Introduction
Bladder cancer is a major public health concern,
accounting for 90% of all primary bladder tumors, and is the fourth
most common cancer in men (1). The
estimated number of new cases of bladder cancer and associated
mortalities for 2016 were 76,960 and 16,390, respectively, in the
USA (2). In 2004, the World Health
Organization recommended a new histological grading system based on
malignancy (3), as follows: Papillary
urothelial neoplasms of low malignant potential (PUNLMP), low-grade
and high-grade urothelial carcinomas. The independent prognostic
factors for survival and local tumor control have been shown to
involve the entire urethra, tumor size, histological grade, stage,
nodal status and disease site (3).
Therefore, a high histological grade is associated with a worse
prognosis.
Immunoglobulin (Ig) is the most established family
of immune molecules. Over the past 500 million years, Ig has become
increasingly complex, resulting in the formation of complicated and
diverse structures (4,5). Furthermore, infinite variations are
found within the variable region (V) of Ig. It was originally
thought that Ig acts strictly as an antibody and is only secreted
by B lineage cells. However, increasingly, evidence has suggested
that Igs, including IgM, IgG and IgA, are expressed in many cell
types other than those of the B-cell lineage, including epithelial
cells, germ cells and neurons (6–10).
Importantly, the non-B-cell-derived Igs, particularly IgG, have
recurrently been shown to be overexpressed in the cells of many
cancers, including breast, colon, lung, liver and stomach cancers
(6,11–14).
Furthermore, unlike classical IgG, which has an antibody function,
cancer cell-derived IgG is predominantly involved in the survival
and progression of cancer cells (6,15).
In order to address the multitude of distinct
antigens in the environment, the mechanism of Ig gene rearrangement
yields a vast repertoire of antigen receptor-binding specificities.
This process involves two stages of rearrangement characterized by
the assembly of the V, diversity (D) and joining (J) gene segments
of the Ig heavy chain (H), and the V and J gene segments of the
light chain (γ or κ), in the developing B-cell (16). Therefore, B-cell-Igs typically display
a huge diversity within a given individual. In our previous
studies, VHDJH or VκJκ rearrangements in
non-B-cell-Igs isolated from neurons, epithelial cells from the
breast and colon, and spermatogenic cells from humans or mice were
analyzed, and were compared with those in B-cell-Igs (17–19). The
results suggested that, unlike B-cell-Igs, non-B-cell-Igs display a
restricted and unique VHDJH or VκJκ
recombination pattern, and that the antigen epitopes of
non-B-cell-derived IgG may be different from those of classical
IgG.
In our previous study, commercial antibodies against
IgG were able to recognize circulating IgG, but they were not
specific for the non-B-cell-derived IgG (6). RP215 was originally generated by Lee
et al (20) using the cell
lysate of the OC-3-VGH ovarian cancer cell line as an immunogen. In
our previous studies, it was determined that the RP215 antibody
specifically recognizes a glycosylated epitope of a
non-B-cell-expressed IgGH (RP215-recognized IgG) (21–23).
Liang et al (24) found that IgG was expressed in bladder
cancer cells using a commercial anti-human IgG, but its
significance remains unclear. In the present study, IgG and its
transcripts were shown to be expressed in bladder cancer cells
using RP215 and reverse transcription-polymerase chain reaction
(RT-PCR), respectively. Notably, functional IgG transcripts with
unique VDJ rearrangements were found in these cancer cells. The
knockdown of IgG in bladder cancer cell lines resulted in the
significant inhibition of cell proliferation, migration and
invasion. Furthermore, it was demonstrated that high IgG expression
was significantly correlated with histological grade and
recurrence.
Materials and methods
Ethics statement
This study was approved by the ethics committee of
Peking University People's Hospital (Beijing, China). All patients
provided written informed consent.
Patients and clinical samples
The clinical samples, including 77 bladder cancer
specimens, 3 cystitis glandularis tissues and 4 normal tissues,
were obtained from patients who underwent surgical resection of
primary tumors at Peking University People's Hospital between April
2011 and August 2012. Patients who received preoperative
radiotherapy or adjuvant chemotherapy were excluded from this
study. The biopsy tissues for immunohistochemical staining were
fixed immediately in 10% buffered formalin and, 24 h later, were
dehydrated in increasing concentrations of alcohol, coagulated and
embedded in paraffin.
Cell culture
The bladder cancer cell line 5637 was obtained from
American Type Culture Collection (Manassas, VA, USA). The bladder
cancer cell lines BIU87 and EJ were obtained from the urology
department of Peking University First Hospital (Beijing, China).
The cells were cultured in RPMI-1640 medium (Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine
serum (FBS; Hyclone; GE Healthcare Life Sciences) and incubated at
37°C in a humidified atmosphere containing 5% CO2.
Immunofluorescence
RP215 was provided by Professor Gregory Lee of the
University of British Columbia in Vancouver, Canada. The 5637,
BIU87 and EJ cells were seeded into 12-well plates upon reaching
60–70% confluence and were maintained in an incubator at 37°C
containing 5% CO2. The cells were fixed in acetone for 5
min at room temperature, after which they were blocked with 10%
normal goat serum (Hyclone; GE Healthcare Life Sciences) for 30 min
and incubated with 12.5 µg/ml purified RP215 as a primary antibody
at 4°C for 45 min. The cells were then incubated with the
fluorescein isothiocyanate-conjugated goat anti-mouse polyclonal
secondary antibody (1:400; cat. no. ab97022; Abcam, Cambridge, UK)
at 4°C for 30 min. Images were captured using an inverted
fluorescence microscope subsequent to mounting with 50%
glycerin.
Immunohistochemical analysis
Tissue sections (4-µm) from the clinical samples
were deparaffinized, rehydrated and then heated in 10 mmol/l
citrate buffer (pH 6.0) for antigen retrieval. Subsequently, the
sections were washed in PBS, blocked with 10% normal goat serum for
30 min and incubated with 7.5 µg/ml purified RP215 in a humidified
chamber overnight at 4°C. Inmunodetection was performed using the
Envision™ ABC kit (GeneTech Co., Ltd., Shanghai, China). After
staining with hematoxylin, the tissues were dehydrated and mounted.
A pathologist independently evaluated the extent and intensity of
RP215 staining and was blinded with respect to the clinical
data.
The relative number of positive cells and the
intensity of staining were assessed in five random 200x microscopic
fields. The percentage of stained cells per field was scored as
follows: 0, 0% (negative); 1, 1–25%; 2, 26–50%; and 3, 51–100%. The
staining intensity was scored on a 4-tiered scale, as follows: 0,
absence of signal; 1, low-intensity signal (light brown); 2,
moderate-intensity signal (brown); and 3, high-intensity signal
(dark brown). The percentage score and intensity score were
multiplied to obtain the score for each field, and the final score
for each case was the average score of the five fields. The score
for RP215 staining was described as follows: negative (−) when the
score was 0–1; ‘low expression’ (+) when the score was 2–3; and
‘high expression’ when the score was 4–6 and 7–9 (++ and +++,
respectively). All evaluations were conducted using a Leica
DM4000B/M microscope (Leica Microsystems, Inc., Buffalo Grove, IL,
USA).
