Introduction
Selenium, an essential biological trace element, has
received considerable attention as an important micronutrient for
humans. Selenium serves a role in several cellular and
physiological processes, including thyroid hormone production and
immune responses (1). It has been
reported that selenium has antioxidant properties (2). Food and drink are the primary natural
sources of selenium. High levels of selenium are present in
cereals, seafood and meat products, whereas there is little found
in vegetables, milk or fruits (3).
Selenium deficiency has been associated with several degenerative
diseases (4). Selenium has been
widely used to treat several pathophysiological conditions,
including cervical, renal and liver cancer (5).
Nasopharyngeal carcinoma is an endemic disease in
South Asia. There are several conventional treatments available,
including radiotherapy. Chemotherapy has gained importance in the
treatment of nasopharyngeal carcinoma (6). However, the complications are high.
Chemotherapy treatment frequently fails due to multidrug resistance
and cancer treatment requires selective action on the targeted site
(6). Cisplatin is a widely used
therapeutic agent that acts as a radiation sensitizer and a
cytotoxic compound (6).
Cisplatin-acquired resistance in patients may lead to the failure
of chemotherapy treatment (6). Drug
resistance and the establishment of cisplatin-resistant cells are
considered to be possible ways to understand the chemoresistance
mechanism (6).
To the best of our knowledge, cisplatin-resistant
nasopharyngeal carcinoma cell establishment and associated studies
are limited. Studies on selenium treatment against
cisplatin-induced nasopharyngeal cancer are also limited. The
ability of selenium to neutralize the toxicity of cisplatin in the
nasopharyngeal tissue has yet to be investigated. The present study
examines the impact of selenium on cisplatin-induced toxicity.
Materials and methods
Materials
Dimethyl sulfoxide was purchased from Sigma- Aldrich
(Merck Millipore, Darmstadt, Germany). Dulbecco's modified Eagle's
medium, fetal bovine serum (FBS), penicillin-streptomycin and
trypsin-ethylenediaminetetraacetic acid were obtained from Welgene,
Inc. (Gyeongsan-si, South Korea). Acridine orange (AO), ethidium
bromide (EB) and 2′,7′-dichlorofluorescein diacetate (DCFH-DA) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Primers were obtained from Macrogen Inc. (Seoul, South Korea).
Animals
Healthy, male, albino rats were purchased from the
Department of Otorhinolaryngology, Jiangsu Provincial People's
Hospital Affiliated to Nanjing Medical University (Nanjing, China).
Those weighing 160–180 g were selected for the present study. The
rats were kept in polypropylene cages, at a temperature of
25±0.5°C, at a relative humidity of 60±5% and at a photoperiod of
12 h/day. The male albino rats were grouped into 4 groups of 6 rats
each.
Experimental induction of cancer
The application of carcinogens was achieved by means
of cisplatin treatment prior to the experiments. Cisplatin (10 µg)
was administered for 45 days for the induction of diabetes with
final tumor incidences close to 100%.
Treatment
The experimental groups were designated as follows:
Group I, normal saline (10 µl); Group II, 10 µg cisplatin; Group
III, 10 µg selenium+10 µg cisplatin; and Group IV 20 µg selenium+20
µg cisplatin. The drug was administered intraperitoneally for 45
days. The dose level and duration of the experiment was selected
based on our preliminary study. The preliminary study was conducted
with a different dose level (range, 1–100 µg). The animals were
sacrificed by decapitation, and nasopharyngeal tissue was
surgically removed. Animal tissues were homogenized using a
Potter-Elvehjem glass-Teflon homogenizer and a Dounce hand
homogenizer (Sigma-Aldrich; Merck Millipore). This method is rapid
and poses little risk to proteins, other than the release of
proteases from other cellular compartments. Proteolytic degradation
was reduced by adding protease inhibitors to the homogenization
buffers. The prepared tissue homogenate was used immediately in
subsequent investigations.
In vivo studies
Determination of lipid peroxidation
Lipid peroxidation (LPO) was determined using the
Lipid Peroxidation (MDA) assay kit (ab118970; Abcam, Cambridge, MA,
USA). This was based on the spectrophotometric method of
Pandurangan et al (7).
Malondialdehyde (MDA) was measured by determining the
thiobarbituric acid reactive species. The absorbance of the
resulting product was measured at 534 nm (Cary 100 UV-Vis
spectrophotometer; Agilent Technologies, Inc., Santa Clara, CA,
USA).
Determination of reduced glutathione
Glutathione (GSH) level was measured using the
gluathione assay kit (Abcam). This was based on the
spectrophotometric method of Pandurangan et al (7). The yellow product color was measured at
405 nm (Cary 100 UV-Vis spectrophotometer; Agilent Technologies,
Inc.).
Determination of superoxide dismutase (SOD) and
catalase enzyme activities
SOD, catalase and lactate dehydrogenase (LDH) enzyme
activities were determined using the antioxidant enzyme assay kit
method (Abcam) which was based on the method of Pandurangan et
al (7).
Quantitative polymerase chain reaction
(qPCR)
The qPCR was performed using a cDNA equivalent of 10
ng total RNA from each sample, withspecific primers for p53
(forward, 5′-TAACAGTTCCTGCATGGGCGGC-3′ and reverse,
5′-AGGACAGGCACAAACACGCACC-3′), bax (forward,
5′-TGGAGCTGCAGAGGATGATTG-3′ and reverse,
5′-GAAGTTGCCGTCAGAAAACATG-3′), caspase 3 (forward,
5′-TTAATAAAGGTATCCATGGAGAACACT-3′ and reverse,
5′-TTAGTGATAAAAATAGAGTTCTTTTGTGAG-3′) and a housekeeping gene,
glyceraldehyde 3-phosphate dehydrogenase (forward,
5′-GGTCACCAGGGCTGCTTTT-3′ and reverse,
5′-ATCTCGCTCCTGGAAGATGGT-3′). Time, temperature and cycles were
performed as previously described (8). The reaction was performed in a 10 µl
reaction volume using SYBR Green Master mix (Bioneer Corporation,
Daejeon, Korea) according to the manufacturer's protocol (8).
Caspase activity assay
Caspase 3 enzyme activity was measured using an
activity assay kit (Sigma-Aldrich; Merck Millipore) based on the
method of Muthuraman (9).
In vitro studies
Cell culture
HK1 cells were obtained from the American Type
Culture Collection (Manassas, VA, USA). 10% FBS and 1% antibiotics
(1% penicillin-streptomycin) were used for cell growth. The cells
were grown to 90% confluence in a CO2 incubator at 37°C
with 5% CO2.
Fluorescence microscopy
The HK1 cells (2.5×104) were cultured in
6-well plates and treated for 48 h with either 10 µg/ml selenium,
10 µg/ml selenium+10 µg/ml cisplatin or 20 µg selenium+20 µg
cisplatin. Control cells were incubated with growth medium only.
The cells were examined with a fluorescence microscope (10) (Axiovert 2000; Carl Zeiss AG,
Oberkochen, Germany).
Confocal laser scanning (CLS) microscopy
The HK1 cells (2.5×104) were grown at a
volume of 2×104 cells/well in 6-well plates. The cells
were treated for 48 h with either 10 µg/ml selenium, 10 µg/ml
selenium+10 µg/ml cisplatin or 20 µg selenium+20 µg cisplatin.
Control cells were incubated with growth medium only. Cells were
stained with EB and AO stains. The cells were viewed immediately
under a CLS microscope (1X81R Motorized Inverted
Microscope; Olympus Corporation, Tokyo, Japan) (10).
Determination of reactive oxygen species (ROS)
production
The HK1 cells (2.5×104) were cultured
in6-well plates and treated for 48 h with either 10 µg/ml selenium,
10 µg/ml selenium+10 µg/ml cisplatin or 20 µg selenium+20 µg
cisplatin. Control cells were incubated with growth medium only.
