Introduction
Ovarian cancer is one of the most frequently
occurring malignant tumors in the female reproductive organs, with
its morbidity rate ranked third, just behind cervical cancer and
endometrial cancer, but ranking first among the gynecological
tumors. Since the onset of ovarian cancer is not clear and without
specific symptoms at the early stage, the majority of clinical
cases are detected at the advanced stage. Although patients may
initially experience good post-operative reactions, they finally
succumb to recurrence and metastasis, and the 5-year survival rate
is only 25–30% (1,2).
Rhizoma Paridis is the rhizome of Paris
polyphylla Smith var. chinensis (Franch) Hara, which
belongs to the Liliaceae family. In traditional Chinese medicine,
the root is suggested to exhibit effects that include
heat-clearance, detoxification, the relief of swelling and pain,
the cooling of the liver and the arrest of convulsions. Modern
pharmacological studies have shown that Rhizoma Paridis displays
extensive pharmacological antitumor, immune regulation and
cardiovascular effects, and is generally used in treating malignant
lymphoma, lung cancer, nasopharyngeal carcinoma, cerebral tumors
and digestive system neoplasms. It has previously been found that
Paris saponins and extracts of Rhizoma Paridis show
significant antitumor activity in in vivo and in
vitro experiments, with multiple targets and pathways. The
mechanism may be associated with its own cytotoxicity and its
effects in accelerating apoptosis, affecting the cell cycle of
tumor cells, inhibiting the generation of the tumor vasculature and
regulating immune function (3,4).
In a previous in vitro study, we demonstrated
that polyphyllin I (PPI) resulted in growth inhibition, accelerated
apoptosis and anti-metastasis in a highly metastatic human ovarian
cancer HO-8910PM cell line (5).
Simultaneously, screening of a gene chip was conducted and it was
found that compared with the control group, there were 123
differentially-expressed genes in the cells treated by PPI; 70 were
downregulated and 53 were upregulated, and they were associated
with processes such as apoptosis, proliferation, migration,
invasion and angiogenesis. C-jun, Caspase-9 and Wnt5A in particular
were validated at the gene and protein level. The expression of
Caspase-9 and Wnt5a decreased with an increased dosage of PPI, and
the dose-effect association was marked. By contrast, the expression
of C-jun increased with increased dosage and lengthened active time
of the drug (6).
The main purpose of the present study was to observe
the in vivo antitumor effects of PPI and analyze the changed
expression of key molecules, such as C-jun, Caspase-9 and Wnt5a,
which were identified in previous in vitro studies. The
study aimed to discuss whether these molecules are involved in the
antitumor effect of PPI and the molecular mechanism involved by a
further nude mouse tumorigenicity assay in vivo.
Materials and methods
Experimental reagents
PPI (National Institute for the Control of
Pharmaceutical and Biological Products, Beijing, China; batch
number, 111590-200402), cis-platinum (DDP; Yunnan, China), reverse
transcription-polymerase chain reaction (RT-PCR) kit (PrimeScript™
RT reagent kit; Takara Biotechnology Co., Ltd., Dalian, China),
primers (synthesized by Takara Biotechnology Co., Ltd.) and PCR kit
(SYBR Premix Ex Taq™II: catalog no., DRR820A; Dalian, China) were
used in the present study, as well as primary antibodies against
Caspase-9 (ab32539), C-jun (ab31419) and Wnt5a (ab72583), which
were obtained from Abcam (Cambridge, UK) and used at 1:80 dilution.
The secondary antibody, which was the working solution from the
AuraStain SP Mouse/Rabbit IHC test kit (P003IH-1) was acquired from
Auragene Bioscience Corporation, Inc. (Changsha, China).
In vivo animal experiments
A total of 32 female BALB/c nude mice (4 weeks old,
weighing 17–20 g) were obtained from Shanghai Slack Laboratory
Animal Co., Ltd. (Shanghai, China; license no., SCXK (Shanghai)
2007–0005). The animals were maintained in a specific pathogen-free
environment. The animal experiments were approved by the
Institutional Animal Care and Use Committee of Zhejiang Chinese
Medicine University (Hangzhou, Zhejiang, China).
Nude mouse HO-8910PM transplantation tumor mass,
which was generated in our previous study, was inoculated
subcutaneously into the back of nude mouse (7). The mass was cut after it grew up and
0.5–1 cm3 of the mass was inoculated subcutaneously into
the back of the right upper extremity of each nude mouse. It took 2
weeks for the mass to grow to soybean-size (0.3 cm3).
