Introduction
The incidence of renal cancer ranks eighth among all
malignant diseases worldwide (1),
while its mortality rate ranks sixth (2). Clear cell carcinoma is the dominant
pathological type of renal cancer (1). Early renal carcinoma (RC) has atypical
clinical symptoms and signs, which are easily overlooked. This,
coupled with the lack of specific tumor markers, makes early
diagnosis of RC remarkably difficult (3). Furthermore, it is easy for RC to
progress into a late stage, since transfer or invasion of real
cancer cells into the surrounding organs occurs at an early stage,
as the kidney does not have a serous layer and is located adjacent
to the trachea, aorta and other important organs. Clinically,
>1/2 of patients with RC had missed the opportunity to remove
the tumor at diagnosis, which is a challenge for the clinicians
(3). At present, the treatment of RC
that has been widely used is the combined strategy of surgical
excision, radiotherapy and chemotherapy, but its efficiency is
still unsatisfactory, since the 5-year survival rate following
surgery is only 20–40% (4,5).
Apoptosis is not only a normal cell cycle process,
but it is also vital for homeostasis, since it can remove excessive
damaged and aging mutant cells (6).
Apoptosis disorders are involved in the pathogenesis of a variety
of malignant tumors, and are considered to be the key reason for
the occurrence and development of tumors, being closely associated
with tumor drug resistance (3).
Inhibition of apoptosis leads to the accumulation of mutations in
the cell, which initiates differentiation (7). Malignant cells do not die if
insufficient apoptosis occurs (8).
The members of the family of inhibitor of apoptosis
proteins (IAPs), which contains eight members, are the key
regulators of cytokinesis, apoptosis and signal transduction, and
have been demonstrated to be closely associated with oncogenesis
(9). Apollon, namely baculoviral IAP
repeat containing 6 (BIRC6), is a membrane-associated protein that
localizes to the Golgi compartment and the vesicular system, and is
the largest member of the IAP family (9). It inhibits apoptosis by binding to
cysteine aspartate-specific proteases (caspases) directly. Its
anti-apoptotic function is much stronger than that of the proteins
of the B-cell lymphoma-2 family, mainly through the function of
Smac, high temperature requirement A protein serine peptidase 2 and
caspase (10,11). Increasing evidence has shown that
upregulation of Apollon may be important in tumor generation
(12–16). However, the association between RC and
Apollon is unclear.
In the present study, Apollon expression was
detected by immunohistochemistry, western blotting and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) in
RC tissues, adjacent non-cancerous tissues and normal tissues
respectively, in order to analyze the association between Apollon
expression and the clinicopathological features of RC, and to
highlight the link between Apollon expression and the occurrence,
development and prognosis of RC.
Materials and methods
Patients and specimens
The patients enrolled in the present study were
recruited from the Third Affiliated Hospital of Southern Medical
University (Guangzhou, China) from February 2010 till February
2014. The patients were diagnosed as RC by >2 pathologists.
Specimens, including 50 cases of cancer tissue, 42 cases of
adjacent non-cancerous tissue and 30 cases of paired normal tissue,
were obtained by intraoperative radical renalectomy. The patients
included 48 males and 2 females, who were <75 years old. All
patients were first-diagnosed cases, without chemotherapy,
radiotherapy or other treatments prior to being sampled, and their
clinical and pathological data were complete and reliable. The
present study was approved by the Third Affiliated Hospital of
Southern Medical University. The usage of information and the
collection of specimens was conducted once informed consent from
the patients or their families was obtained.
Reagents and equipment
Anti-Apollon antibody was purchased from Abcam
(Cambridge, UK) (ab84429). The PowerVision two-step
immunohistochemistry kit was purchased from Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd. (Beijing, China) (PV-6001).
TRIzol, for total RNA extraction, was purchased from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) (15596–026). The
RT kit was purchased from Promega Corporation (Madison, WI, USA)
(A5001). The RT-qPCR kit was purchased from Promega Corporation
(A6001). Paraffin-embedding equipment, paraffin-slicing equipment
and the automatic upright microscope system (DM5000 B) were
purchased from Leica Microsystems GmbH (Wetzlar, Germany). The 400W
ultraviolet imaging system was purchased from Kodak (Rochester, NY,
USA). The PCR equipment (ABI PRISM 7500 Sequence Detection System)
was purchased from Applied Biosystems (Thermo Fisher Scientific,
Inc.). The Total Protein Extraction kit, polyvinylidene difluoride
membranes and Beyo-ECL Plus Detection System were purchased from
Beyotime Institute of Biotechnology (Haimen, China).
