Introduction
Nasopharyngeal carcinoma (NPC) is the most common
malignant tumor in South China (1).
The majority of studies have reported that the etiologic factors of
NPC are associated with genetic susceptibility, Epstein-Barr virus
infection and other environmental factors (2,3). However,
the exact genetic changes, such as in microRNAs (miRNAs or miRs),
that influence NPC progression remain largely unknown.
miRNAs are small non-coding RNAs of 20–22
nucleotides in length. miRNAs are recognized as novel markers that
are important in multiple biological functions of various human
cancers, including cell growth, migration, differentiation,
apoptosis, metastasis and angiogenesis (4–6). miRNAs
negatively regulate gene expression levels post-transcriptionally
by targeting the 3′-untranslated region (UTR) of messenger RNAs
(mRNAs) in a sequence-specific manner (7,8). Based on
previous studies on the regulation of miRNAs in the biological
progression of multiple cancers, miRNAs are presently considered as
potential novel targets for anti-cancer therapies (5,9,10).
miR-143 is located on chromosome 5q32, and its
expression is decreased in a variety of tumors. As a
tumor-suppressor gene, miR143 expression is negatively correlated
with malignant processes and patient's survival, since it inhibits
cell proliferation, migration and metastasis (11–16).
However, the mechanism that explains the regulation of cell
proliferation in NPC by miR-143 remains unknown.
The present study noticed low miR-143 expression in
NPC specimens and NPC cell lines. These results suggest that
miR-143 affects tumor proliferation, invasion and metastasis, and
that miR-143 is associated with NPC development. It was also
observed that miR-143 targeted the oncogene cyclin-dependent kinase
(CDK) 6 directly, downregulating it, and that CDK6 is essential for
the regulation of miR-143 in NPC cells in vitro. Our study
demonstrates that miR-143 inhibits cell proliferation and
tumorigenicity in NPC by targeting CDK6 and suppressing its
expression.
Materials and methods
NPC cell lines and treatments
The NPC cell lines CNE1, CNE2, 6–10B, 5–8F and HONE1
were obtained from the American Type Culture Collection (Manassas,
VA, USA) and cultured in Dulbecco's modified Eagle's medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (HyClone; GE Healthcare
Life Sciences, Logan, UT, USA) and 1% penicillin/streptomycin. All
cells were maintained in a humidified atmosphere at 37°C with 5%
CO2. NP69 cells (used as control) were kindly offered by
Professor Libing Song (State Key Laboratory of Oncology in Southern
China, Sun Yat-sen University, Guangzhou, China and Department of
Experimental Research, Sun Yat-sen University Cancer Center,
Guangzhou, China). The miR-143 mimic, miR-143 mimic mutant
(miR-143-mut), miR-143 inhibitor and negative control (NC) miRNA
were purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
For silencing CDK6 expression, a specific small interfering siRNA
(siRNA) was synthesized and purified by Guangzhou RiboBio Co., Ltd.
Transfection of oligonucleotides and siRNA was performed using
Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol.
NPC specimens
Eight pairs of snap-frozen NPC and adjacent tissue
were collected from patients undergoing curative resection and
diagnosed histopathologically at PLA421 Hospital (Guangzhou, China)
from 2006 to 2010. The present study was conducted on eight pairs
of NPC tumors and matched normal tissue from adjacent regions,
which were diagnosed histopathologically by experienced
pathologists. All samples were immediately frozen and stored in
liquid nitrogen until further analysis. All samples were obtained
with informed consent and approved by the Institutional Research
Ethics Committee of PLA421 Hospital.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cultured cells
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocol. RT-qPCR was performed using SYBR
Green I Nucleic Acid Gel Stain (Invitrogen; Thermo Fisher
Scientific, Inc.) in an ABI 7500 Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Gene expression data
were normalized to the geometric mean of the β-actin housekeeping
gene to control for variability in the expression levels, and
calculated as 2−[(Cq of gene)−(Cq of
β−actin)], where Cq represents the quantification cycle
for each transcript. The primers selected were as follows:
p21Cip1 forward 5′-CGATGCCAACCTCCTCAACGA-3′,
p21Cip1 reverse 5′-TCGCAGACCTCCAGCATCCA-3′,
p27Kip1 forward 5′-TGCAACCGACGATTCTTCTACTCAA-3′,
p27Kip1 reverse 5′-CAAGCAGTGATGTATCTGATAAACAAGGA-3′,
cyclin D1 forward 5′-AACTACCTGGACCGCTTCCT-3′, cyclin D1 reverse
5′-CCACTTGAGCTTGTTCACCA-3′, β-actin forward
5′-TGGCACCCAGCACAATGAA-3′ and β-actin reverse
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′.