The mice were then randomly assigned into 4 groups (8 mice in each
group) as follows: Saline group (containing the same quantity of
dimethyl sulfoxide, 1.5 µl/ml), PPI low-concentration group (1.5
mg/kg PPI), PPI high-concentration group (3 mg/kg PPI) and
DDP-positive control group (2 mg/kg). DDP is widely used in ovarian
cancer; thus, it was used in the present study as a positive
control. The volume of the drug for intraperitoneal injection was
limited at 1% of the body weight, and the drug was administered
every 7 days for 7 weeks in total. The maximum longitudinal
diameter (a) and transverse diameter (b) of the transplant
subcutaneous sarcoma were measured using Vernier calipers every 7
days. The mean tumor volume of each group was determined according
to V=1/2ab2 and the growth curves were drawn. The mice
were also weighed on an electronic scale every 7 days.
Histological experiment
After 49 days, the animals were sacrificed by
cervical dislocation and dissections were performed to obtain
hearts, livers, spleens, lungs and kidneys. White metastases the
size of rice grains could be observed by the naked eye in the
lungs, but there were no abnormalities observed in other organs.
The organs were divided into two sections, one of which was stored
in liquid nitrogen, while the other was added to 10% neutral
buffered formalin (pH 7.4) prior to tissue chips (2 mm) being made
by Google Organisms Technology Co., Ltd. (Wuhan, China).
Hematoxylin and eosin staining was performed on the sections and an
analysis of the tissue chips was performed using iViewer (Beijing
Unic-tech Co., Ltd., Beijing, China).
Immunohistochemistry
The expression conditions of Caspase-9, C-jun and
Wnt5a proteins of subcutaneous tumor tissues and lung metastatic
tumor tissue with metastasis were analyzed. Paraffin-embedded
tissue chips were treated according to immunohistochemical
processes, and antigen and antibody were bound together through
high-pressure thermal remediation. Rabbit anti-Caspase-9, C-jun and
Wnt5a were all used at 1:80 dilution (Abcam). The subsequent
procedures would be performed as per the instructions of the
AuraStain SP Mouse/Rabbit IHC test kit (Auragene Bioscience, Inc.).
Staining amount (A) was scored as follows: 0, no stained cells; 1,
1–10% stained cells; 2, 11–50% stained cells; 3, 51–80% stained
cells; 4, 81–100% stained cells. Staining intensity score (B) was
scored as follows: 0, negative; 1, weakly-positive; 2,
moderately-positive; 3, strongly-positive. The immunohistochemistry
staining score (HIS) was calculated as follows: HIS = A × B. HIS of
9–12, strongly-positive; 5–8, moderately-positive; 1–4,
weakly-positive; and 0, negative.
RT-PCR
The tissue was ground with liquid nitrogen and
TRIzol was added to extract RNA by a conventional method (8). The RNA was reverse-transcribed into cDNA
according to the instructions of the RT-PCR kit. Quantitative PCR
was conducted on an Applied Biosystems® 7500 Real-Time
PCR System (Thermo Fisher Scientific, Inc., Waltham, MA, USA) using
SYBR Green with specific primers (Table
I). The PCR amplifications conditions were as follows: 95°C for
10 min, 95°C for 15 sec and 60°C for 1 min (for 40 cycles). Using a
solubility curve to check whether there was non-specific banding in
the PCR amplified products, the amplification conditions were as
follows: 95°C for 15 sec and 60°C for 1 min, for 40 cycles. The Cq
value was automatically generated and analyzed. The relative
expression of the genes was calculated using the 2−ΔΔCq
method (9). The experiment was
repeated three times.
 | Table I.Primer sequences for target genes. |
Table I.
Primer sequences for target genes.
| Gene name | Primer sequences |
|---|
| β-actin |
|
|
Forward |
5′-TGGCACCCAGCACAATGAA-3′ |
|
Reverse |
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ |
| C-jun |
|
|
Forward |
5′-CACGTGAAGTGACGGACTGTTCTA-3′ |
|
Reverse |
5′-CAGGGTCATGCTCTGTTTCAGG-3′ |
| Wnt5a |
|
|
Forward |
5′-TTCGCCCAGGTTGTAATTGAAG-3′ |
|
Reverse |
5′-CTGCATGTGGTCCTGATACAAGTG-3′ |
| Caspase-9 |
|
|
Forward |
5′-GCCATATCTAGTTTGCCCACACC-3′ |
|
Reverse |
5′-CACTGCTCAAAGATGTCGTCCA-3′ |
Data processing and statistical
analysis
The data were processed by SPSS18.0 software (IBM
SPSS, Armonk, NY, USA). The relative expression was represented as
the mean plus standard deviation. A one-way analysis of variance
and least significant difference test was used to compare the
normally distributed data between groups, with the non-parametric
method used to test the abnormally distributed data. Differences
were statistically significant between each group when
P<0.05.