Immunohistochemistry
The rapid PowerVision two-step staining method was
used with the following specifications: Paraffin slices were
obtained with a thickness of 5 µm, which were next incubated at
65°C for 60 min, followed by dewaxing, hydration and
high-temperature antigen retrieval in a microwave by incubation in
a 0.1 M citrate solution (pH 6.0) for 10 min. Next, the slides were
incubated with 3% H2O2 at room temperature
for 20 min, followed by incubation with goat serum (ab7481; Abcam)
at room temperature for 20 min and incubation with anti-Apollon
antibody (1:400; ab19609; Abcam) at 4°C overnight. The slides were
heated up the following day prior to being incubated with an
anti-rabbit antibody at room temperature for 20 min. Subsequently,
the slides were subjected to 3,3′-diaminobenzidine and hematoxylin
staining, prior to being mounted for light microscopic examination.
Immunohistochemical staining was scored independently by two
pathologists without knowledge of the patients' characteristics.
Any discrepancy was solved by consensus review. The score of
immunoreactivity was performed by calculating the extent and
intensity of positive staining of cells in a semi-quantitative
manner. Score interpretation was performed as described previously
(17). The standards for evaluation
included the following: Positive staining intensity (0, negative;
1, weak positive; 2, moderate positive; and 3, strong positive) and
proportion of positive areas (0, 0%; 1, 1–30%; 2, 30–70%; and 4,
70–100%). The final score was obtained by multiplication of the
aforementioned two scores, with 0 regarded as negative, 1–2
regarded as weak positive, 3–4 regarded as moderate positive and ≥5
regarded as strong positive. The negative and weak-moderate
positive results were considered as low expression of Apollon,
while the strong positive results were considered as Apollon
overexpression. RC patients were divided into a low-expression
group or a high-expression group, based on their final score, which
was considered as high expression if its value was >5.
Western blotting
Tissues were lysed with the Total Protein Extraction
kit (Beyotime Institute of Biotechnology), and the protein lysates
were resolved on 12% SDS-PAGE prior to being transferred to
polyvinylidene difluoride membranes (IPFL00010; EMD Millipore,
Billerica, MA, USA). The membranes were blocked with 5% milk and
then incubated for 2 h with primary antibodies against Apollon
(ab19609; Abcam) and GAPDH (ab8245; Abcam) at 37°C. The membranes
were washed with PBS containing Tween 20 and then incubated with
the secondary antibody (ab131366; Abcam). The Beyo-ECL Plus
Detection System (Beyotime Institute of Biotechnology) was employed
to visualize the bands. The Apollon protein expression levels were
quantified by Image Lab Software 5.1 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and represented as the densitometric ratio of
the targeted protein to GAPDH.
RT-qPCR
Total RNA was extracted from 1 mg renal tissue using
TRIzol (Invitrogen (Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. Upon treatment with DNA-free DNase
(Ambion; Thermo Fisher Scientific, Inc.) to remove the chromosomal
DNA, complementary (c)DNA was synthesized using an RT kit (Promega
Corporation) and stored at −20°C until use. The messenger RNA
(mRNA) expression levels of Apollon and β-actin were determined by
RT-qPCR using the ABI PRISM 7500 Sequence Detection System. The
primer sequences (sense/anti-sense) were: Apollon,
5′-TGACAGGGCATACATCACAG-3′/5′-GCAACAATCTCCCACTGAAG-3′ and β-actin,
5′-GCACCACACCTTCTACAATGAG-3′/5′-GATAGCACAGCCTGGATAGCA-3′. The mRNA
expression levels of the target genes were normalized to the
β-actin signal, which served as a housekeeping gene. All the
reactions were performed in triplicate using 20-µl samples
containing 50 ng cDNA. The reaction protocol involved heating for
10 min at 95°C, followed by 40 cycles of amplification (15 sec at
95°C and 1 min at 60°C). The data were analyzed using the ABI PRISM
7500 Sequence Detection software version 1.2 (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The levels of Apollon expression
in unknown samples were calculated as a ratio of Apollon to
β-actin. Determinations were performed three times for each
sample.
Statistical analysis
The patients were categorized into two groups
according to their level of Apollon expression (high Apollon
expression group and low Apollon expression group). The differences
in Apollon expression with respect to the clinical factors at
diagnosis, including gender, age, alcohol consumption history,
tumor size, histological grade, T-stage, N-stage and
tumor-node-metastasis (TNM)-stage, were analyzed. The expression
levels are presented as median values. The quantitative data were
compared using one-way analysis of variance and unpaired t test,
while the qualitative data were compared using Pearson's
χ2 test. Statistical analyses were conduced with SPSS
19.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Patients
In total, 50 patients (males, 48; females, 2) were
enrolled in the study. Their mean age was 58.6 years (range, 42–74
years). According to the 7th edition of the International Union
Against Cancer's renal cancer staging manual (2009 edition), the
enrolled patients were classified as follows: 4 cases of stage I,
20 cases of stage II and 26 cases of stage III (12).