Western blot analysis
Protein was extracted using lysis buffer containing
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc., Rockford, IL, USA), 1% Triton X-100, 0.1% SDS, 1% sodium
deoxycholate, 0.15 M NaCl and 10 mM Tris (pH 7.2). The protein
concentration was assessed using a Pierce Bicinchoninic Acid Assay
kit (Thermo Fisher Scientific, Inc.). Protein samples (50 µg) were
separated by 12.5% SDS-PAGE and transferred onto a nitrocellulose
membrane (EMD Millipore, Bedford, MA, USA). The membrane was
blocked with 5% non-fat milk for 1 h and then incubated with the
RP215 and anti-GAPDH (cat. no. TA-08; OriGene Technologies, Inc.,
Rockville, MD, USA) primary antibodies (7.5 µg/ml) overnight at
4°C. The blots were then incubated with horseradish
peroxidase-conjugated anti-mouse IgG secondary antibody (1:3,000;
cat. no. ab97040; Abcam) for 1 h at room temperature.
Immunoreactive bands were visualized using the SuperSignal West
Pico Chemiluminescent Substrate (Thermo Fisher Scientific,
Inc.).
RT-PCR
Total RNA was extracted from the bladder cancer cell
lines EJ, 5637 and BIU87 using TRIzol reagent (Thermo Fisher
Scientific, Inc.), treated with DNase (Magen, Guangzhou, China),
and then reverse transcribed using the RevertAid First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The primers for the constant region of the
IgGH were as follows: Forward, 5′-CAGGACTGGCTGAATGGC-3′ and
reverse, 5′-GGCGTGGTCTTGTAGTTGTT-3′. The primers for GAPDH were:
Forward, 5′-CAAGGTCATCCATGACAACTTTG-3′; and reverse,
5′-GTCCACCACCCTGTTGCTGTAG-3′.
In order to amplify the human IgVH gene by nested
PCR, the first round of PCR was performed using upstream primers to
VH1 (5′-GAGGTGCAGCTCGAGGAGTCTGGG-3′), VH2
(5′-CAGGTGCAGCTCGAGCAGTCTGGG-3′), VH3
(5′-CAGGTACAGCTCGAGCAGTCAGG-3′) and VH4
(5′-CAGGTGCAGCTGCTCGAGTCGGG-3′), coupled with a CH1 region primer
(5′-ACACCGTCACCGGTTCGG-3′). PCR was performed using 2X Taq PCR
Master Mix kit (Biomed, Beijing, China). The reaction conditions of
the first round of PCR were as follows: Pre-denaturation at 94°C
for 5 min; followed by denaturation at 94°C for 30 sec, annealing
at 59–47°C for 30 sec for the first 18 cycles (with decreases of
2°C increments at each step); then 20 cycles of denaturation at
94°C for 30 sec, annealing at 47°C for 30 sec and polymerization at
72°C for 30 sec with a final elongation step for 7 min at 72°C. A
total of 38 cycles was performed. For the second round of PCR, an
upstream primer targeting the framework 2 region
[5′-TGG(A/G)TCCG(A/C/G) CAG(G/C)C(T/C)CC(A/C/G/T)GG-3′], coupled
with a JH primer (5′-AACTGCAGAGGAGACGGTGACC-3′), was used. The
reaction conditions for the second round of PCR were as follows:
Pre-incubation at 95°C for 15 min, followed by 38 cycles of
denaturation at 94°C for 1.5 min, annealing at 57°C for 1.5 min and
polymerization at 72°C for 3 min, with a final elongation step for
5 min at 72°C. PCR products were subjected to electrophoresis on
1.5% agarose gels containing 0.5 µg/ml ethidium bromide.
Sequencing and analysis of rearranged
genes
PCR products were cloned into a pGEM-T Easy Vector
(Promega Corporation, Madison, WI, USA) and sequenced on an ABI
3100 Genetic Analyzer (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The VHDJH sequences were
compared with those found in the Basic Local Alignment Search Tool
(https://blast.ncbi.nlm.nih.gov/Blast.cgi) and the
International Immunogenetics information system (http://www.imgt.org/) databases in order to identify
the best matched germline gene segments and V(D)J junctions. V gene
sequences belonging to a set of VHDJH
recombinants were defined on the basis of identical VH, DH and JH
gene usage and V-D and D-J junction sequences. The repertoire of
the cancer-derived Ig V genes was compared with that of published
B-cell-derived Ig V genes (17).
Cell transfection with small
interfering (si)RNA
siRNAs against the constant region of the Ig γ-chain
(siRNA1, 5′-GGUGGACAAGACAGUUGAG-3′; and siRNA2,
5′-AGUGCAAGGUCUCCAACAA-3′) and the non-silencing control RNA (NC,
5′-UUCUCCGAACGUGUCACGU-3′) were produced by Shanghai GenePharma Co.
Ltd. (Shanghai, China). The siRNAs and NC were transfected into the
5637 and BIU87 cell lines using Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
knockdown efficiency of IgG was verified by western blot
analysis.
Cell proliferation assay
The 5637 and BIU87 cells were transfected with siRNA
(50 µg/ml) in 96-well plates and incubated at 37°C. Cell
proliferation was analyzed using the Cell Counting kit-8 (CCK8)
Cell Proliferation Assay (Invitrogen; Thermo Fisher Scientific,
Inc.). Briefly, CCK8 reagent (5 µl) was added to each well for 2 h,
after which the number of viable cells was calculated by measuring
the absorbance at 450 nm. Each condition was assessed in
triplicate.
Cell migration assay
Migration assays were performed in 24-well Transwell
plates (Corning Incorporated, Corning, NY, USA) containing a
polycarbonate filter (8-µm pore size). In total, 105
5637 and BIU87 cells from each group were added to the Transwell
chamber. Conditioned medium supplemented with 10% FBS was added to
the bottom of the chamber. The cells were incubated in an incubator
containing 5% CO2 at 37°C for 24 h. Following
incubation, the cells in the upper chamber that were attached and
had not migrated were removed, and the cells that had migrated to
the bottom of the filter were fixed in methanol and stained with
hematoxylin. The number of cells was counted in at least 6
randomized fields under a light microscope. The results were
obtained from at least three individual experiments.