Results
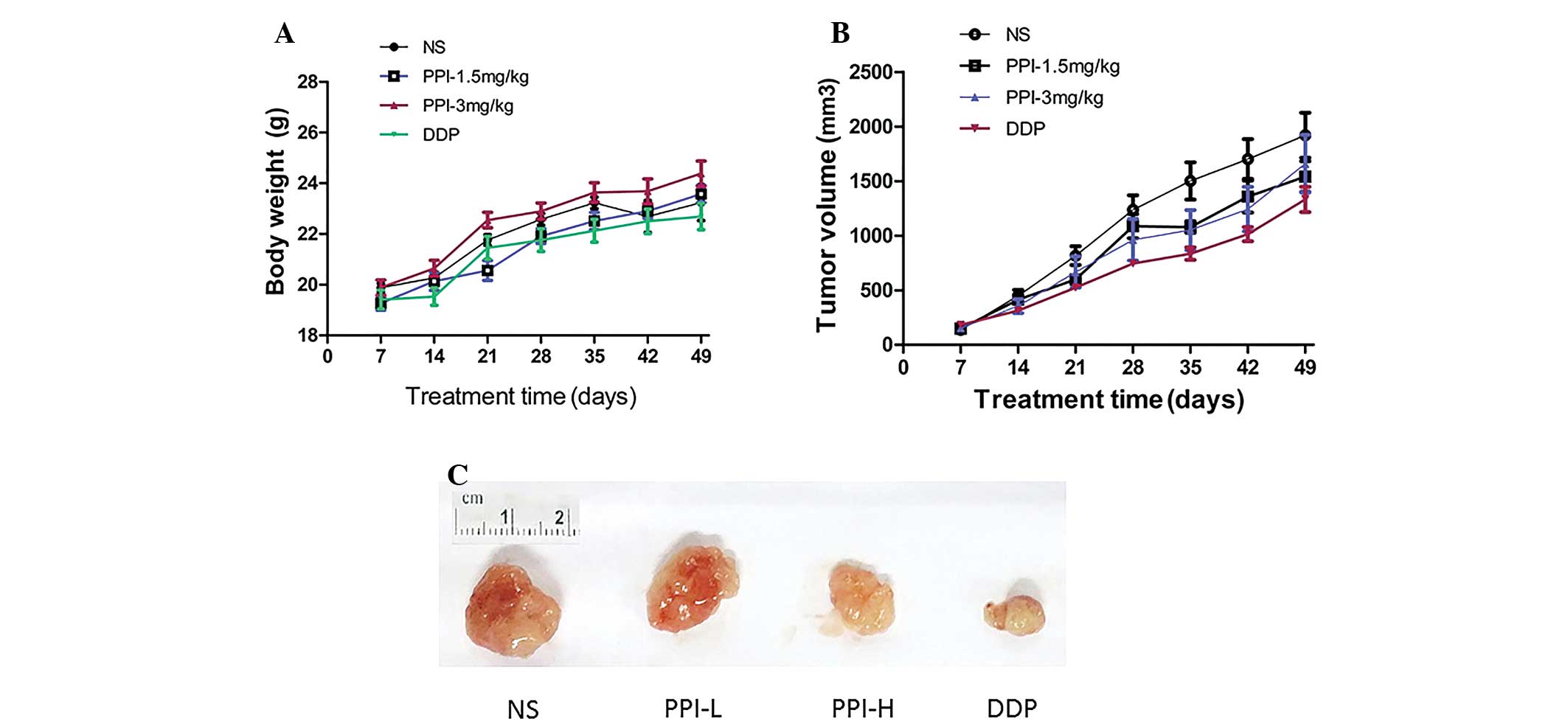
Effects of PPI on body weight and
tumor growth
Within 7 weeks, the body weights of the nude mice in
the groups differed. During the experiment, there was 1 mouse in
the PPI high concentration group that died on day 40, while the
others all survived. The toxic effect in the DDP group was the most
significant, with reduced activity and declined food intake; in the
other groups, the mice were as normal. The body weight of the mice
in the DDP and NS groups increased at first and then decreased,
particularly in the DDP group. In the PPI groups with different
dosages, the body weight increased continuously, but without a
significant difference compared with the control group. There was a
significant difference between the DDP and PPI high concentration
groups (P=0.02) (Fig. 1A).