Western blotting
Cell lysates were extracted using
radioimmunoprecipitation assay buffer [50 mM Tris-Hcl (pH 8.0), 150
mM NaCl, 1% Triton X-100 and protease inhibitor cocktail;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany]. Equal amounts
of protein (40 µl) were separated by 12% SDS-PAGE and transferred
onto polyvinylidene fluoride membranes. The membranes were blocked
using 5% non-fat milk, and probed with antibodies against cyclin D1
(1:1,000; cat. no. ab134175), p21Cip1 (1:1,000; cat. no.
ab109520), p27Kip1 (1:1,000; cat. no.
ab32034), retinoblastoma protein (Rb) (1:1,000; cat. no. ab181616),
phosphorylated (p)-Rb (1:1,000; cat. no. ab47763) and CDK6
(1:1,000; cat. no. ab124821; all Abcam, Cambridge, UK) for 2 h at
room temperature. After washing three times with TBST for 5 min
each, the membranes were incubated with the secondary antibody
(1:5,000; cat. nos. A9169 and A9044; Sigma-Aldrich; Merck
Millipore) for 1 h at room temperature. After washing the membranes
with TBST three times for 5 min each, the membranes were stripped
and reprobed with anti-β-actin mouse monoclonal antibody (1:1,500;
cat. no. A5441; Sigma-Aldrich; Merck Millipore) as a loading
control. The Immobilon Western Chemiluminescent HRP Substrate
(Merck Millipore) was used to visualize the bands.
MTT assay
MTT assay was performed to quantify NPC cell
viability. Cells were stained with 100 µl sterile MTT (0.5 mg/ml;
Sigma-Aldrich; Merck Millipore) for 4 h at 37°C. The culture medium
was then removed and formazan crystals in the cells were
solubilized using dimethyl sulfoxide (Sigma-Aldrich; Merck
Millipore) with plate incubation for 30 min. Cells were incubated
for 36 h at 37°C in a humidified 5% CO2 atmosphere.
Absorbance was measured at a 490-nm wavelength. The experiments
were performed independently in triplicate.
Colony formation assay
Cells were seeded on a 6-well plate
(1.0×103 cells/well) and cultured for 10 days. Colonies
were fixed for 5 min with 10% formaldehyde and stained with 1.0%
crystal violet for 1 min. The experiments were performed
independently in triplicate.
Flow cytometry
Cells were harvested by trypsinization, washed in
ice-cold PBS and fixed in 75% ice-cold ethanol. Cells were then
treated with bovine pancreatic RNAase (2 µg/ml; Sigma-Aldrich;
Merck Millipore) at 37°C for 30 min, followed by incubation with
propidium iodide (20 µg/ml; Sigma-Aldrich; Merck Millipore) for 20
min. Cell cycle analysis was determined using an LSR II flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA) with FACSDiva
6.0 software (BD Biosciences).
Luciferase assay
The region of the human CDK6 3′-UTR containing
miR-143 binding site was amplified by PCR from genomic DNA and
cloned into the pGL3 vector (Promega Corporation, Madison, WI, USA)
using Sac II and Pst I restriction enzymes. The
primers used were as follows: CDK6 3′UTR upstream,
5′-TCCCCGCGGAAATGCAGCTGTTCTGAACTGG-3′ and downstream,
3′-AACTGCAGAAATGCCCTAACGGCAAGAC-5′. The cycling conditions were as
follows: Initial denaturation at 94°C for 5 min; followed by 35
cycles of denaturation at 94°C for 15 sec, annealing at 60°C for 45
sec and elongation at 72°C for 30 sec; and finally elongation at
72°C for 5 min. Cells were seeded in triplicate in a 24-well plate
and allowed to settle for 24 h. pGL3-CDK6-luciferase plasmid (100
ng) was transfected into CNE1 cells using Lipofectamine 2000
reagent. Luciferase, and control signals were measured at 48 h
after transfection using the Dual-Luciferase Reporter Assay System
(Promega Corporation), according to the protocol provided by the
manufacturer.