Cell invasion assay
Invasion assays were performed in 24-well invasion
chambers (Corning Incorporated) containing a polycarbonate filter
(8-µm pore size). In total, 105 5637 and BIU87 cells
from each group were added to the invasion chamber. Conditioned
medium supplemented with 10% FBS was added to the bottom portion of
the chamber. The cells were incubated in an incubator with 5%
CO2 at 37°C for 24 h. Following incubation, the cells in
the upper chamber that were attached and had not migrated were
removed, and the cells that had migrated to the bottom of the
filter were fixed in methanol and stained with hematoxylin. The
number of cells was counted in at least 6 randomized fields under a
light microscope. The results were obtained from at least three
individual experiments.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical analyses were performed using the
χ2 test and Student's t-test. P<0.05 was considered
statistically significant. All statistical evaluations were
performed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA).
Results
IgG is found in transitional
epithelial cancer cells
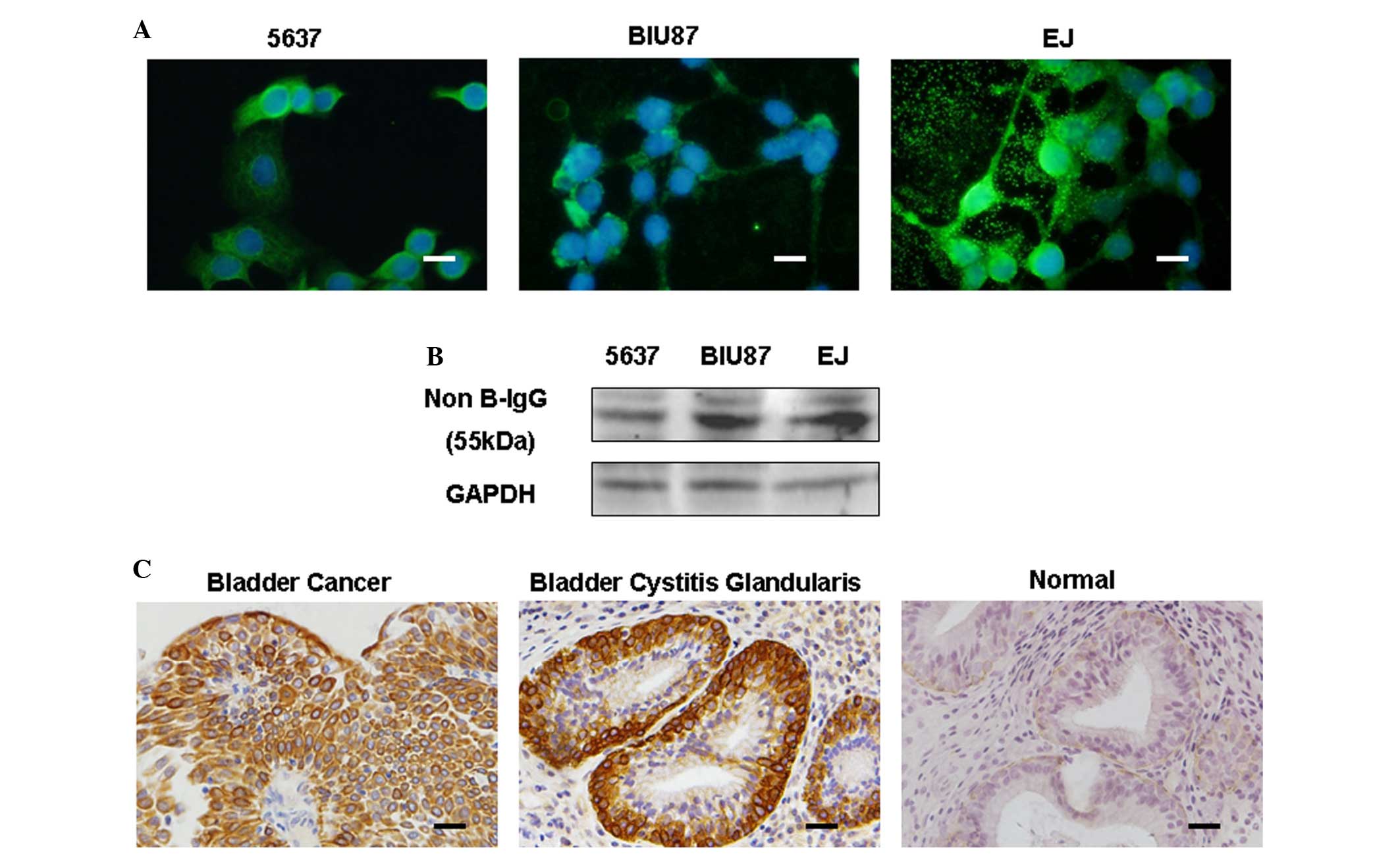
In the present study, IgG expression was first
detected in bladder cancer cell lines (EJ, 5637 and BIU87) by
immunofluorescence. IgG staining was observed in the cell
cytoplasm, filopodia-like structures and the extracellular area
(Fig. 1A). Furthermore, IgG
expression was also detected in these bladder cancer cell lines by
western blotting (Fig. 1B).
Subsequently, immunohistochemistry demonstrated that IgG
immunoreactivity was recurrently localized to the cytoplasm of
tumor cells in bladder cancer tissues (58/77, 75.3%) and to the
cytoplasm of cells in cystitis glandularis tissues (3/3, 100%), but
not to the transitional epithelial cells of normal tissues (0/4)
(Fig. 1C).