The cells were incubated with 5 µM of DCFH-DA in a growth medium
(Sigma-Aldrich; Merck Millipore) for 30 min at 37°C and 5%
CO2. The fluorescence was measured at 485/525 nm (Ex/Em)
based on the method of Muthuraman et al (10) (Axiovert 2000; Carl Zeiss AG).
Statistical analysis
All values are expressed as the mean ± standard
deviation. Test and control values were compared using the
Student's t-test (SPSS 16, Statistical Package; SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
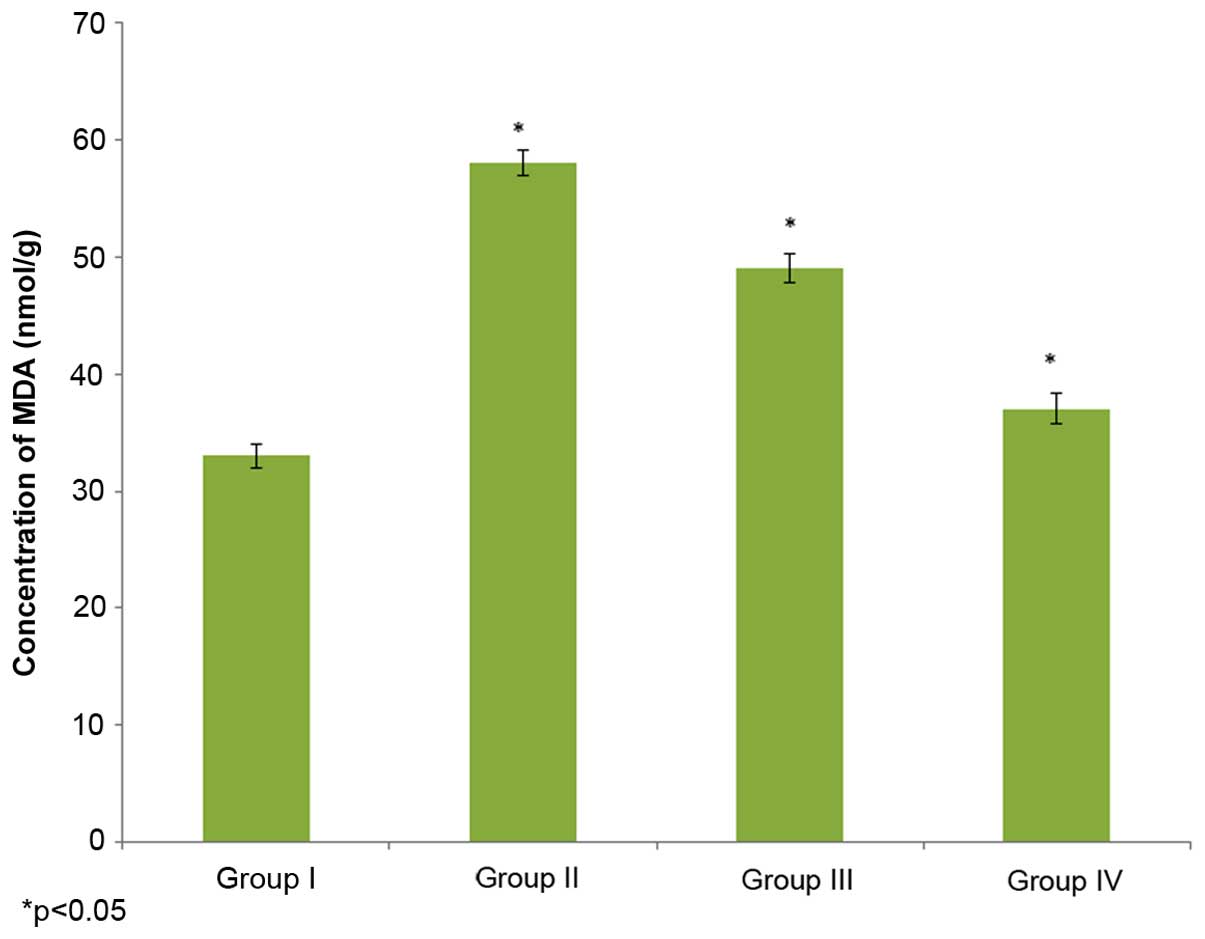
Effect of selenium on MDA
concentration
The effect of selenium on MDA concentration in the
male albino rats is demonstrated in Fig.
1. The MDA concentration in the control rats (group I) was 33±1
nmol/g, whereas it increased to 58±1.1 nmol/g in the
cisplatin-induced rats (group II). The administration of 10 µg/ml
selenium with 10 µg/ml cisplatin (group III) significantly reduced
(49±1.2 nhmol/g) the concentration of MDA in the rats compared to
the control group (group I) (P=0.02132; Fig. 1). The administration of 20 µg/ml
selenium with 20 µg/ml cisplatin (group IV) also significantly
reduced the concentration of MDA (37±1.3 nmol/g) in the rats
compared to the control group (group I) (P=0.03562; Fig. 1).
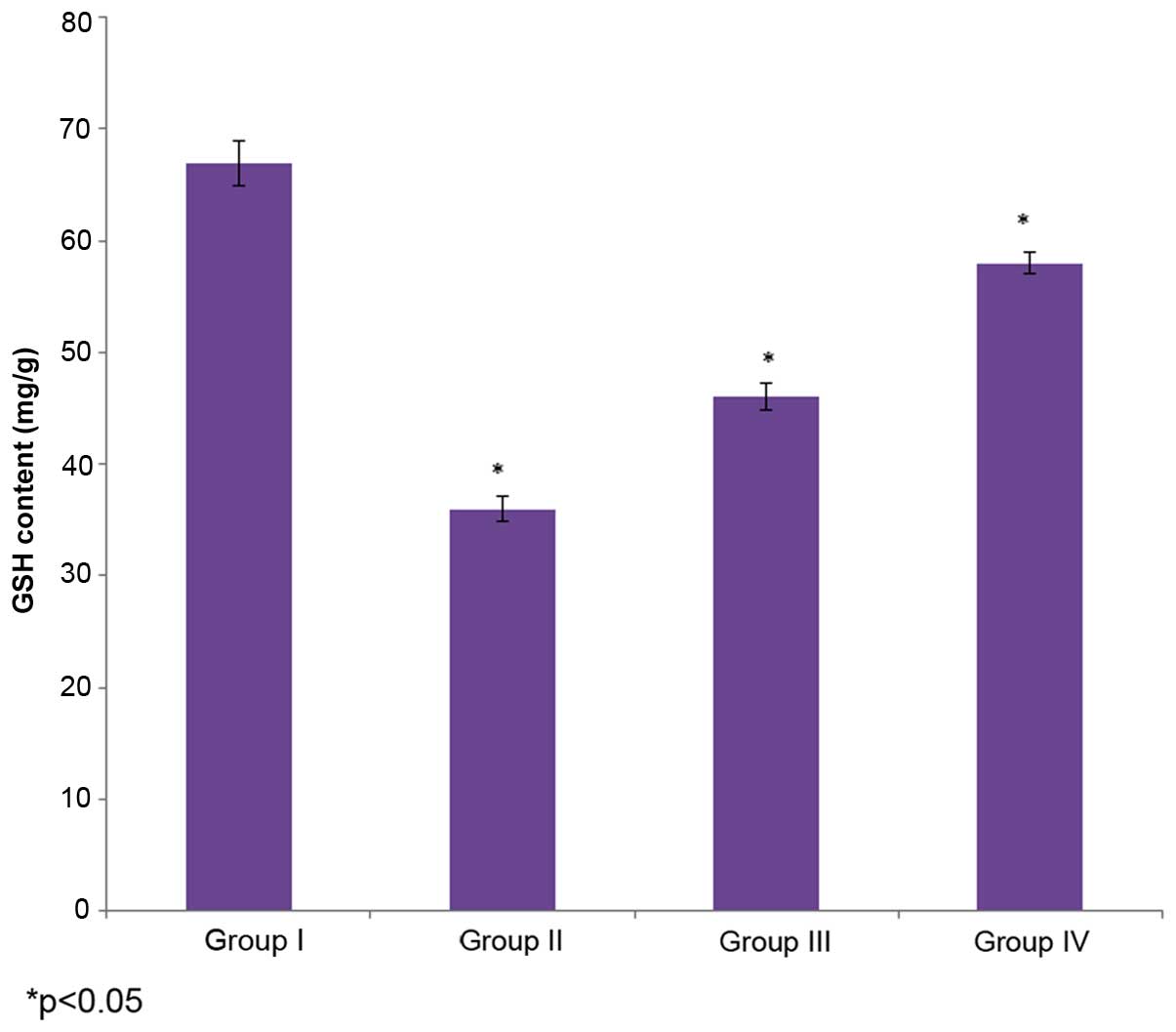
Effect of selenium on level of
GSH
The effect of selenium on GSH content in the male
albino rats is demonstrated in Fig.