The growth of transplant subcutaneous sarcoma in
each group showed a rising trend by varying degrees following
administration, being fastest in the NS group and slowest in the
DDP group. There was a significant difference in the size of the
tumor among the four groups (F=3.418, P=0.038). When the NS group
was compared with PPI high concentration and DDP groups, there were
significant differences (P=0.0370 and P=0.008, respectively)
(Fig. 1B). It could be observed that
in the PPI high and low concentration groups, the tumor sizes were
significant smaller than that in the NS control group, and in the
DDP-positive group, the tumor size was the smallest (Fig. 1C).
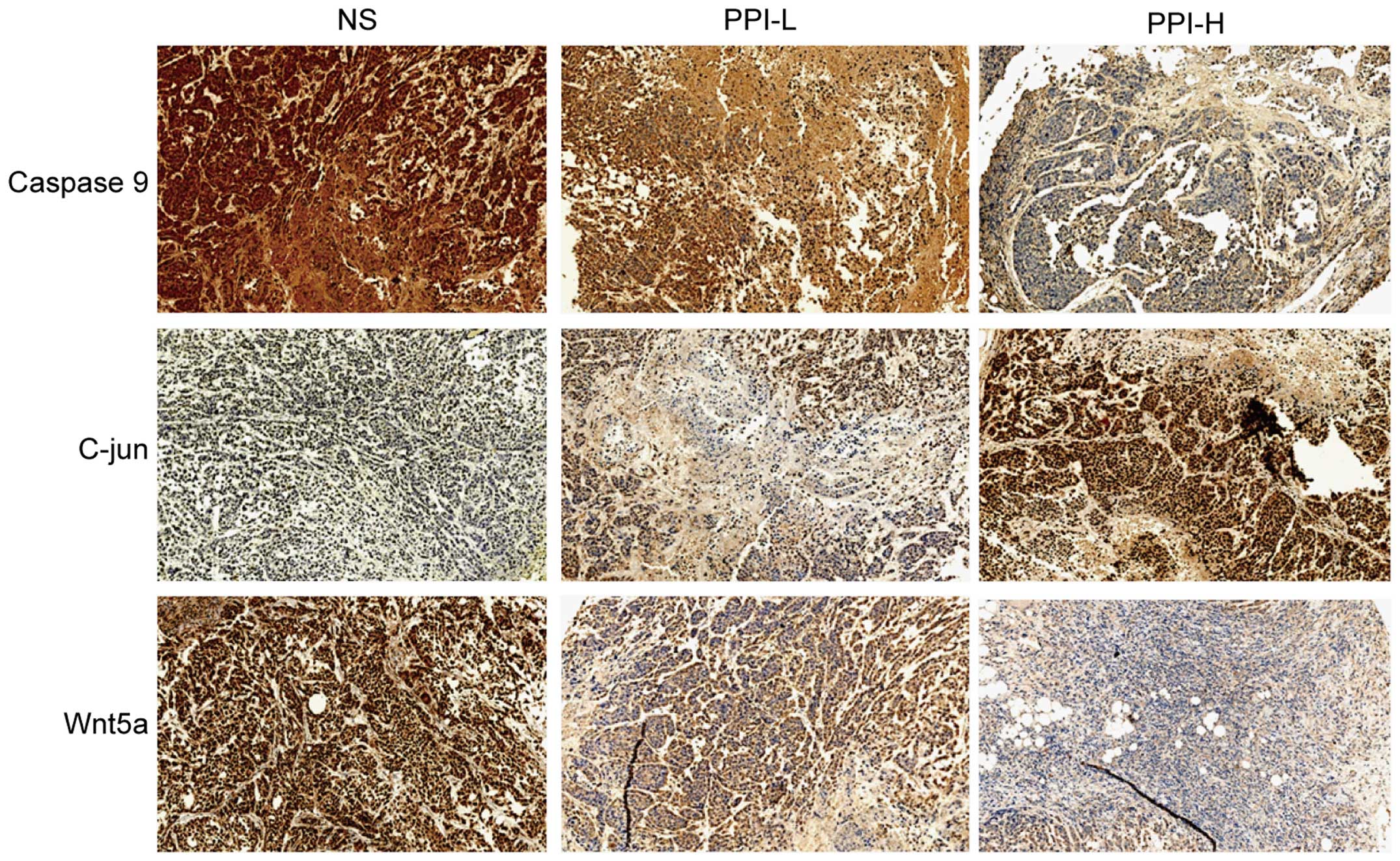
Caspase-9, C-jun and Wnt5a protein
expression
In our previous in vitro experiment, it was
noted that three key molecules (Caspase-9, C-jun and Wnt5a) were
regulated by PPI, which may be associated with the antitumor
effect. Therefore, in vivo experiments were performed in the
present study for confirmation. The immunohistochemical results
showed that the expression of Caspase-9 was decreased in the PPI
groups in the tumor tissue, and that Wnt5a expression was also
decreased in the PPI groups, while C-jun expression was increased
(Fig. 2).