IgG transcripts with unique patterns
of VHDJH rearrangements are found in bladder
cancer cells
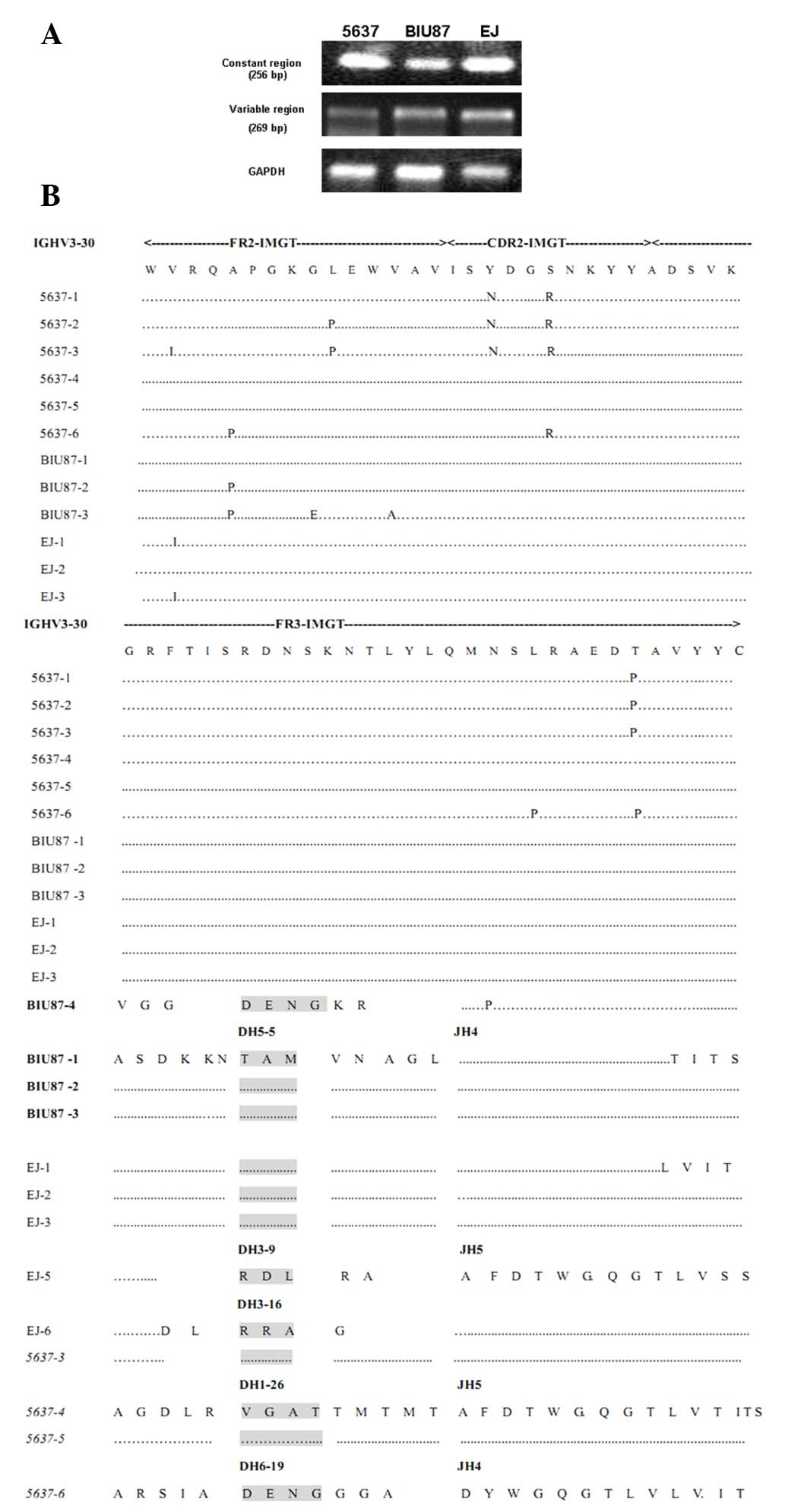
In order to determine whether IgG was produced by
the cancer cells themselves and was not the result of uptake from
the extracellular environment, the transcription of the IgGH in EJ,
5637 and BIU87 cells was examined by RT-PCR using primers targeting
both the constant and V regions. The results showed that the
transcript of the IgGH was expressed in these three cancer cell
lines (Fig. 2A). Subsequently, the
sequences of these VHDJH rearrangements were
analyzed by a comparison with the best matched functional germline
IgVH, IgDH and IgJH genes. The results demonstrated that, similar
to the B-cell-Igs, all of the bladder cancer-IgG transcripts
displayed classical and functional VHDJH
rearrangement patterns. However, unlike B-cell-derived IgVH, which
has great diversity, several sets of VHDJH
rearrangements were frequently shared among these cell lines, and
even among different cell lines (VH3-30/D6-6/JH4 was shown in 3/6
5637 cells, VH3-30/D1-26/JH5 was shown in 2/6 5637 cells and
VH3-30/D5-5/JH4 was shared between 3/4 BIU87 cells and 3/5 EJ
cells). The bladder cancer-VHDJH
rearrangements showed restricted VH, DH and JH usage, and a unique
VHDJH pattern, including the VH3 region,
which was dominantly used (14/16 VHDJH
rearrangements analyzed in this study). In particular, the VH3-30
region was dominantly expressed in 5637 (8/9), BIU87 (3/4) and EJ
(3/5) cells. Furthermore, among the germline IgHJ1-6 genes, only
IgHJ4 (11/16) and IgHJ5 (5/16) were frequently used. Conversely,
IgDH showed diversity within each cell line, as follows: DH6-6,
DH6-19 and DH1-26 were shown in 5637 cells, DH5-5 and DH6-19 were
shown in BIU87 cells, and DH5-5, DH3-16 and DH3-9 were shown in EJ
cells. However, DH6-19 was shown in 5637 cells and in BIU87 cells,
whereas DH5-5 was shown in BIU87 and EJ cells (Table I, Fig.
2B).
 | Table I.Assignment of likely matching
germline variable region genes to the VHDJH
recombinants from different bladder cancer cell lines and analysis
of the V gene somatic mutation rate. |
Table I.
Assignment of likely matching
germline variable region genes to the VHDJH
recombinants from different bladder cancer cell lines and analysis
of the V gene somatic mutation rate.
| Cell lines | No. of
patterns |
VHDJH | No. of clones | Mutation rate
(%) |
|---|
| 5637 | 4 |
IgHV3-30/IgHD6-6/IgHJ4 | 3 | 2.7 |
|
|
|
IgHV3-30/IgHD1-26/IgHJ5 | 2 | 1.6 |
|
|
|
IgHV3-30/IgHD6-19/IgHJ4 | 1 | 3.7 |
|
|
|
IgHV3-15/IgHD4-17/IgHJ4 | 1 | 3.1 |
| BIU87 | 2 |
IgHV3-30/IgHD5-5/IgHJ4 | 3 | 1.0 |
|
|
|
IgHV4-61/IgHD6-19/IgHJ5 | 1 | 3.2 |
| EJ | 3 |
IgHV3-30/IgHD5-5/IgHJ4 | 3 | 2.1 |
|
|
|
IgHV3-15/IgHD3-9/IgHJ5 | 1 | 6.6 |
|
|
|
IgHV1-18/IgHD3-9/IgHJ5 | 1 | 5.8 |
To enhance IgG affinity, the B-cell-derived IgVH of
IgG is usually hypermutated (25).
Therefore, the present study analyzed the mutation pattern in
bladder cancer-derived IgVH and compared the sequence homology
among VHDJH rearrangements from three cancer
cell lines. It was demonstrated that the bladder cancer-derived
IgVH showed only a low frequency of mutation (Table I). Furthermore, the same mutations
were frequently present among different VHDJH
rearrangements, which resulted in a high homology among
VHDJH rearrangements in IgVH (Fig. 2). These results suggested that the
conservative domain of IgVH, as well as IgHJ4 and IgHJ5, may exert
a similar framework effect in different VHDJH
rearrangements in bladder cancer cells, but that IgDH determines
the unique biological activity of each VHDJH
rearrangement.