Statistical analysis
Statistical analyses were performed using SPSS 20.0
software (IBM SPSS, Armonk, NY, USA). The Student t-test was used
to evaluate significant differences between two groups of data in
all relevant experiments. All results are presented as means ±
standard deviation. P<0.05 (two-tailed paired t-test) was
considered to indicate a statistically significant difference.
Results
miR-143 is downregulated in NPC cell
lines and clinical tissues
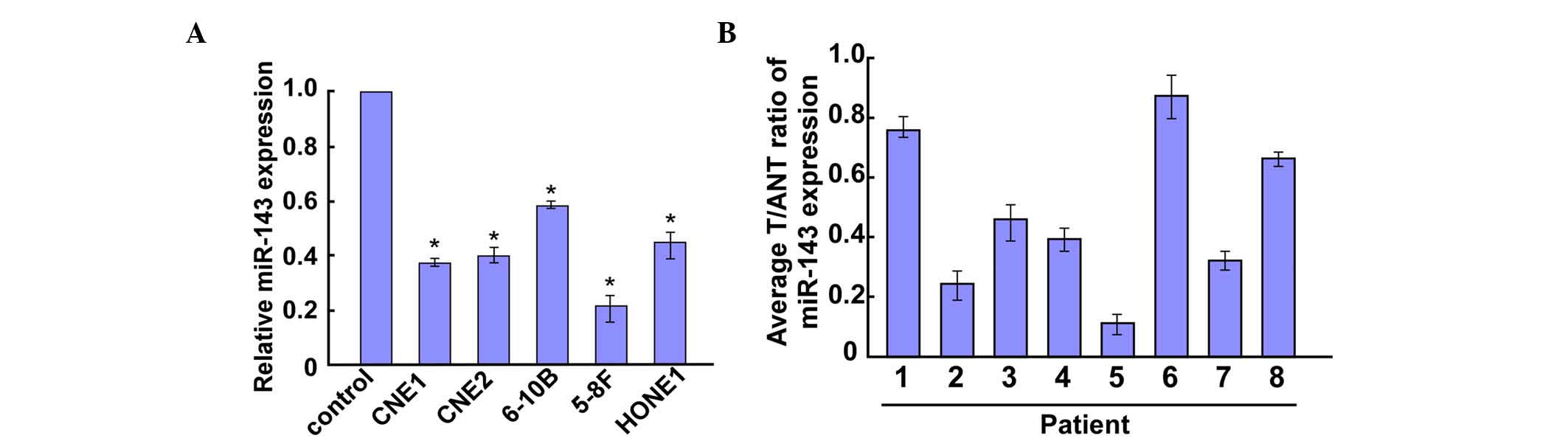
RT-qPCR revealed that miR-143 was markedly
downregulated in all five NPC cell lines compared with the control
cell line (NP69) (Fig. 1A).
Furthermore, miR-143 was significantly downregulated in eight NPC
tumor tissues compared with the control tissues (Fig. 1B). Taken together, these data
demonstrate that miR-143 expression is decreased in NPC tissues and
cell lines.