Increased Apollon protein and mRNA
expression in RC tissues
Immunohistochemical staining
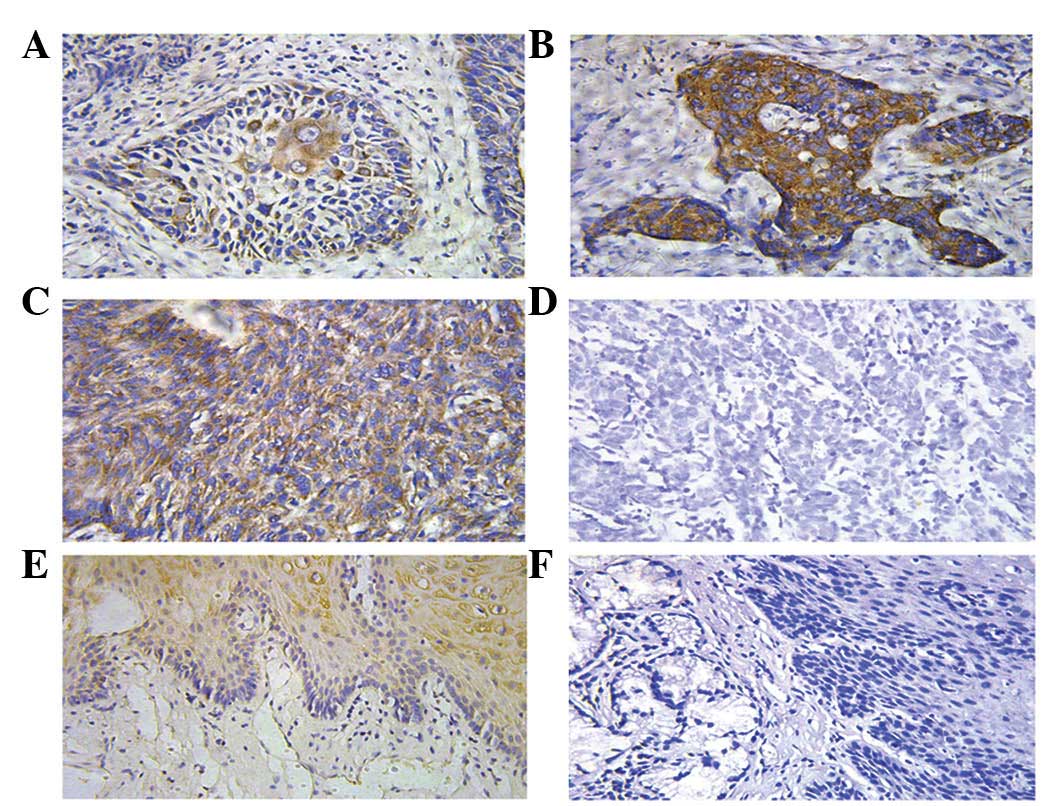
Apollon protein was detected by immunohistochemical
staining (Fig. 1). Apollon protein
was expressed in RC tissues, adjacent non-cancerous tissues and
normal renal tissues. Apollon expression was detected mainly in the
cytoplasm of the carcinoma cells and in the cytoplasm of the clear
cell carcinoma cells or underlying basal cells of the adjacent or
normal tissues.
The positive reactants of Apollon were yellow or
brown substances that mainly existed in the cytoplasm (Fig. 1). According to the aforementioned
standards, the expression rate of Apollon was 56% (28/50) in the
tumor tissue, while it was 5% (2/42) in the adjacent non-cancerous
tissue and 0% (0/30) in the normal renal mucosa epithelial tissue.
This difference was statistically significant (P<0.001)
(Table I).
 | Table I.Expression of Apollon in tumor,
adjacent and normal epithelial tissues. |
Table I.