2. The level of GSH in the control rats (group I) was 67±2
mg/g, whereas it decreased to36±1.1 mg/g in the cisplatin-induced
rats (group II). The administration of 10 µg/ml selenium with 10
µg/ml cisplatin (group III) significantly increased (46±1.2 mg/g)
the level of GSH in the rats compared to the control group (group
I) (P=0.4329; Fig. 2). The
administration of 20 µg/ml selenium with 20 µg/ml cisplatin (group
IV) also significantly increased the level of GSH (58±1 mg/g) in
the rats compared to the control group (group I) (P=0.02147;
Fig. 2).
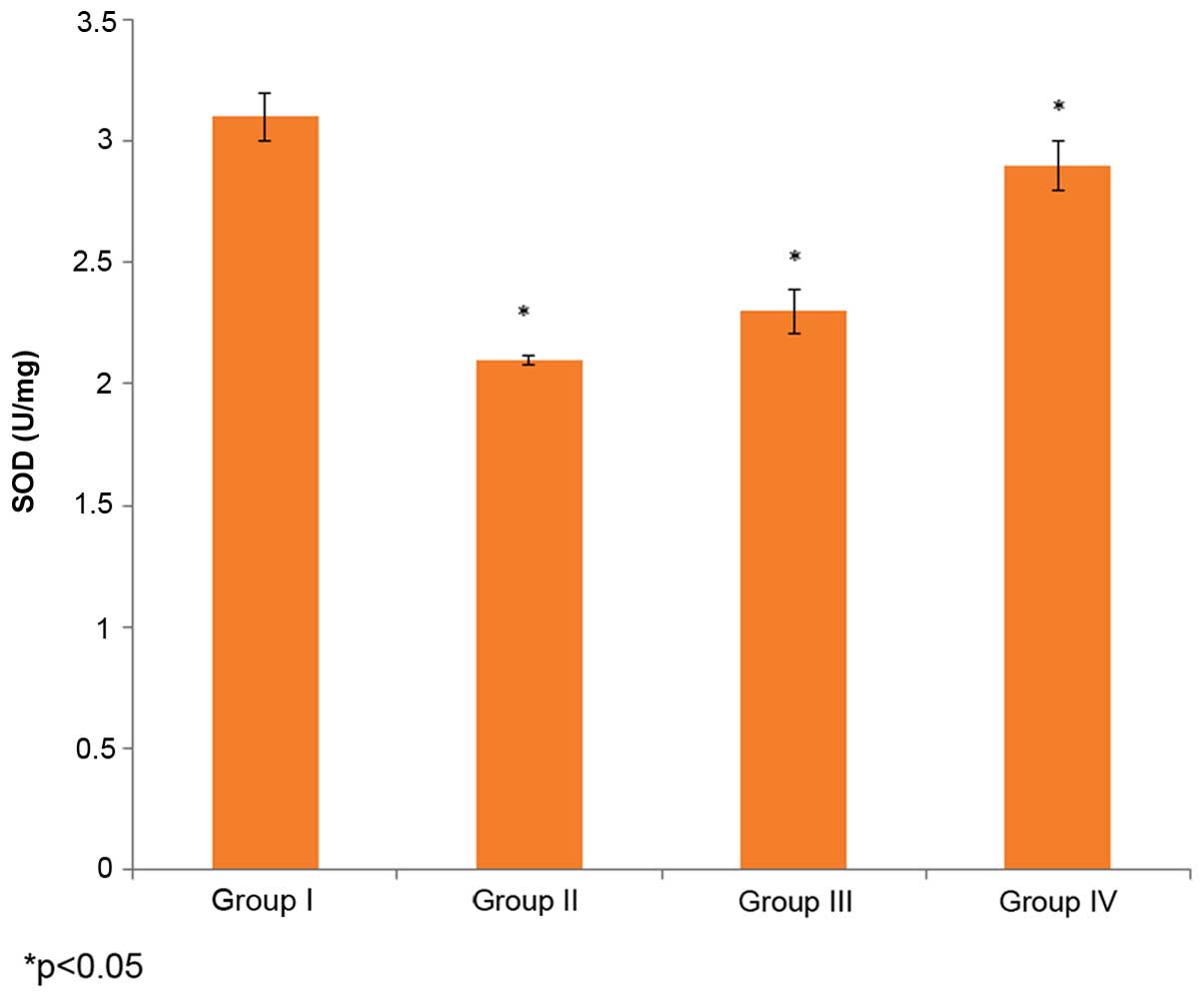
Effect of selenium on SOD
activity
The effect of selenium on SOD activity in the male
albino rats is demonstrated in Fig.
3. The SOD activity in the control rats (group I) was 3.1±0.1
U/mg, whereas it decreased to 2.1±0.02 U/mg in the
cisplatin-induced rats (group II). The administration of 10 µg/ml
selenium with 10 µg/ml cisplatin (group III) significantly
increased the SOD activity in the rats compared to the control
group (group I) (P=0.03253; Fig. 3).
The administration of 20 µg/ml of selenium with 20 µg/ml of
cisplatin (group IV) also significantly increased the SOD activity
(58±1 mg/g) in the rats compared to the control group (group I)
(P=0.03821; Fig. 3).