Ectopic miR-143 regulates NPC cell
proliferation
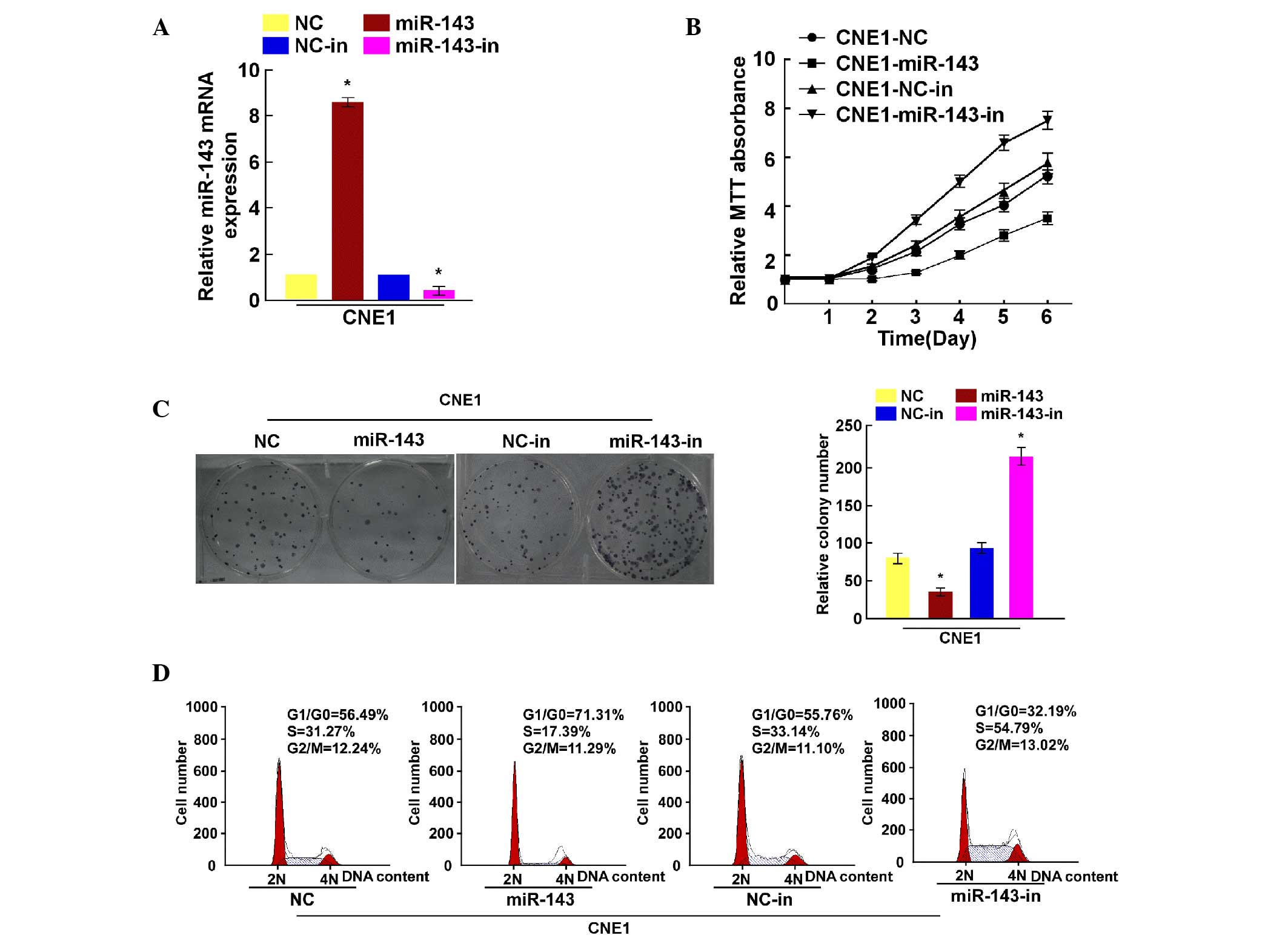
To explore the biological function of miR-143 in NPC
progression, miR-143 expression was determined by RT-qPCR in CNE1
cells transfected with miR-143 mimic and inhibitor (Fig. 2A). Cell viability was then measured by
MTT assay, revealing that miR-143 overexpression decreased the CNE1
cell growth rate, while inhibiting miR-143 expression increased it
(Fig. 2B). The colony formation assay
demonstrated that miR-143 upregulation inhibited the colony
formation capacity of CNE1 cells, while miR-143 downregulation
promoted it (Fig. 2C). Flow cytometry
revealed decreased miR-143 expression in the S phase compared with
that observed in the control cells (Fig.
2D). Collectively, these results suggest that downregulation of
miR-143 enhances NPC cell proliferation.
CDK6 is a direct target of miR-143 in
NPC cells
miRNAs regulate mRNAs by targeting their 3′-UTRs.