Expression of Apollon in tumor,
adjacent and normal epithelial tissues.
| Apollon
expression | Cancer tissue | Adjacent tissue | Normal tissue |
|---|
| Total, N |
50 |
42 |
30 |
| − |
8 |
28 |
20 |
| + |
6 |
6 |
7 |
| ++ |
8 |
6 |
3 |
| +++ |
28 |
2 |
0 |
| P-value | 0.002 | 0.012 | 0.010 |
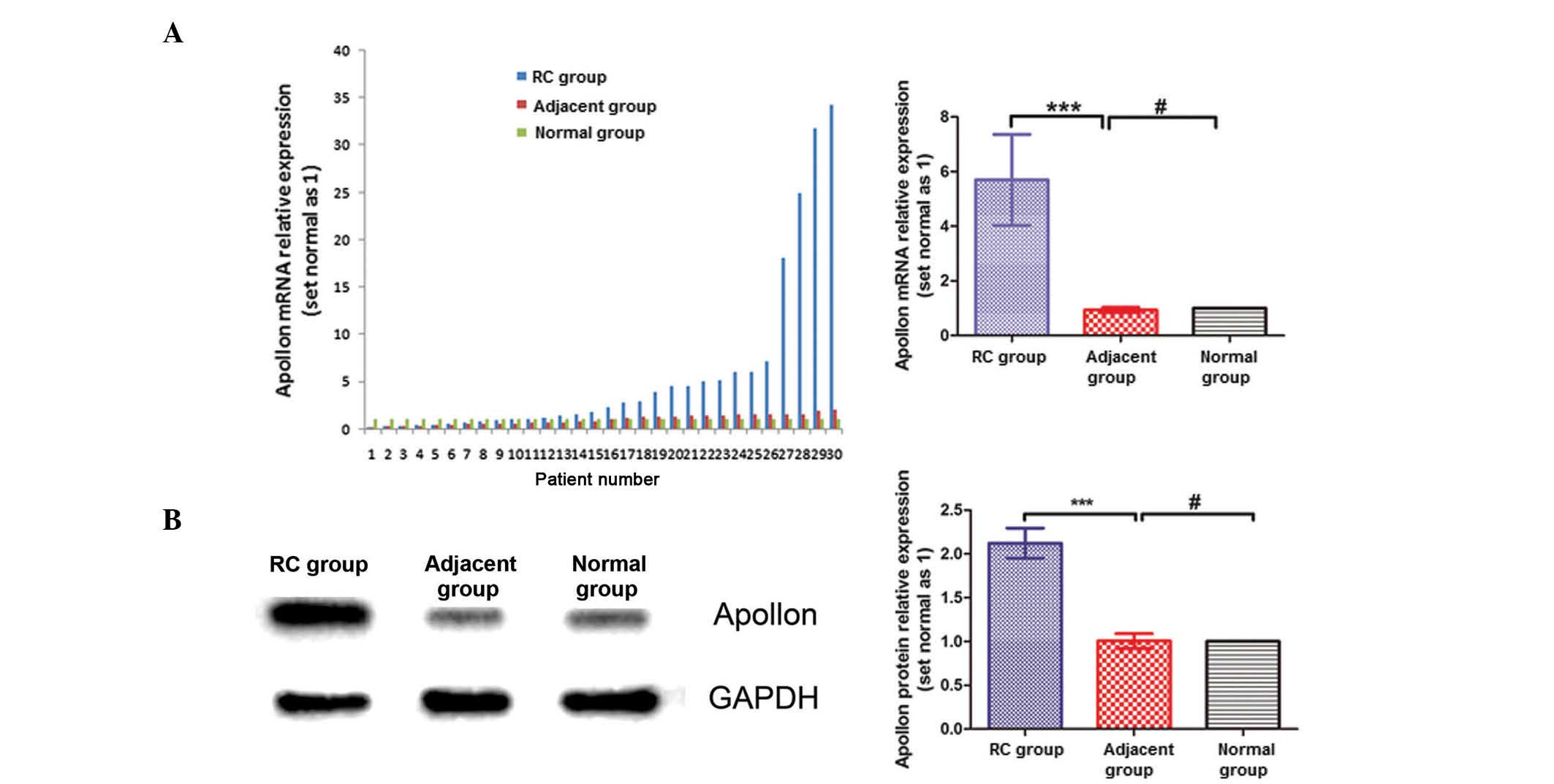
RT-qPCR and western blotting
The mRNA expression of Apollon in each group was
normalized against the endogenous mRNA expression of the
housekeeping gene β-actin. The relative expression of Apollon mRNA
was described as 2−ΔΔCq (18). Apollon expression in RC tissues was
significantly higher than that in adjacent non-cancerous tissues
and normal renal tissues (P=0.012) (Fig.
2A). Western blotting analysis also revealed that Apollon
protein expression in RC tissues was significantly higher than that
in adjacent non-cancerous tissues and normal tissues (Fig. 2B).
Apollon expression and clinicopathological
variables
The association between Apollon high expression
(score ≥5) and various clinicopathological features was analyzed as
aforementioned (Table II). There was
no correlation between Apollon overexpression and age (P=0.823),
gender (P=0.201), histological grade (P=0.156), tumor size
(P=0.749) or alcohol consumption history (P=0.976). Significant
associations were observed between Apollon overexpression and nodal
involvement (P=0.007), T-stage (P=0.006) and TNM-stage (P=0.035)
(Table II).
 | Table II.Associations between Apollon
expression and clinicopathological characteristics of renal cancer
patients. |
Table II.