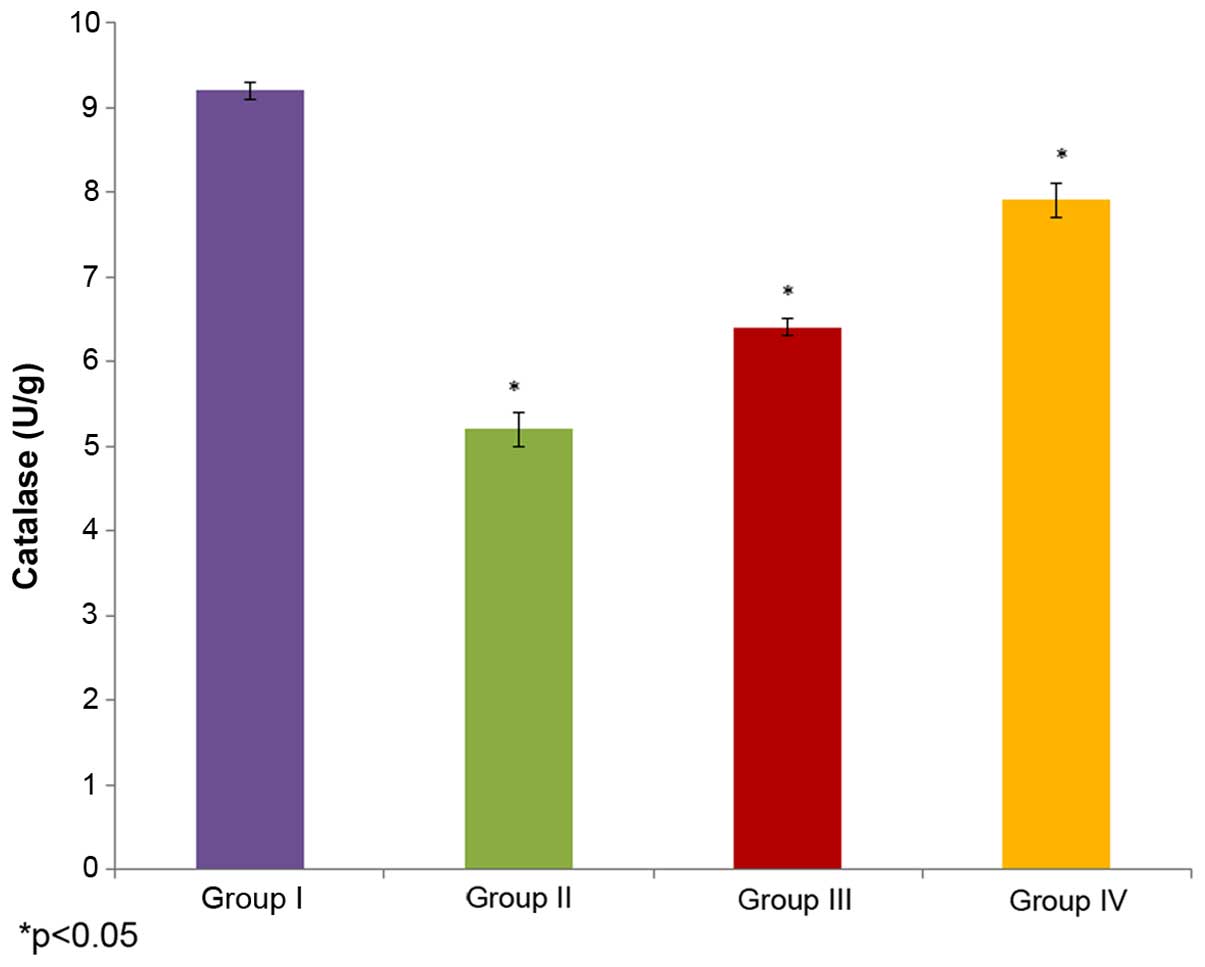
Effect of selenium on catalase
activity
The effect of selenium on catalase activity in the
male albino rats is demonstrated in Fig.
4. The catalase activity in the control rats (group I) was
9.2±0.1 U/g, whereas it decreased to 5.2±0.2 U/g in the
cisplatin-induced rats (group II). The administration of 10 µg/ml
selenium with 10 µg/ml cisplatin (group III) (P=0.02225; Fig. 4) significantly increased (6.4±0.1 U/g)
the catalase activity in the rats compared to the control group
(group I). The administration of 20 µg/ml selenium with 20 µg/ml
cisplatin (group IV) also significantly increased the catalase
activity (7.9±0.2 U/g) in the rats compared to the control group
(group I) (P=0.03312; Fig. 4).
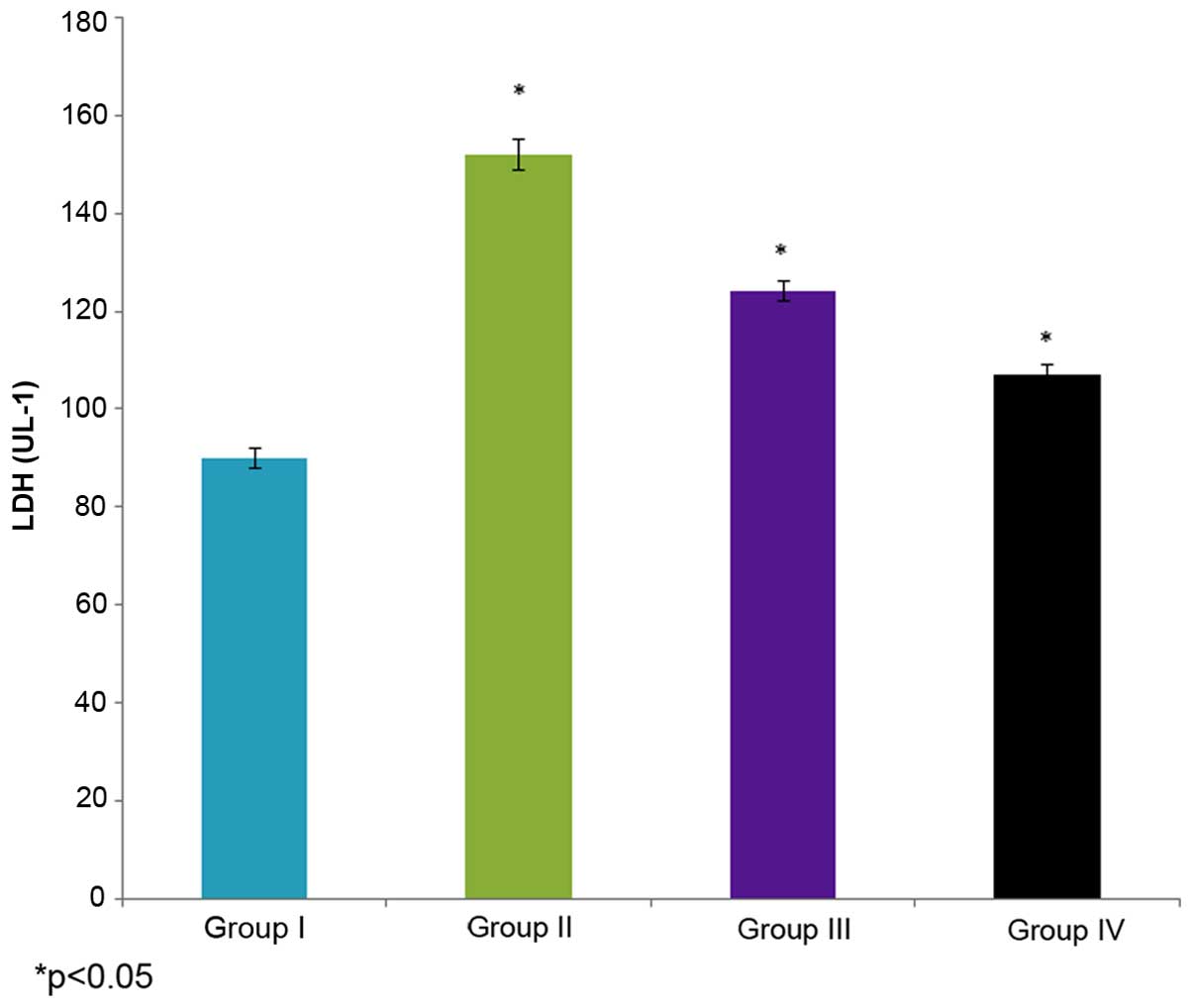
Effect of selenium on LDH
activity
The effect of selenium on LDH activity in the male
albino rats is demonstrated in Fig.
5. The LDH activity in the control rats (group I) was 90±2 U/l,
whereas it increased to 152±3 U/l in the cisplatin-induced rats
(group II). The administration of 10 µg/ml selenium with 10 µg/ml
cisplatin (group III) significantly decreased (124±2 U/l) the LDH
activity in the rats compared to the control group (group I)
(P=0.02412; Fig. 5). The
administration of 20 µg/ml selenium with 20 µg/ml cisplatin (group
IV) also significantly decreased LDH activity (107±2 UL-1) in the
ratscompared to the control group (group I) (P=0.04412; Fig. 5).