Thus, TargetScan (www.targetscan.org/), PicTar (http://pictar.mdc-berlin.de/) and miRanda (http://www.microrna.org/microrna/home.do) were used to
predict the potential targets of miR-143 in humans. The analysis
revealed a miR-143 binding site in the CDK6 mRNA 3′-UTR (Fig. 3A). As predicted, western blotting
revealed that miR-143 downregulated CDK6 expression in CNE1 cells,
and that miR-143 inhibition upregulated it (Fig. 3B). To confirm the direct regulation of
CDK6 by miR-143, a pGL3-CDK6-3′-UTR-luciferase reporter vector
containing the miR-143 binding site was constructed. The luciferase
assay demonstrated that ectopic miR-143 expression decreased the
luciferase activity, and that miR-143 downregulation increased the
pGL3-CDK6-3′-UTR-luciferase reporter activity. By contrast, in our
control experiment, mutant miR-143 had no effect on the luciferase
expression driven by the pGL3-CDK6-3′-UTR-luciferase reporter
vector (Fig. 3C). Collectively, these
results suggest that miR-143 directly targets CDK6 in NPC
cells.
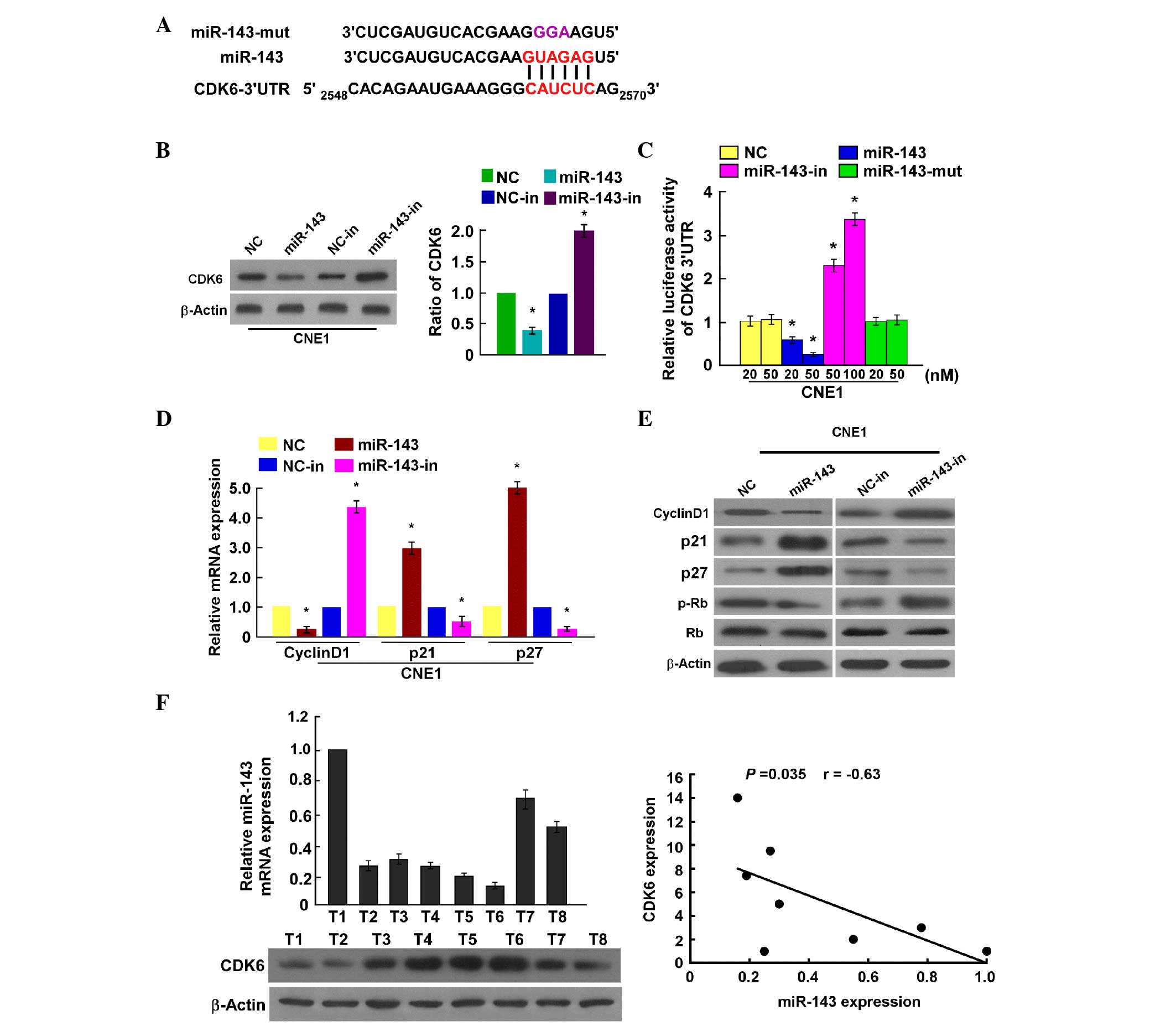 | Figure 3.CDK6 is a direct target of miR-143.