Associations between Apollon
expression and clinicopathological characteristics of renal cancer
patients.
|
|
| Apollon |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | N | High
expression | Low expression | P-value |
|---|
| Gender |
|
|
| 0.201 |
|
Male | 48 | 26 | 22 |
|
|
Female | 2 | 2 | 0 |
|
| Age, years |
|
|
| 0.212 |
|
≥55 | 34 | 17 | 17 |
|
|
<55 | 16 | 11 | 5 |
|
| Alcohol consumption
history (≥10 years) |
|
|
| 0.976 |
|
Yes | 41 | 23 | 18 |
|
| No | 9 | 5 | 4 |
|
| Tumor size, cm |
|
|
| 0.749 |
| ≥4 | 26 | 14 | 12 |
|
|
<4 | 24 | 14 | 10 |
|
| Histological
grade |
|
|
| 0.156 |
|
Well | 16 | 6 | 10 |
|
|
Moderate | 26 | 16 | 10 |
|
|
Poor | 8 | 6 | 2 |
|
| T-stage |
|
|
| 0.006 |
| T1 | 11 | 3 | 8 |
|
| T2 | 16 | 6 | 10 |
|
| T3 | 18 | 15 | 3 |
|
| T4 | 5 | 4 | 1 |
|
| N-stage |
|
|
| 0.007 |
| N0 | 17 | 5 | 12 |
|
|
N1+N2+N3 | 37 | 23 | 10 |
|
| TNM-stage |
|
|
| 0.035 |
| I | 4 | 2 | 2 |
|
| II | 20 | 7 | 13 |
|
|
III | 26 | 19 | 7 |
|
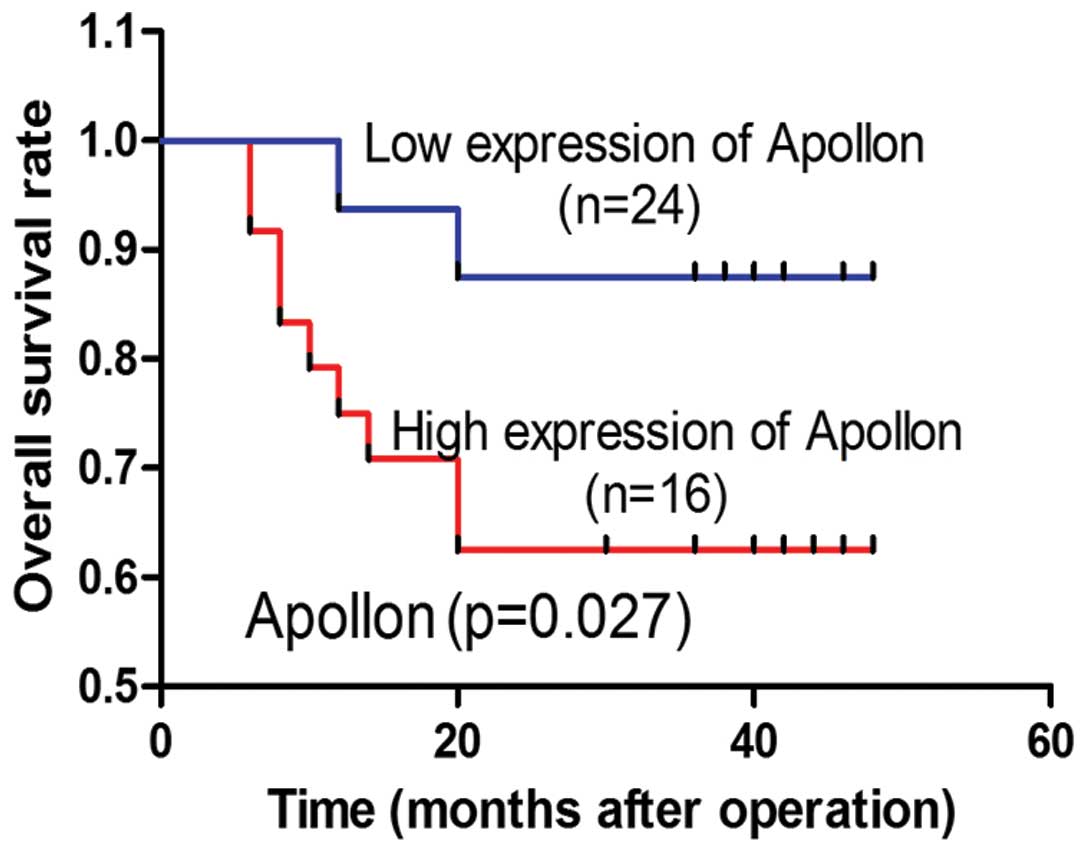
Survival analysis
In total, 80% (40 of 50) of the patients were
followed up successfully (the remaining 10 patients could not be
contacted). To evaluate the effect of Apollon expression on
survival, Kaplan-Meier analysis and log-rank test were used. The
results indicated that high level of Apollon expression was
correlated with short overall survival (OS) (P=0.017) (Fig. 3).