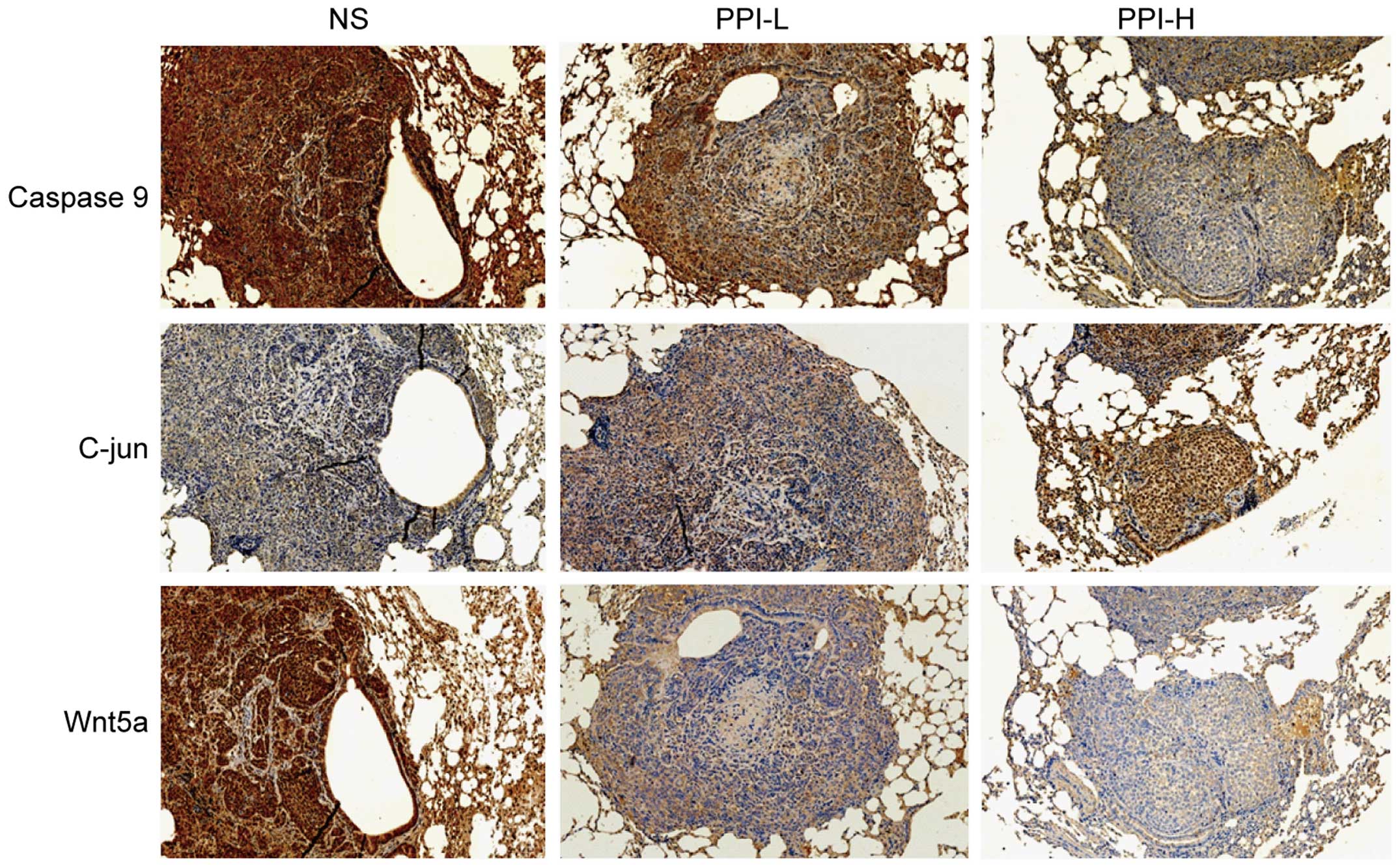
Similarly, the change tendency was the same in the
lung metastasis tumor tissue, in which the expression of Caspase-9
and Wnt5a was also decreased in PPI groups, while that of C-jun was
increased (Fig. 3).