(A) Sequence alignment of miR-143, miR-143 mutant and putative CDK6
3′-UTR, and miR-143 binding sites. (B) Western blotting analysis of
CDK6 expression levels in nasopharyngeal carcinoma cell lines
overexpressing miR-143 or treated with miR-143 inhibitor compared
with control cells. (C) CDK6 luciferase reporter activity in CNE1
cells transfected with miR-143 mimic, miR-143 mutant, miR-143
inhibitor or negative control. (D) RT-qPCR analysis of p21, p27 and
cyclin D1 mRNA expression in CNE1 cells. (E) Western blot
determination of p21, p27, cyclin D1, p-Rb and Rb expression in
CNE1 cells. (F) RT-qPCR and western blot analysis of miR-143 and
CDK6 expression, respectively. The bottom panel depicts the results
of the statistical analysis of the correlation between miR-143 and
CDK6 expression levels. Bars represent the mean ± standard
deviation of three independent experiments. *P<0.05. miR,
microRNA; CDK, cyclin-dependent kinase; UTR, untranslated region;
NC, negative control; mut, mutant; in, inhibitor; mRNA, messenger
RNA; Rb, retinoblastoma protein; p-, phosphoryulated; T, tumor;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction. |
Our results reveal that miR-143 may regulate NPC
cell proliferation. Subsequently, its effects on the expression
level of genes associated with the cell cycle and cell
proliferation were evaluated. It was observed that the mRNA levels
of p21Cip1 and p27Kip1 were significantly
upregulated, while those of cyclin D1 were significantly
downregulated (Fig. 3D). Furthermore,
the protein expression levels of p21Cip1 and
p27Kip1 were upregulated, while those of cyclin D1 were
downregulated, and p-Rb was decreased in miR-143-overexpressing
cells compared with NC cells. By contrast, the expression levels of
p21Cip1 and p27Kip1 were downregulated, while
cyclin D1 was upregulated and Rb phosphorylation was increased in
cells transfected with miR-143 inhibitor (Fig. 3E). These results further confirm that
miR-143 is important in NPC cell proliferation by regulating CDK6
and certain cell cycle-related regulators.
Clinical relevance of miR-143
downregulation and CDK6 expression in NPC
To examine the clinical association between miR-143
and CDK6, miR-143 levels and CDK6 expression were examined in eight
fresh clinical NPC tissue samples. Statistical analysis
demonstrated that miR-143 expression was inversely correlated with
CDK6 (r=−0.63, P=0.035; Fig.
3F).