Univariate analysis revealed that Apollon
expression, T-stage, TNM-stage and tumor size were associated with
OS, while tumor differentiation, age or alcohol consumption history
had no prognostic significance for OS (Table III).
 | Table III.Cox proportional hazards regression
model was used in univariate analysis. |
Table III.
Cox proportional hazards regression
model was used in univariate analysis.
|
Characteristics | Hazard ratio (95%
CI) | P-value |
|---|
| T-stage | 1.040 | 0.883 |
| (T1 vs. T2 vs. T3
vs. T4) | (0.612–1.768) |
|
| TNM-stage | 2.458 | 0.011 |
| (III vs. I and
II) | (1.229–4.917) |
|
| Tumor size | 1.106 | 0.663 |
| (≥4 cm vs. <4
cm) | (0.702–1.744) |
|
| Apollon
expression | 0.855 | 0.005 |
| (positive vs.
negative) | (0.758–0.953) |
|
Discussion
Apollon is the largest member of the IAP family
(19), and it was identified by Chen
et al in 1999 (19). The
Apollon gene locates on 2p21-22, and encodes a markedly large
protein (530 kDa) that contains a single BIR domain and an
ubiquitin-conjugating enzyme domain. Apollon is abundant in brain,
placenta, testis, lymphatic cells and secretory organs (19). Different studies have reported that
Apollon is a dual regulator of cell proliferation and cell death,
with multiple functions due to its different functional domains and
diverse binding patterns (9). Apollon
has been reported to serve a significant role in apoptosis
resistance in a variety of cancers (10,19–23),
including breast cancers, colon cancers, gliomas, fibrosarcomas,
osteosarcomas, lung cancers, cervical cancers and prostate cancers.
However, a role for Apollon in renal cancer has rarely been
reported. In the present study, Apollon was observed to also serve
a role in apoptosis resistance in RC. These results are in
agreement with those of Zhang et al (24). This process may facilitate further
studies on RC.
Regarding the expression of IAPs in RC, previous
studies revealed a significant overexpression of IAPs in cancer
tissues and normal squamous epithelia, including cellular (c)IAP-1,
cIAP-2, XIAP and survivin. However, the expression of these
proteins was an early event in renal cancer, and did not correlate
with the histological type of cancer or stage of the tumors
(25–27). The potential roles of IAP family
proteins in the homeostasis of normal tissues as well as in the
pathogenesis of RC are still unknown, since studies on Apollon and
RC are limited. In the present study, the differences in Apollon
expression were compared in a variety of malignant renal tissues
and adjacent non-cancerous tissues and benign renal tissues.
Immunohistochemical staining revealed a markedly high frequency of
Apollon expression in the majority of cancer cells. By contrast, in
normal mucosa, the presence of positive cells was partial, and in
certain cases, the signals were negative (Fig. 1). Thus, differences in the positive
cell ratio would result in differences in intensity of mRNA
expression for Apollon, indicating that Apollon could have a
significant role in renal cancer.
According to our immunohistochemistry data, Apollon
protein was detected in the majority of cancer samples (42 of 50,
84%). The protein was located in the cytoplasm, which confirmed a
previous study that reported that Apollon localized to the Golgi
compartment and the vesicular system (28). The expression of Apollon was observed
in numerous cases of normal squamous epithelial tissue, mainly in
the basal layer (28). The expression
of Apollon is ubiquitous in fetal tissues, but becomes restricted
during development, and appears to be negligible in the majority of
terminally differentiated adult tissues, which confirms its role in
caryomitosis (29). From the clinical
data, the present study noticed that the expression of Apollon was
not associated with age, gender, tumor size or tumor
differentiation, but exhibited a strong association with TNM-stage,
T-staging and nodal involvement. These results indicated that high
expression of Apollon leads to increased malignant biological
characteristics, regardless of the degree of tumor differentiation,
which is inconsistent with previous studies (29–33)
reporting that Apollon expression indicates poor differentiation.
