activated cytokine-induced killer
cells enhance the anti-tumor effect of chemotherapy on non-small
cell lung cancer in patients after surgery. Cytotherapy.
11:1076–1083. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Yu W, Li H, Yu J, Zhang X, Ren X
and Cao S: Can the dual-functional capability of CIK cells be used
to improve antitumor effects? Cell Immunol. 287:18–22. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Price JD, Hotta-Iwamura C, Zhao Y,
Beauchamp NM and Tarbell KV: DCIR2+ cDC2 DCs and Zbtb32 restore
CD4+ T cell tolerance and inhibit diabetes. Diabetes. 64:3521–3531.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmeel LC, Schmeel FC, Coch C and
Schmidt-Wolf IG: Cytokine-induced killer (CIK) cells in cancer
immunotherapy: Report of the international registry on CIK cells
(IRCC). J Cancer Res Clin Oncol. 141:839–849. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mao Q, Li L, Zhang C, Sun Y, Liu S and Cui
S: Clinical effects of immunotherapy of DC-CIK combined with
chemotherapy in treating patients with metastatic breast cancer.
Pak J Pharm Sci. 28:(Suppl 3). S1055–S1058. 2015.
|
|
25
|
Wang S and Wang Z: Efficacy and safety of
dendritic cells co-cultured with cytokine-induced killer cells
immunotherapy for non-small-cell lung cancer. Int Immunopharmacol.
28:22–28. 2015. View Article : Google Scholar : PubMed/NCBI
|