Knockdown of IgG significantly reduces
the proliferation of 5637 and BIU87 cells
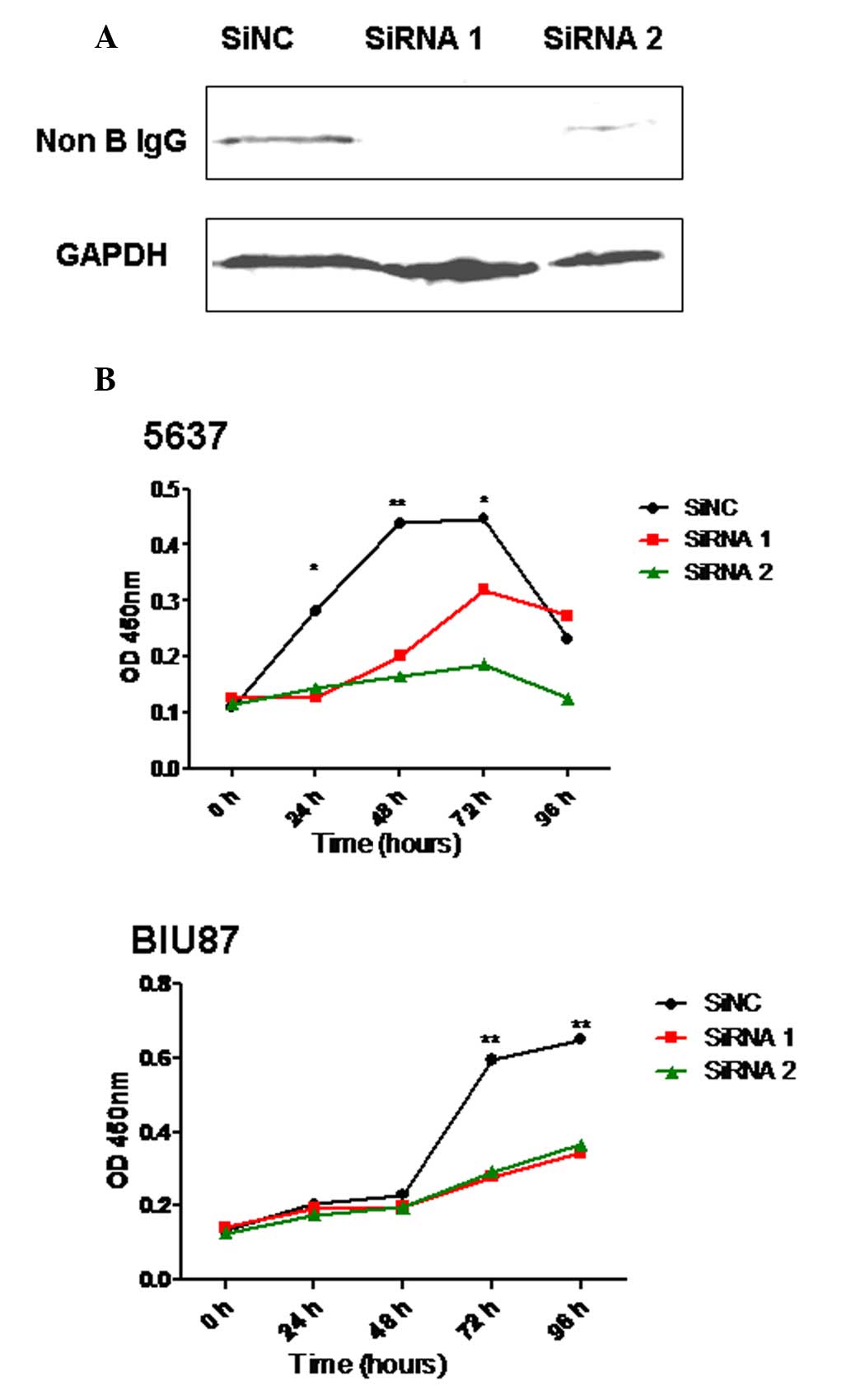
In order to investigate whether IgG has a role in
the proliferation of bladder cells, two siRNAs to knockdown IgG
expression in bladder cancer cell lines were designed and the
proliferation of these cells was analyzed following transfection.
The results showed that the ability of these cells to proliferate
was significantly downregulated following the knockdown of IgG
heavy chain expression in 5637 and BIU87 cells (P<0.05; Fig. 3).
Knockdown of IgG significantly reduces
the migration and invasion of 5637 and BIU87 cells
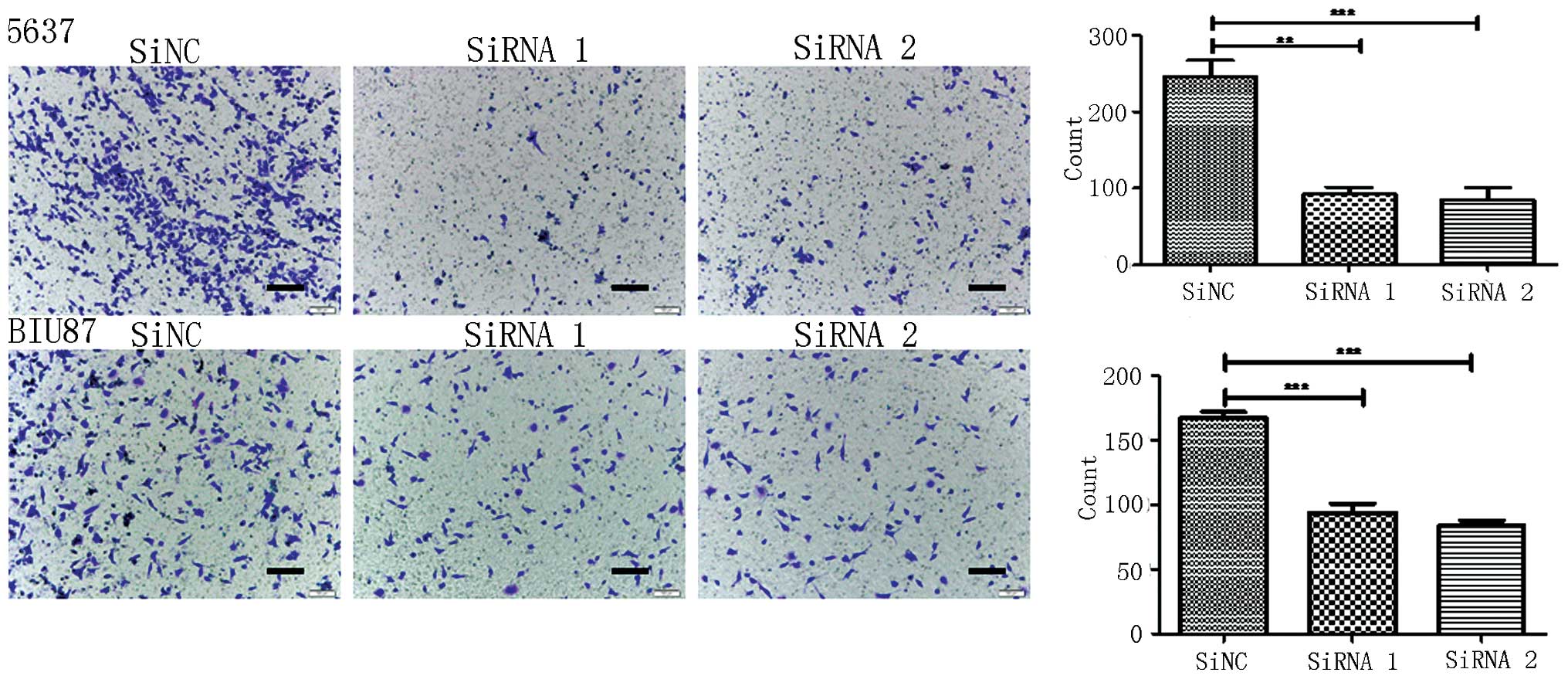
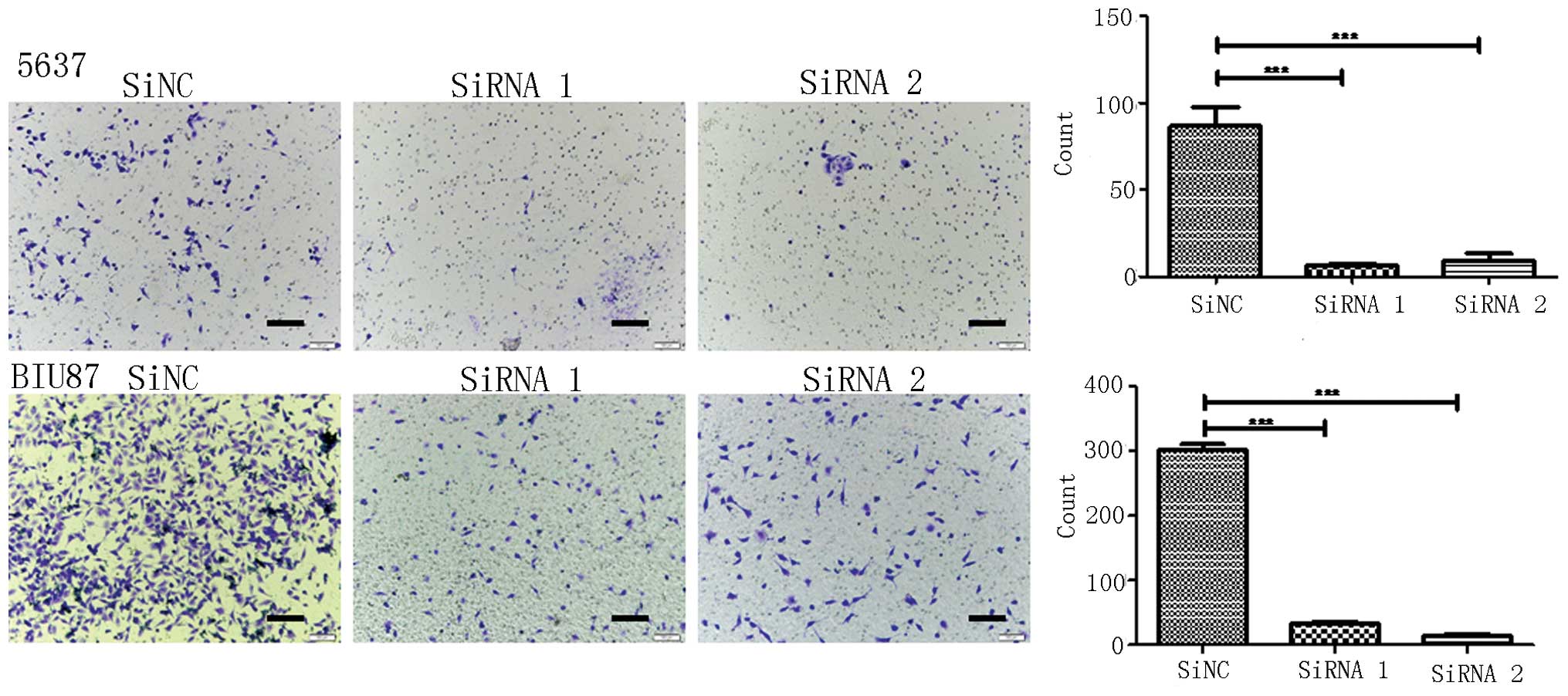
The present study investigated whether IgG is
involved in the migration and invasiveness of bladder cancer cells.
Migration and invasion assays were performed in 24-well invasion
chambers using the same number of 5637 and BIU87 cells in each
group. It was demonstrated that the migration and invasion of 5637
and BIU87 cells was significantly decreased following knockdown of
IgG expression (P<0.05; Figs. 4
and 5).
IgG expression significantly
correlates with a high histological grade and recurrence
Next, the present study aimed to determine whether
the expression of IgG was correlated with a high histological
grade, the size of the tumor and/or the recurrence of bladder
cancer. A high positivity of IgG staining was found in patients
with high-grade bladder cancer (32/36, 88.9%) compared with
patients with low-grade bladder cancer (63.4%, 26/41), and this
difference was statistically significant (P=0.045; Table II). Following a comparison of the
frequency of positivity between larger tumors (>3 cm) and
smaller tumors (≤3 cm), it was found that the rate of IgG
positivity in patients with large tumors was 94.1% (16/17), which
was significantly higher than patients with smaller tumors (42/60,
70.0%) (P=0.003; Table II).
Furthermore, IgG expression was significantly associated with the
recurrence of bladder cancer (P=0.012). The rate of positivity (++
and +++) in patients who experienced bladder cancer recurrence was
62.9% (17/27), whereas the rate of positivity (++ and +++) in
patients with non-recurrence was 22% (11/50) (P=0.012; Table II).
 | Table II.Clinicopathological variables and
evaluation of non-B-IgG immunostaining in bladder cancer
tissues. |
Table II.