By contrast, similar results to the ones reported in the present
study were reported by Low et al (23), who studied the association between
prostate cancer and Apollon. The results of that study indicated
that increased expression of Apollon was a late event in prostate
cancer and did not correlate with Gleason grade (23). Our data suggested that Apollon is
functionally an indicator of metastasis or prognosis in renal
cancer.
The increase in Apollon expression in renal cancer
suggests an important role for this protein in the development and
progression of the disease. In view of the pro-survival function of
Apollon in RC cells and other systems (12–14,20), a
cytoprotective advantage to RC cells is expected to be provided by
elevated expression of Apollon, thus promoting RC development and
progression. Numerous previous studies have demonstrated that the
expression level of Apollon was significantly associated with
patients' reaction to therapy, recurrence, OS, disease-free
survival (DFS) and prognosis (29–33). A
previous multivariate analysis revealed that Apollon was an
independent prognostic factor for OS and DFS in human epithelial
ovarian cancer (29), which is in
agreement with our results. In addition, various in vitro
studies have demonstrated that Apollon overexpression indicates a
stronger resistance to chemotherapy, since silencing its expression
can sensitize cancer cells to chemotherapy, which can be
interpreted by the cytoprotective advantage of Apollon and the
chemotherapeutic mechanism (20,21,30–32).
However, the role of Apollon in renal cancer remains uncertain. The
present study demonstrated that Apollon may be a therapeutic target
and a potential predictor of prognosis in RC. Future studies are
required to expand the therapeutic arsenal in the fight against
renal cancer and to improve the quality of life of RC patients.
In conclusion, the present study indicates for the
first time that Apollon expression is associated with the
biological characteristics of renal cancer, and it is potentially a
valuable predictor and novel target for renal cancers. Although the
mechanisms of Apollon function in tumorigenesis and metastasis are
still unclear, the present study provides evidence for further
studies in vitro and in vivo to clarify the
regulatory mechanisms of IAP expression in renal cancer in
association with the apoptotic signaling pathways.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Naito M: Molecular mechanism of apoptosis
inhibition by IAPs and its implication to cancer therapy.
Seikagaku. 78:525–528. 2006.(In Japanese). PubMed/NCBI
|
|
4
|
Mariette C, Piessen G and Triboulet JP:
Therapeutic strategies in oesophageal carcinoma: Role of surgery
and other modalities. Lancet Oncol. 8:545–553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lerut T, Coosemans W, Decker G, De Leyn P,
Nafteux P and Van Raemdonck D: Cancer of the esophagus and
gastro-esophageal junction: Potentially curative therapies. Surg
Oncol. 10:113–122. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giancotti FG and Tarone G: Positional
control of cell fate through joint integrin/receptor protein kinase
signaling. Annu Rev Cell Dev Biol. 19:173–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Evan G: Cancer-a matter of life and cell
death. Int J Cancer. 71:709–711. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hauser HP, Bardroff M, Pyrowolakis G and
Jentsch S: A giant ubiquitin-conjugating enzyme related to IAP
apoptosis inhibitors. J Cell Biol. 141:1415–1422. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiu XB and Goldberg AL: The
membrane-associated inhibitor of apoptosis protein, BRUCE/Apollon,
antagonizes both the precursor and mature forms of Smac and
caspase-9. J Biol Chem. 280:174–182. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qiu XB, Markant SL, Yuan J and Goldberg
AL: Nrdp1-mediated degradation of the gigantic IAP, BRUCE, is a
novel pathway for triggering apoptosis. EMBO J. 23:800–810. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pohl C and Jentsch S: Final stages of
cytokinesis and midbody ring formation are controlled by BRUCE.
Cell. 132:832–845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren J, Shi M, Liu R, Yang QH, Johnson T,
Skarnes WC and Du C: The Birc6 (Bruce) gene regulates p53 and the
mitochondrial pathway of apoptosis and is essential for mouse
embryonic development. Proc Natl Acad Sci USA. 102:565–570. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chu L, Gu J, Sun L, Qian Q, Qian C and Liu
X: Oncolytic adenovirus-mediated shRNA against Apollon inhibits
tumor cell growth and enhances antitumor effect of 5-fluorouracil.
Gene Ther. 15:484–494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lopergolo A, Pennati M, Gandellini P,
Orlotti NI, Poma P, Daidone MG, Folini M and Zaffaroni N: Apollon
gene silencing induces apoptosis in breast cancer cells through p53
stabilisation and caspase-3 activation. Br J Cancer. 100:739–746.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Van Houdt WJ, Emmink BL, Pham TV, Piersma
SR, Verheem A, Vries RG, Fratantoni SA, Pronk A, Clevers H, Rinkes
IH Borel, et al: Comparative proteomics of colon cancer stem cells
and differentiated tumor cells identifies BIRC6 as a potential
therapeutic target. Mol Cell Proteomics. 10:M111.011353. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Y, Fu D, Xi J, Ji Z, Liu T, Ma Y,
Zhao Y, Dong L, Wang Q and Shen X: Expression and clinical
significance of UCH37 in human renal carcinoma. Dig Dis Sci.