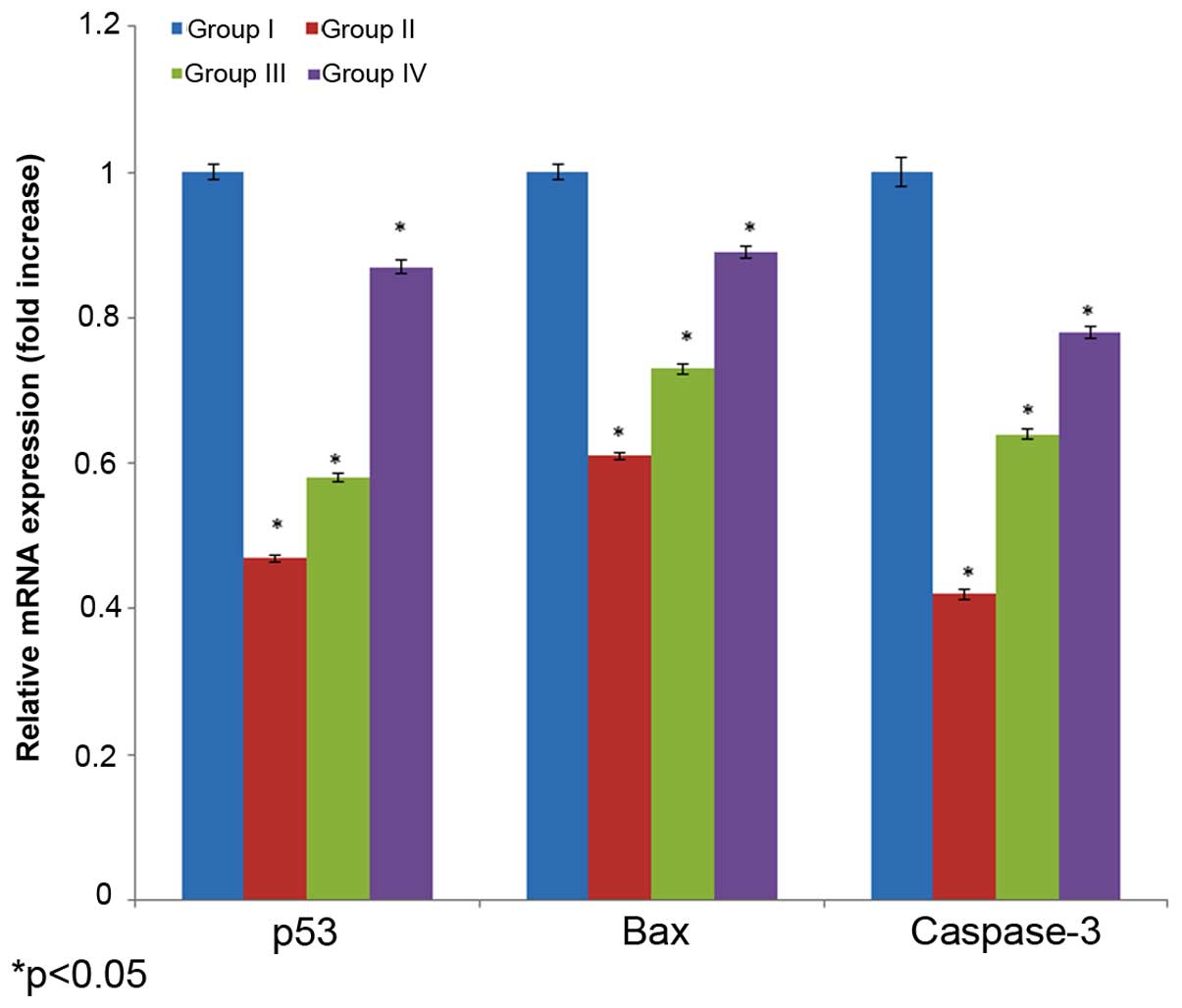
Effect of selenium on mRNA
expression
The effect of selenium on mRNA expression in the
male albino rats is shown in Fig. 6.
The mRNA expression of p53, bax and caspase 3 decreased 0.53-, 0.39
and 0.48-fold, in the cisplatin-induced rats (group I) compared
with their respective controls (group I). The administration of 10
µg/ml selenium with 10 µg/ml cisplatin (group III) significantly
increased mRNA expression of p53, bax and caspase 3 (0.42-, 0.27-
and 0.36-fold, respectively) in the rats compared to the control
group (group I) (P=0.03312; Fig. 6).
The administration of 20 µg/ml of selenium with 20 µg/ml of
cisplatin (group IV) significantly increased mRNA expression
(0.13-, 0.11- and 0.22-fold, respectively) in the rats compared
towith the control group (P=0.05113; Fig.
6).
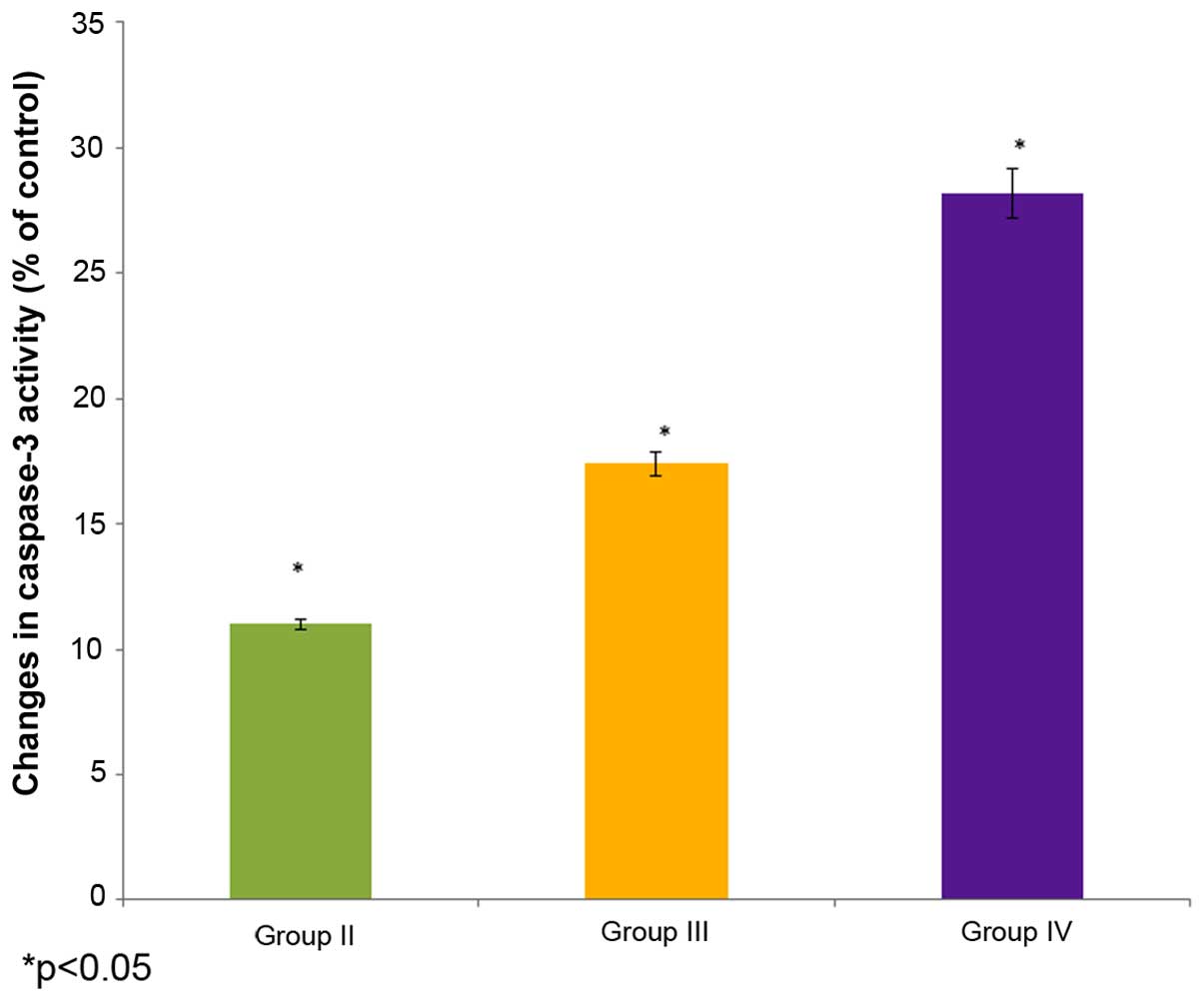
Effect of selenium on caspase 3
activity
The effect of selenium on caspase 3 activity in the
male albino rats is shown in Fig. 7.