Clinicopathological variables and
evaluation of non-B-IgG immunostaining in bladder cancer
tissues.
|
|
| Scores for
non-B-IgG signals |
|
|---|
|
|
|
|
|
|---|
| Variables | No. of
patients | (−) | (+) | (++) | (+++) | P-value |
|---|
| Age (years) |
|
|
|
|
| 0.4933 |
|
>60 | 53 | 12 | 21 | 7 | 13 |
|
|
≤60 | 24 | 7 | 9 | 4 | 4 |
|
| Gender |
|
|
|
|
| 0.9635 |
|
Male | 66 | 15 | 28 | 9 | 14 |
|
|
Female | 11 | 4 | 2 | 2 | 3 |
|
| Histological
grade |
|
|
|
|
| 0.0451 |
|
High-grade | 36 | 4 | 17 | 5 | 10 |
|
|
Low-grade | 41 | 15 | 13 | 6 | 7 |
|
| Tumor size, cm |
|
|
|
|
| 0.0028 |
|
>3 | 17 | 1 | 4 | 3 | 9 |
|
| ≤3 | 60 | 18 | 26 | 8 | 8 |
|
| Tumor number |
|
|
|
|
| 0.9815 |
|
<8 | 62 | 16 | 23 | 9 | 14 |
|
| ≥8 | 11 | 3 | 4 | 2 | 2 |
|
| Recurrence |
|
|
|
|
| 0.0120 |
|
Yes | 28 | 4 | 7 | 5 | 12 |
|
| No | 37 | 3 | 23 | 6 | 5 |
|
Discussion
The present study demonstrated that IgG, with a
unique VHDJH pattern, was expressed in
bladder cancer cells. Furthermore, it was determined that IgG was
involved in the proliferation, migration and invasiveness of
bladder cancer cells. Importantly, IgG expression was correlated
with histologically high-grade cancer, a large tumor size and
recurrence.
To date, IgG has been considered to be produced
solely by B-cells and to function strictly as an antibody (26). Since Qiu et al (6) first reported that IgG was expressed in
non-B-cell-derived cancer cells, evidence has accumulated in
support of Ig expression in non-B-cells (7,10,27). Igs have been shown to be overexpressed
in numerous cancer cells, including many types of cancer cells
derived from epithelial and mesenchymal tissues (12,13). In
addition, in our previous study, it was demonstrated that IgG
expression was strongly correlated with poor-differentiation, local
invasion, metastasis and a poor prognosis in patients with lung
adenocarcinoma, which suggested that IgG may serve as a novel
prognostic biomarker for lung adenocarcinoma (15). In the future, the authors of the
present study will investigate the role of IgG in the prognosis of
patients with bladder cancer. The present study further confirmed
that IgG is expressed in primary bladder cancer cells and in cancer
cell lines at both the protein and mRNA levels. Furthermore,
immunohistochemical analysis detected IgG expression in
transitional epithelial cells of cystitis glandularis tissues.
However, IgG staining was either weak or negative in normal
transitional epithelial cells. These results suggested that
overexpression of IgG may be induced by various pathological
factors, including factors that promote cancer or proinflammatory
factors. However, it cannot be determined whether IgG is expressed
in normal human transitional epithelial cells based on the few
cases used in the present study because of the small sample
size.
The VHDJH rearrangement is the
best evidence for IgG expression, which, for a long time, has been
considered to occur only in B-cells (28). In the present study, whether the
VHDJH rearrangement could be identified in
bladder cancer cell lines without B-cell contamination was
investigated. The results clearly showed that the germline IgVH,
IgDH and IgJH gene segments were selected and rearranged to form
VHDJH rearrangements with a classical
junction pattern in these bladder cancer cell lines. Furthermore,
the bladder cancer-VHDJH rearrangements had a
distinct usage and features that differed from those of
B-cell-derived IgG. It has been reported that each B-cell expresses
a unique VHDJH recombination, including a
random N region sequence (16).
Therefore, the frequency of identical junction sequences from two
independent B-cell clones present in an individual should be <1
in 4 million. However, the bladder
cancer-VHDJH rearrangements showed conserved
IgVH and IgJH, but not IgDH, among the different bladder cancer
cell lines. The usage of VH3 and IgJH4 was frequent in the cancer
cells, although the primers used for the PCR were designed to be
applicable to almost all VHDJH
rearrangements. In addition, the sequence differences among the
VHDJH rearrangements were primarily in the D
region and not in the V and J regions. These results suggested an
unknown mechanism that allows bladder cancer cells to express
several dominant VHDJH sequences.
It has previously been reported that the expression
of IgG in cancer cells is associated with growth factor-like
activity, which can promote the proliferation and survival of
cancer cells (6), and is involved in
the carcinogenesis of breast cancer (29). The present study demonstrated that
bladder cancer cell-derived IgG was able to promote cell
proliferation. Tumor metastasis is the most important cause of
cancer-associated mortality worldwide, and the migration and
invasion of cancer cells is a prerequisite for metastasis (30). In the present study, an important
finding was that the bladder cancer cell-derived IgG showed
significant effects in the promotion of cell migration and
invasion, which suggested that IgG may be involved in the
metastasis and prognosis of bladder cancer.
A high histological grade has been associated with a
poor prognosis (31). However, to
date, no independent prognostic factor has been shown to be useful
for the prediction of bladder cancer prognosis. The present study
further analyzed the relationship between IgG expression and the
histological grade of bladder cancer. It was found that IgG
expression was significantly correlated with a high histological
grade. Furthermore, recurrence and tumor size are indicators for
survival of patients with bladder cancer (32,33). The
present study also found that IgG expression was related to tumor
size and the recurrence of bladder cancer, which suggests that IgG
has the potential to be a novel independent predictor of bladder
cancer prognosis.
In conclusion, the present study demonstrated that
IgG was overexpressed in bladder cancer and was involved in the
proliferation, migration and invasion of bladder cancer cells.
Furthermore, IgG transcripts with unique patterns of
VHDJH rearrangements were found in bladder
cancer cells, although the underlying mechanism remains unclear.
The results of the present study suggested that IgG expression may
serve as a potential target in cancer therapies and might be used
as a prognostic marker.
Acknowledgements
The authors would like to thank Peking University
Center for Human Disease Genomics for providing the RP215 antibody,
and the Department of Pathology of Peking University People's
Hospital for their technological support in the immunohistochemical
analysis and staining evaluation. This study was supported by
grants from the National Natural Science Foundation of China (nos.
81272237, 91229102 and 81472393) and the Beijing Natural Science
Foundation (no. 7152149).
References
|
1
|
Bos MK, Marmolejo RO, Rasch CR and Pieters
BR: Bladder preservation with brachytherapy compared to cystectomy
for T1-T3 muscle-invasive bladder cancer: A systematic review. J
Contemp Brachytherapy. 6:191–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seitz M, Zaak D, Knuchel-Clarke R and
Stief C: Urinary bladder tumours. The new 2004 WHO classification.
Urologe A. 44:1073–1086. 2005.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Das S, Hirano M, Tako R, McCallister C and
Nikolaidis N: Evolutionary genomics of immunoglobulin-encoding Loci
in vertebrates. Curr Genomics. 13:95–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirano M, Guo P, McCurley N, Schorpp M,
Das S, Boehm T and Cooper MD: Evolutionary implications of a third
lymphocyte lineage in lampreys. Nature. 501:435–438. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qiu X, Zhu X, Zhang L, Mao Y, Zhang J, Hao
P, Li G, Lv P, Li Z, Sun X, et al: Human epithelial cancers secrete
immunoglobulin g with unidentified specificity to promote growth
and survival of tumor cells. Cancer Res. 63:6488–6495.