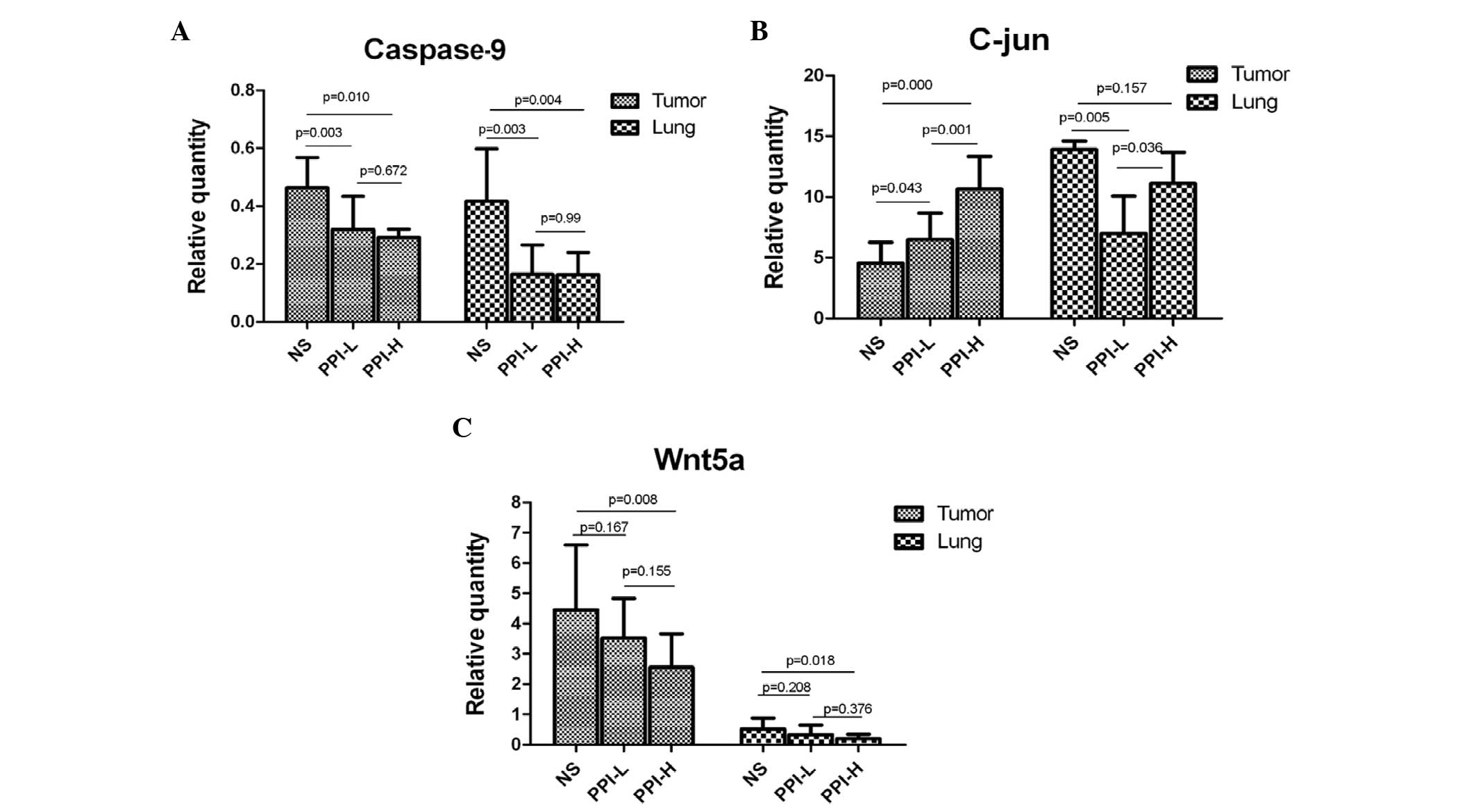
C-jun, Caspase-9 and Wnt5a gene
expression
Furthermore, the changes in C-jun, Caspase-9 and
Wnt5a gene expression were also detected at the transcriptional
level. The results showed that there were significant differences
in C-jun, Caspase-9 and Wnt5a gene expression in the tumor and lung
tissues among the NS, PPI-L and PPI-H groups (Fig. 4A-C). For transplant subcutaneous
sarcoma, Caspase-9 expression in the drug groups was lower than
that in the control group (P=0.003 and P=0.010, respectively),
while C-jun expression was higher (P=0.043 and P<0.001,
respectively). Wnt5A expression was lower in the high dosage group
and exhibited no significant change in the low dosage group
(P=0.167 and P=0.155, respectively) (Fig.