The caspase 3 activity decreased to 11% of the control in the
cisplatin-induced rats (group II). The administration of 10 µg/ml
selenium with 10 µg/ml cisplatin (group III) significantly
increased (17.4%) the caspase 3 activity in the rats compared to
the control group (group I) (P=0.04451; Fig. 7). The administration of 20 µg/ml
selenium with 20 µg/ml cisplatin (group IV) also significantly
increased the caspase 3 activity (28.2%) in the rats compared to
the control group (group I) (P=0.04123; Fig. 7).
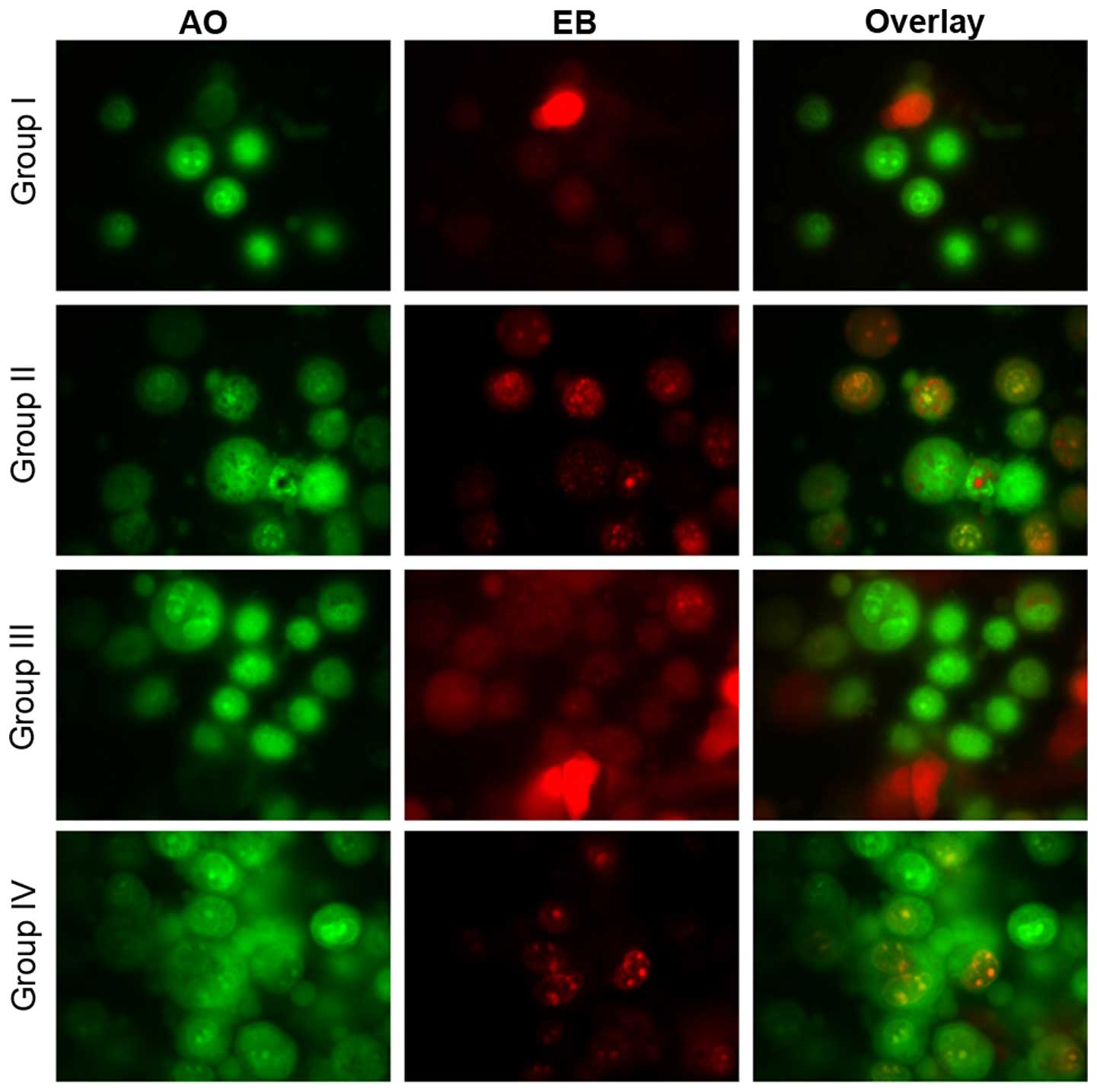
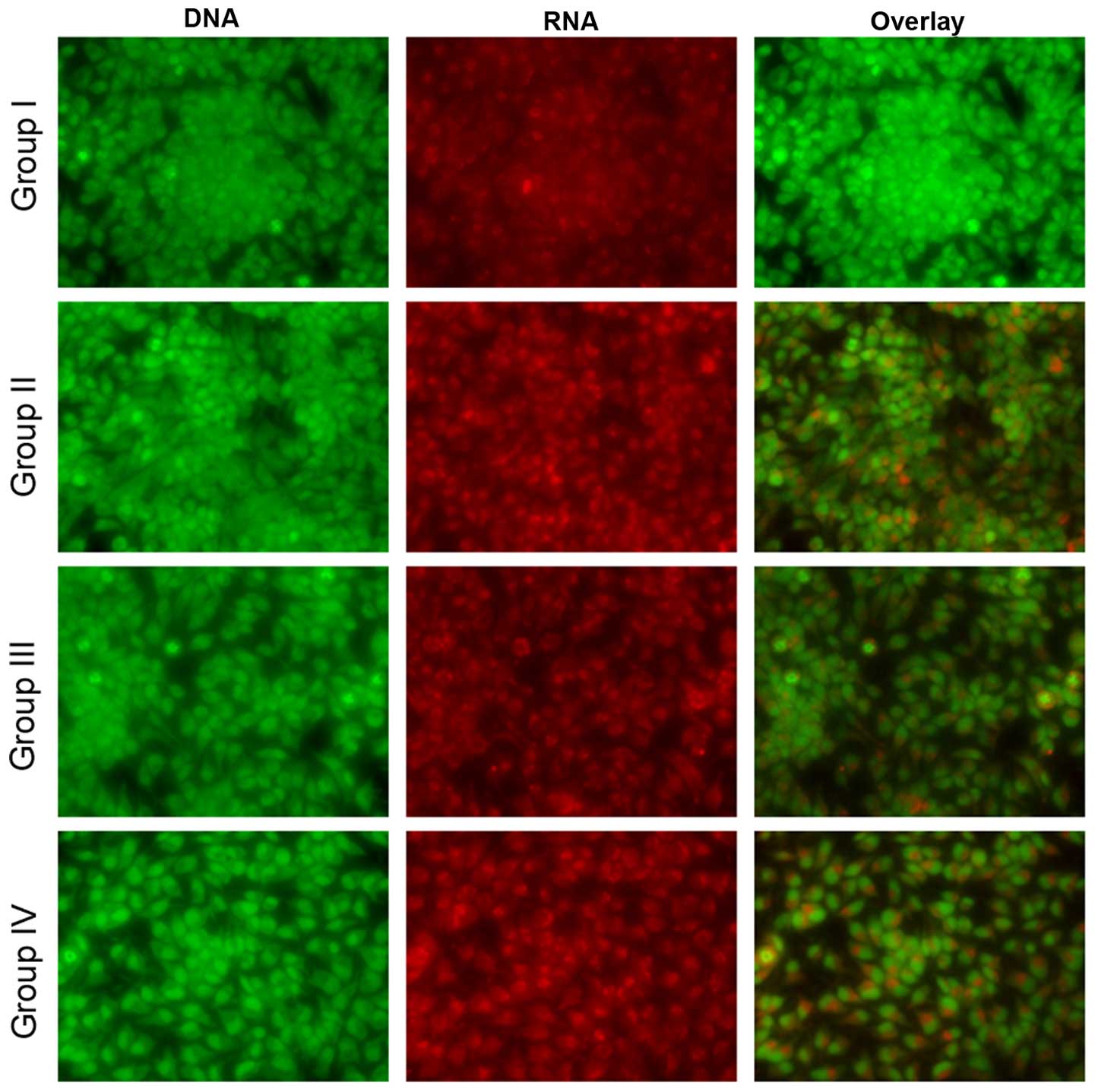
Effect of selenium on apoptosis
Microscopy was performed to determine whether the
anticancer activity of selenium is associated with the induction of
apoptosis, a morphological feature of cell death. Chromatin
condensation in the stained nucleus was used to differentiate
viable, apoptotic and necrotic cells. The anticancer activity of
cisplatin in the HK1 cells is shown in Figs. 8 and 9.