57:2310–7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Z, Naito M, Hori S, Mashima T, Yamori
T and Tsuruo T: A human IAP-family gene, apollon, expressed in
human brain cancer cells. Biochem Biophys Res Commun. 264:847–854.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hao Y, Sekine K, Kawabata A, Nakamura H,
Ishioka T, Ohata H, Katayama R, Hashimoto C, Zhang X, Noda T, et
al: Apollon ubiquitinates SMAC and caspase-9, and has an essential
cytoprotection function. Nat Cell Biol. 6:849–860. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lamers F, Schild L, Koster J, Speleman F,
Øra I, Westerhout EM, van Sluis P, Versteeg R, Caron HN and
Molenaar JJ: Identification of BIRC6 as a novel intervention target
for neuroblastoma therapy. BMC Cancer. 12:2852012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bianchini M, Levy E, Zucchini C, Pinski V,
Macagno C, De Sanctis P, Valvassori L, Carinci P and Mordoh J:
Comparative study of gene expression by cDNA microarray in human
colorectal cancer tissues and normal mucosa. Int J Oncol. 29:83–94.
2006.PubMed/NCBI
|
|
23
|
Low CG, Luk IS, Lin D, Fazli L, Yang K, Xu
Y, Gleave M, Gout PW and Wang Y: BIRC6 protein, an inhibitor of
apoptosis: Role in survival of human prostate cancer cells. PLoS
One. 8:e558372013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang S, Tang W, Weng S, Liu X, Rao B, Gu
J, Chen S, Wang Q, Shen X, Xue R and Dong L: Apollon modulates
chemosensitivity in human renal carcinoma. Oncotarget. 5:7183–7197.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nemoto T, Kitagawa M, Hasegawa M, Ikeda S,
Akashi T, Takizawa T, Hirokawa K and Koike M: Expression of IAP
family proteins in esophageal cancer. Exp Mol Pathol. 76:253–259.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou S, Ye W, Shao Q, Qi Y, Zhang M and
Liang J: Prognostic significance of XIAP and NF-κB expression in
esophageal carcinoma with postoperative radiotherapy. World J Surg
Oncol. 11:2882013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vaishlia NA, Zinov'eva MV, Sass AV,
Kopantsev EP, Vinogradova TV and Sverdlov ED: Increase of BIRC5
gene expression in non-small cell lung cancer and renal carcinoma
does not correlate with expression of genes SMAC/DIABLO and PML
encoding its inhibitors. Mol Biol (Mosk). 42:652–661. 2008.(In
Russian). PubMed/NCBI
|
|
28
|
Bartke T, Pohl C, Pyrowolakis G and
Jentsch S: Dual role of BRUCE as an antiapoptotic IAP and a
chimeric E2/E3 ubiquitin ligase. Mol Cell. 14:801–811. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang L, Chen YJ, Hou J, Wang YY, Tang WQ,
Shen XZ and Tu RQ: Expression and clinical significance of BIRC6 in
human epithelial ovarian cancer. Tumour Biol. 35:4891–4896. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sung KW, Choi J, Hwang YK, Lee SJ, Kim HJ,
Lee SH, Yoo KH, Jung HL and Koo HH: Overexpression of Apollon, an
antiapoptotic protein, is associated with poor prognosis in
childhood de novo acute myeloid leukemia. Clin Cancer Res.
13:5109–5114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ismail EA, Mahmoud HM, Tawfik LM, Habashy
DM, Adly AA, El-Sherif NH and Abdelwahab MA: BIRC6/Apollon gene
expression in childhood acute leukemia: Impact on therapeutic
response and prognosis. Eur J Haematol. 88:118–127. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang W, Xue R, Weng S, Wu J, Fang Y, Wang
Y, Ji L, Hu T, Liu T, Huang X, et al: BIRC6 promotes hepatocellular
carcinogenesis: Interaction of BIRC6 with p53 facilitating p53
degradation. Int J Cancer. 136:E475–E487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dong X, Lin D, Low C, Vucic EA, English
JC, Yee J, Murray N, Lam WL, Ling V, Lam S, et al: Elevated
expression of BIRC6 protein in non-small-cell lung cancers is
associated with cancer recurrence and chemoresistance. J Thorac
Oncol. 8:161–170. 2013. View Article : Google Scholar : PubMed/NCBI
|