4A-C). For the lung tissues, Caspase-9 and C-jun expression in
the drug groups was lower than that of the control group (P=0.003,
P=0.004 and P=0.005, respectively), and Wnt5a was lower in the high
dosage group, with no significant change in the low dosage group
(P=0.208) (Fig. 4A-C). These results
were in compliance with the aforementioned immunohistochemical
detection.
Discussion
In the present study, it was found that PPI markedly
inhibited the growth of ovarian cancer transplant sarcoma in
vivo, which confirmed the antitumor effect on ovarian cancer
that was indicated in previous in vitro studies (9). The antitumor effects of PPI in lung
cancer have been reported a number of times; for example, PPI
increased the radiation sensitivity of gefitinib-resistance lung
cancer cell lines (10), PPI markedly
inhibited the proliferation and growth of non-small cell lung
cancer (11), and PPI suppressed the
metastasis of lung cancer (12) in
previous studies. More importantly, it was reported that PPI
promoted the apoptosis of ovarian cancer cells through the
mitochondrial pathway (13).
Therefore, these results indicated that PPI exhibits good antitumor
effects in vitro and in vivo.
In order to investigate the mechanism of PPI, in our
previous in vitro studies, screening of genes by microarray
was conducted and it was found that compared with the control
group, there were 123 differentially-expressed genes in the cells
treated by PPI; 70 were downregulated and 53 were upregulated, and
they were associated processes such as apoptosis, proliferation,
migration, invasion and angiogenesis. C-jun, Caspase-9 and Wnt5A in
particular were validated at the gene and protein levels. The
expression of Caspase-9 and Wnt5A decreased with increased dosage
of PPI, and the dose-effect association was marked. By contrast,
the expression of C-jun increased with increased dosage and
prolonged time of drug administration (6). The present study also found that the
changes of the three genes in vivo were the same as those
in vitro, indicating that these molecules may play important
roles in the antitumor effect of polyphyllins.
It was reported that PPI promoted mitochondrial
apoptosis, as observed by decreases in the expression of BCL2,
increases in the expression of BAX, the release of cytochrome
c, and the activation and cleavage of Caspase-9 (10,13); while
these downstream molecular changes occurred, the upstream changes
were not clear. Our studies revealed that the change in expression
of C-jun, Caspase-9 and Wnt5A may be associated with the effect of
promoting apoptosis. The decreased expression of Caspase-9 observed
in the present study, which is due to cleavage induced by the drug,
promoted the apoptosis cascade reaction. There are three main types
of Wnt signaling pathways: i) The canonical Wnt/β-catenin pathway;
ii) the Wnt/JNK pathway (also known as the planar cell polarity
pathway); and iii) the Wnt/Ca+2 pathway, which can be
selectively activated by extracellular molecules, thus becoming
effector molecules. Activation of JNK can further induce the
activity of transcription factors such as C-jun (14,15). C-jun
is the specific substrate of JNK, and the activation of this
pathway results in the release of apoptosis factor cytochrome
c and ultimately mitochondrial apoptosis (16). The present study found that the
expression of C-jun increased in the PPI groups, which may be
associated with mitochondrial apoptosis. Furthermore, Wnt5A was
closely associated with tumor development. The overexpression of
Wnt5A may promote the metastasis of tumors and
epithelial-mesenchymal transition (17,18). Wnt5A
has also been shown to be closely involved in the invasion of
prostate cancer cells (19). The
present study found significantly decreased expression of Wnt5A
when administering PPI, which may suggest its anti-metastatic
effect.
In conclusion, the present study found that PPI
exhibited a marked anti-ovarian cancer effect in vivo, with
little impact on the body weight of nude mice, which indicated its
safety and efficacy, and thus meant that PPI could be considered
for further development. The study confirmed expression alterations
of three key molecules (Caspase-9, C-jun and Wnt5A) at the
molecular level, and that polyphyllins inhibited the expression of
Caspase-9 and Wnt5A while promoting the expression of C-jun. The
results in this study also provided a basis for further
investigating the effects and mechanism of PPI, and will be of
great significance.
Acknowledgements
This study was supported by a grant (no. LQ12H16015)
from the Zhejiang Province Natural Science Fund of Youth in
China.