The analysis revealed normal cell size and morphology in the
control cells (group I), whereas the cisplatin-incubated HK1 cells
demonstrated altered cell morphology, including apoptosis and
necrosis (group II). The administration of 10 µg/ml selenium with
10 µg/ml cisplatin (group III) significantly reduced the apoptosis
and necrosis of the cells compared to the control group (group II).
The administration of 20 µg/ml selenium with 20 µg/ml cisplatin
(group IV) (P=0.03421; Figs. 8 and
9) significantly reduced the
occurrence of apoptosis and necrosis towards normal levels compared
to the control group (group II) (P=0.03411; Figs. 8 and 9).
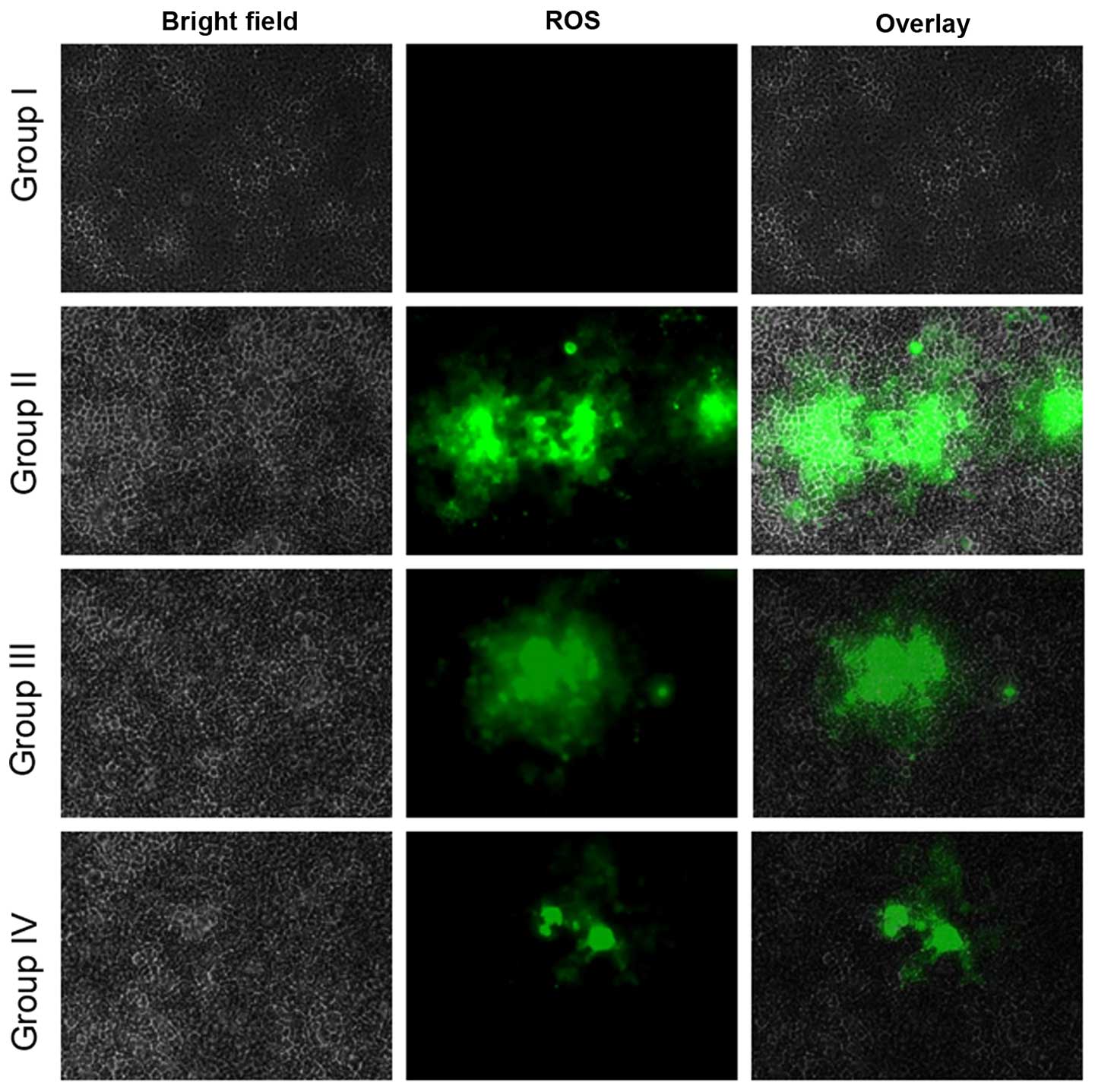
Effect of selenium on reactive oxygen
species (ROS) level
ROS play a vital role in facilitating signal
transduction processes within the intracellular region (10). The fluorescent probe, DCFH-DA,
determined the intracellular ROS generation. Fluorescence studies
indicated no green fluorescence intensity of DCF in the control
cells (group I), whereas increased levels were found in the
cisplatin-incubated cells (group II). The administration of 10
µg/ml selenium with 10 µg/ml cisplatin (group III) significantly
increased the levels of ROS compared with the control group (II).
The administration of 20 µg/ml selenium with 20 µg/ml cisplatin
(group IV) also increased the levels of ROS in the cells compared
with the control group (P=0.04612; Fig.
10).
Discussion
Recently, several studies have been focused on
cisplatin due to its wider uses. Cisplatin induces neurotoxicity,
nephrotoxicity, ototoxicity, myelotoxicity, nausea, vomiting,
hemolytic anemia and electrolyte disturbances (6,11). Chronic
exposure to cisplatin could cause drug resistance and
nasopharyngeal cancer. Selenium has attracted attention from
several researchers due its anticancer potential (11). The present study demonstrated that
chronic exposure to cisplatin could produce nasopharyngeal cancer
in adult rats and that the use of selenium could reduce the effect
of cisplatin.