2003.PubMed/NCBI
|
|
7
|
Huang J, Sun X, Mao Y, Zhu X, Zhang P,
Zhang L, Du J and Qiu X: Expression of immunoglobulin gene with
classical V-(D)-J rearrangement in mouse brain neurons. Int J
Biochem Cell Biol. 40:1604–1615. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Welinder C, Baldetorp B, Blixt O, Grabau D
and Jansson B: Primary breast cancer tumours contain high amounts
of IgA1 immunoglobulin: An immunohistochemical analysis of a
possible carrier of the tumour-associated Tn antigen. PLoS One.
8:e617492013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Upender MB, Dunn JA, Wilson SM and Naegele
JR: Immunoglobulin molecules are present in early-generated
neuronal populations in the rat cerebral cortex and retina. J Comp
Neurol. 384:271–282. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang J, Zhang L, Ma T, Zhang P and Qiu X:
Expression of immunoglobulin gene with classical V-(D)-J
rearrangement in mouse testis and epididymis. J Histochem Cytochem.
57:339–349. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng S, Cao J and Geng L: Structure and
expression of colorectal cancer related Immunoglobulin novel gene
SNC73. Zhonghua Yi Xue Za Zhi. 81:485–488. 2001.(In Chinese).
PubMed/NCBI
|
|
12
|
Chen Z and Gu J: Immunoglobulin G
expression in carcinomas and cancer cell lines. FASEB J.
21:2931–2938. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Z, Huang X, Ye J, Pan P, Cao Q, Yang
B, Li Z, Su M, Huang C and Gu J: Immunoglobulin G is present in a
wide variety of soft tissue tumors and correlates well with
proliferation markers and tumor grades. Cancer. 116:1953–1963.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang C, Huang T, Wang Y, Huang G, Wan X
and Gu J: Immunoglobulin G expression in lung cancer and its
effects on metastasis. PLoS One. 9:e973592014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Liu D, Wang C, Liao Q, Huang J,
Jiang D, Shao W, Yin CC, Zhang Y, Lee G and Qiu X: Binding of the
monoclonal antibody RP215 to immunoglobulin G in metastatic lung
adenocarcinomas is correlated with poor prognosis. Histopathology.
67:645–653. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gritzmacher CA: Molecular aspects of
heavy-chain class switching. Crit Rev Immunol. 9:173–200.
1989.PubMed/NCBI
|
|
17
|
Zheng J, Huang J, Mao Y, Liu S, Sun X, Zhu
X, Ma T, Zhang L, Ji J, Zhang Y, et al: Immunoglobulin gene
transcripts have distinct VHDJH recombination characteristics in
human epithelial cancer cells. J Biol Chem. 284:13610–13619. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang S, Mao Y, Huang J, Ma T, Zhang L,
Zhu X, Zheng J, Wu L, Yin CC and Qiu X: Immunoglobulin gene locus
events in epithelial cells of lactating mouse mammary glands. Cell
Mol Life Sci. 67:985–994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang J, Sun X, Gong X, He Z, Chen L, Qiu
X and Yin CC: Rearrangement and expression of the immunoglobulin
µ-chain gene in human myeloid cells. Cell Mol Immunol. 11:94–104.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee G, Laflamme E, Chien CH and Ting HH:
Molecular identity of a pan cancer marker, CA215. Cancer Biol Ther.
7:2007–2014. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee G: Cancer cell-expressed
immunoglobulins: CA215 as a pan cancer marker and its diagnostic
applications. Cancer Biomark. 5:137–142. 2009.PubMed/NCBI
|
|
22
|
Lee G, Chu RA and Ting HH: Preclinical
assessment of anti-cancer drugs by using RP215 monoclonal antibody.
Cancer Biol Ther. 8:161–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee G and Ge B: Cancer cell expressions of
immunoglobulin heavy chains with unique carbohydrate-associated
biomarker. Cancer Biomark. 5:177–188. 2009.PubMed/NCBI
|
|
24
|
Liang PY, Li HY, Zhou ZY, Jin YX, Wang SX,
Peng XH and Ou SJ: Overexpression of immunoglobulin G prompts cell
proliferation and inhibits cell apoptosis in human urothelial
carcinoma. Tumour Biol. 34:1783–1791. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O'Connor KC, Nguyen K and Stollar BD:
Recognition of DNA by VH and Fv domains of an IgG anti-poly(dC)
antibody with a singly mutated VH domain. J Mol Recognit. 14:18–28.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li W, Yu R, Ma B, Yang Y, Jiao X, Liu Y,
Cao H, Dong W, Liu L, Ma K, et al: Core fucosylation of IgG B cell
receptor is required for antigen recognition and antibody
production. J Immunol. 194:2596–2606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoshimi K, Woo M, Son Y, Baudry M and
Thompson RF: IgG-immunostaining in the intact rabbit brain:
Variable but significant staining of hippocampal and cerebellar
neurons with anti-IgG. Brain Res. 956:53–66. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hozumi N and Tonegawa S: Evidence for
somatic rearrangement of immunoglobulin genes coding for variable
and constant regions. 1976 [classical article]. J Immunol.
173:4260–4264. 2004.PubMed/NCBI
|
|
29
|
Adamovic T, McAllister D, Guryev V, Wang
X, Andrae JW, Cuppen E, Jacob HJ and Sugg SL: Microalterations of
inherently unstable genomic regions in rat mammary carcinomas as
revealed by long oligonucleotide array-based comparative genomic
hybridization. Cancer Res. 69:5159–5167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
International Agency for Research on
Cancer, . World Cancer Report 2014. Stewart BW and Wild CP: WHO
Press; Geneva, Switzerland: pp. 418–419. 2015
|
|
31
|
Chung CK, Stryker JA, Ward SP, Nahhas WA
and Mortel R: Histologic grade and prognosis of carcinoma of the
cervix. bstet Gynecol. 57:636–642. 1981.
|
|
32
|
Lodde M, Mian C, Mayr R, Comploj E, Trenti
E, Melotti R, Campodonico F, Maffezzini M, Fritsche HM and Pycha A:
Recurrence and progression in patients with non-muscle invasive
bladder cancer: prognostic models including multicolor fluorescence
in situ hybridization molecular grading. Int J Urol. 21:968–972.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Droller MJ: Tumor size predicts the
survival of patients with pathologic stage T2 bladder carcinoma: A
critical evaluation of the depth of muscle invasion. J Urol.
163:665–666. 2000.PubMed/NCBI
|