References
|
1
|
Siegel R, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeng H, Zheng R, Guo Y, Zhang S, Zou X,
Wang N, Zhang L, Tang J, Chen J, Wei K, et al: Cancer survival in
China, 2003–2005: A population-based study. Int J Cancer.
136:1921–1930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun J, Liu BR, Hu WJ, Yu LX and Qian XP:
In vitro anticancer activity of aqueous extracts and ethanol
extracts of fifteen traditional Chinese medicines on human
digestive tumor cell lines. Phytother Res. 21:1102–1104. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiao X, Bai P, Bui Nguyen TM, Xiao J, Liu
S, Yang G, Hu L, Chen X, Zhang X, Liu J and Wang H: The antitumoral
effect of Paris Saponin I associated with the induction of
apoptosis through the mitochondrial pathway. Mol Cancer Ther.
8:1179–1188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gu LH, Feng JG, Qian LJ and Ma SL:
Research on proliferation inhibitory effect of Paris Saponin I on
high metastatic human ovarian cancer cell line HO-8910PM in vitro.
Chinese Archives of Traditional Chinese Medicine. 30:2212–2215.
2012.(In Chinese).
|
|
6
|
Gu LH, Feng JG, Xu HY, Luo M and Su D:
Polyphyllin I inhibits proliferation and metastasis of ovarian
cancer cell line HO-8910PM in vitro. J Tradit Chin Med. 33:325–333.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shenhua X, Hanzhou M, Lijuan Q, Yongzheng
S, Chihong Z, Xiaoshu H, Yongliang G and Shanxing D: Establishment
and characterization of a model of highly metastasizing human
ovarian cancer transplanted into subcutis of the nude mice. J Exp
Clin Cancer Res. 14:387–393. 1995.
|
|
8
|
Chomczynski P and Sacchi N: Single-step
method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction. Anal Biochem.
162:156–159. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak and Schmittgen, . Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang H, Zhao P, Feng J, Su D and Ma S:
Effect of Polyphyllin I on radiosensitivity in a
gefitinib-resistant lung adenocarcinoma cell line. Oncol Lett.
7:2059–2064. 2014.PubMed/NCBI
|
|
11
|
Kong M, Fan J, Dong A, Cheng H and Xu R:
Effects of polyphyllin I on growth inhibition of human non-small
lung cancer cells and in xenograft. Acta Biochim Biophys Sin
(Shanghai). 42:827–833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shuli M, Wenyuan G, Yanjun Z, Chaoyi M,
Liu Y and Yiwen L: Paridis saponins inhibiting carcinoma growth and
metastasis in vitro and in vivo. Arch Pharm Res. 34:43–50. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiao X, Bai P, Bui Nguyen TM, Xiao J, Liu
S, Yang G, Hu L, Chen X, Zhang X, Liu J and Wang H: The antitumoral
effect of Paris Saponin I associated with the induction of
apoptosis through the mitochondrial pathway. Mol Cancer Ther.
8:1179–1188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heasley LE and Winn RA: Analysis of
Wnt7a-stimulated JNK activity and cJun phosphorylation in non-small
cell lung cancer cells. Methods Mol Biol. 468:187–196. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang A, He S, Sun X, Ding L, Bao X and
Wang N: Wnt5a promotes migration of humanosteosarcoma cells by
triggering aphosphatidylinositol-3 kinase/Akt signals. Cancer Cell
Int. 14:15–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bates DJ, Lewis LD, Eastman A and Danilov
AV: Vincristine activates c-Jun N-terminal kinase in chronic
lymphocytic leukemia in vivo. Br J Clin Pharmacol. 80:493–501.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dissanayake SK, Wade M, Johnson CE,
O'Connell MP, Leotlela PD, French AD, Shah KV, Hewitt KJ, Rosenthal
DT, Indig FE, et al: The Wnt5A/protein kinase C pathway mediates
motility in melanoma cells via the inhibition of metastasis
suppressors and initiation of an epithelial to mesenchymal
transition. J Biol Chem. 282:17259–17271. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taki M, Kamata N, Yokoyama K, Fujimoto R,
Tsutsumi S and Nagayama M: Down-regulation of Wnt-4 and
up-regulation of Wnt-5a expression by epithelial-mesenchymal
transition in human squamous carcinoma cells. Cancer Sci.
94:593–597. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamamoto H, Oue N, Sato A, Hasegawa Y,
Yamamoto H, Matsubara A, Yasui W and Kikuchi A: Wnt5a signaling is
involved in the aggressiveness of prostate cancer and expression of
metalloproteinase. Oncogene. 29:2036–2046. 2010. View Article : Google Scholar : PubMed/NCBI
|