The accumulation of free radicals and oxidative
stress induction have been reported as the toxic effects of
cisplatin (6). It is well known that
oxidative stress plays a crucial role in cell apoptosis and death.
Increased lipid peroxidation is the primary consequence of this
oxidative stress, mainly involving polyunsaturated fatty acids
(12). In the present study,
cisplatin significantly increased lipid peroxidation in the male
albino rats. This is evidence for the role of free radicals and
oxidative damage in the reduction of cisplatin-induced
nasopharyngeal cancer. Cell membranes may readily bind to cisplatin
and cause lipid peroxidation via the increased generation of free
radicals (13). Treatment of selenium
with cisplatin reduced the concentration of MDA, indicating the
anti-cancer activity of selenium (14). It has been reported that the use of
selenium has a protective effect against myocardial injury
(14).
Cell and tissue integrity and function have been
shown to be safeguarded by the protective action of selenium
against oxidative damage. ROS may also bind to cellular proteins
and can initiate the formation of side chain and readily
susceptible (degradative) amino acids (15). Impairment of cell function may occur
due to the accumulation of oxidized proteins. Yuan and Tang
(16) demonstrated that selenium can
counteract the free radicals and oxidative damage in chickens.
The accumulation of free radicals may affect DNA
structure and stability, leading to DNA damage and cell death
(17). It has been reported that
several toxic compounds can induce DNA damage (18), and the lipid peroxidation product MDA
could bind to DNA (19). It has been
shown that selenium could reduce DNA damage in cells (20). Kara et al (21) have demonstrated the antioxidant
properties of selenium. Antioxidant enzymes are the first line of
cell defense that safeguards cells from oxidative damage. In the
present study, SOD, catalase and LDH activities all significantly
increased following selenium treatment.
GSH is a well-known non-enzymatic antioxidant that
provides a second line of defense against oxidative damage
(22). GSH acts as a substrate for
the glutathione peroxidase and glutathione S-transferase enzymes,
and is involved in the reduction/removal of ROS from cells
(23). Reduced levels of GSH occur
during oxidative stress, which results in the impairment of cell
function and metabolism (24).
In conclusion, in the present study, cisplatin
exhibited carcinogenicity in male albino rats and HK1 cells.
Cellular architecture also returned to normal following treatment.
Morphological and apoptotic changes confirmed that a reduction in
apoptosis occurs following selenium treatment. From these
experimental results, it can be concluded that cisplatin could
exert carcinogenicity, but that treatment with selenium could
significantly reverse this toxicity.
References
|
1
|
Tinggi U: Selenium: Its role as an
antioxidant in human health. Environ Health Prev Med. 13:102–108.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Demerdash FM and Nasr HM: Antioxidant
effect of selenium on lipid peroxidation, hyperlipidemia and
biochemical parameters in rats exposed to diazinon. J Trace Elem
Med Biol. 28:89–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tinggi U, Reilly C and Patterson CM:
Determination of selenium in foodstuffs using spectrofluorometry
and hydride generation atomic absorption spectrometry. J Food Comp
Anal. 5:269–280. 1992. View Article : Google Scholar
|
|
4
|
Rayman M: Selenium and human health.
Lancet. 379:1256–1268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brigelius-Flohé R, Banning A and Schnurr
K: Selenium- dependent enzymes in endothelial cell function.
Antioxid Redox Signal. 5:205–215. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loehrer PJ and Einhorn LH: Drugs five
years later. Cisplatin. Ann Intern Med. 100:704–713. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pandurangan M, Veerappan M and Kim DH:
Cytotoxicity of zinc oxide nanoparticles on antioxidant enzyme
activities and mRNA expression in the cocultured C2C12 and 3T3-L1
cells. Appl Biochem Biotechnol. 175:1270–1280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muthuraman P, Ramkumar K and Kim DH:
Analysis of the dose-dependent effect of zinc oxide nanoparticles
on the oxidative stress and antioxidant enzyme activity in
adipocytes. Appl Biochem Biotechnol. 174:2851–2863. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muthuraman P: Effect of cortisol on
caspases in the co-cultured C2C12 and 3T3-L1 cells. Appl Biochem
Biotechnol. 173:980–988. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muthuraman P, Kim DH, Muthuviveganandavel
V, Vikramathithan J and Ravikumar S: Differential bio-potential of
ZnS nanoparticles to normal MDCK cells and cervical carcinoma HeLa
cells. J Nanosci Nanotechnol. 15:1–8. 2015.PubMed/NCBI
|
|
11
|
Milosavljevic N, Duranton C, Djerbi N,
Puech PH, Gounon P, Lagadic-Gossmann D, Dimanche-Boitrel MT, Rauch
C, Tauc M, Counillon L and Poët M: Nongenomic effects of cisplatin:
Acute inhibition of mechanosensitive transporters and channels
without actin remodeling. Cancer Res. 70:7514–7522. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Windsor RE, Strauss SJ, Kallis C, Wood NE
and Whelan JS: Germline genetic polymorphisms may influence
chemotherapy response and disease outcome in osteosarcoma: A pilot
study. Cancer. 118:1856–1867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Farber JL, Kyle ME and Coleman JB:
Mechanisms of cell injury by activated oxygen species. La Inves.
62:670–679. 1990.
|
|
14
|
Gan L, Liu Q, Xu HB, Zhu YS and Yang XL:
Effects of selenium overexposure on glutathione peroxidase and
thioredoxin reductase gene expressions and activities. Biol Trace
Elem Res. 89:165–175. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Halliwell B and Gutteridge JMC: Oxidative
stress: Adaptation, damage, repair and deathFree Radicals in
Biology and Medicine. 3rd. Oxford University Press; Oxford: pp.
246–350. 2001
|
|
16
|
Yuan X and Tang C: Lead effect on DNA and
albumin in chicken blood and the protection of selenium nutrition.
J Environ Sci Health A. 34:1875–1887. 1999. View Article : Google Scholar
|
|
17
|
Scott D, Galloway SM, Marshall RR,
Ishidate M Jr, Brusick D, Ashby J and Myhr BC: International
commission for the protection against environment mutagens and
carcinogens. Genotoxicity under extreme culture conditions. A
report from ICPEMC task Group 9. Mutat Res. 257:147–205. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cavalcantea DG, Martinez CB and Sofiaa SH:
Genotoxic effects of Roundup on the fish Prochilodus lineatus.
Mutat Res. 655:41–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eder E, Wacker M, Lutz U, Nair J, Fang X,
Bartsch H, Beland FA, Schlatter J and Lutz WK: Oxidative
stress-related DNA adducts in the liver of female rats fed with
sunflowers, rapeseed-, olive- or coconut oil supplemented diets.
Chem Biol Interact. 159:81–89. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
El-Bayoumy K: The protective role of
selenium on genetic damage and on cancer. Mutat Res. 475:123–139.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kara H, Cevik A, Konar V, Dayangac A and
Yilmaz M: Protective effects of antioxidants against
cadmium-induced oxidative damage in rat testes. Biol Trace Elem
Res. 120:205–211. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Priscilla DH and Prince PS:
Cardioprotective effect of gallic acid on cardiac troponin-T,
cardiac marker enzymes, lipid peroxidation products and
antioxidants in experimentally induced myocardial infarction in
Wistar rats. Chem Biol Interact. 179:118–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Townsend DM, Tew KD and Tapiero H: The
importance of glutathione in human disease. Biomed Pharmacother.
57:144–155. 2003. View Article : Google Scholar
|
|
24
|
Hill MF and Singal PK: Right and left
myocardial antioxidant responses during heart failure subsequent to
myocardial infarction. Circulation. 96:2414–2420. 1997. View Article : Google Scholar : PubMed/NCBI
|