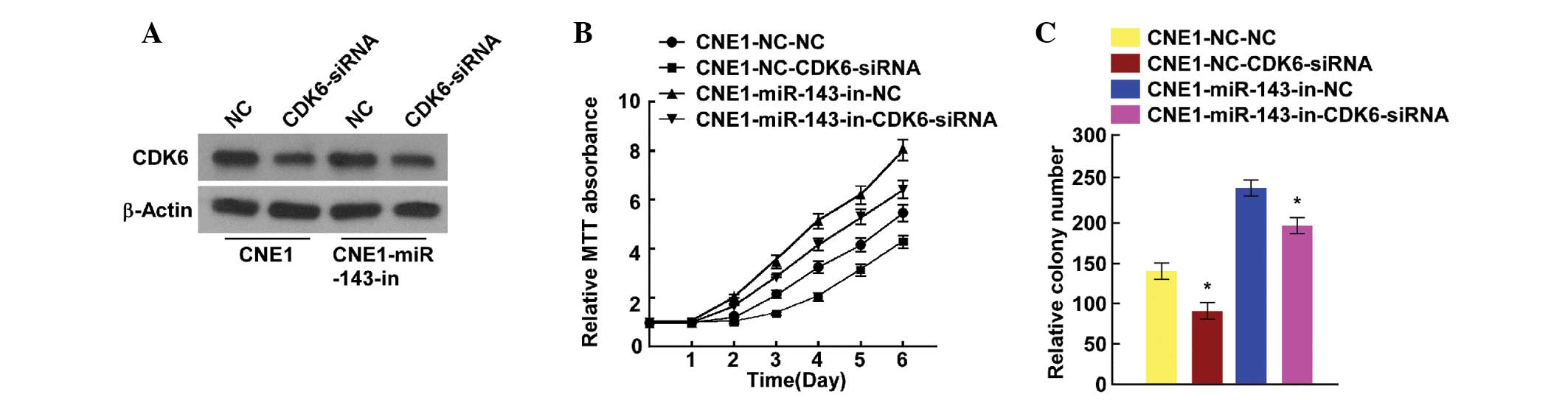
CDK6 suppression is essential for
miR-143-mediated effects in NPC
As reported above, miR-143 targeting of CDK6 was
closely correlated with cell proliferation. Therefore, endogenous
CDK6 expression was suppressed for further investigation. Cell
proliferation was analyzed using MTT and colony formation assays to
further confirm the link between miR-143 and the suppression of
CDK6 in NPC cells. CDK6 was downregulated in miR-143
inhibitor-transfected cells that contained CDK6 siRNA (Fig. 4A). The MTT and colony formation assays
indicated that CDK6 suppression decreased the growth rate of NPC
cells transfected with miR-143 inhibitor (Fig. 4B and C). These data confirmed that
miR-143 inhibits NPC cell proliferation and tumorigenicity by
downregulating CDK6 expression, and that CDK6 suppression is
essential for the miR-143-mediated effects on NPC cell
proliferation.
Discussion
The present study evaluated the function of miR-143
in NPC. In the current study, miR-143 was significantly
downregulated in NPC cells and tumor tissues compared with control
cells and adjacent regions tissues. Furthermore, ectopic miR-143
regulated NPC cell proliferation in vitro. It was also
demonstrated that CDK6 is a direct target of miR-143, and that it
was downregulated through its 3′-UTR. Importantly, miR-143
decreased the expression of the cell cycle regulators cyclin D1 and
CDK6, inhibited Rb phosphorylation, and increased the expression of
p21Cip1 and p27Kip1. These results suggest
that miR-143 may be important in inhibiting NPC carcinogenesis,
progression and cell cycle.
Previous studies have shown that increasing the
expression levels of the cell cycle inhibitors p21Cip1
and p27Kip1 and decreasing the expression levels of the
cell cycle regulator cyclin D1 leads to G1/S arrest (17). CDK6 is an important cell cycle
regulatory factor (18), and is a
member of the cyclin D family of proteins, which are responsible
for early G1 regulation. In the early phase of the cell cycle, G1
transition is mainly regulated by cyclin D1 complexed with CDK4
and/or CDK6 (19).
miRNA regulation of CDK6 has been reported
elsewhere. For example, Wang et al observed that miR-320c
inhibited the tumorous behavior of bladder cancer by targeting CDK6
(20). Furthermore, it has been
reported that miR-145, miR-504 and miR-105 all directly target CDK6
and regulate endogenous CDK6 expression in cancer cells.
Suppressing these miRNAs resulted in increased CDK6 and affected
cell growth (21–23). Recent studies revealed that miR-143,
as a tumor suppressor, targeted the 3′-UTR of KRAS, c-Myc and GLI
family zinc finger 3, which regulate cell DNA damage repair, cell
adhesion ability and cell migration in NPC, respectively (11,24,25). In
the present study, CDK6 was identified to be a direct target of
miR-143, which downregulates its expression. These results support
the hypothesis that miR-143 regulates cell proliferation by
targeting the cell cycle regulator CDK6 and affecting cell
function. Furthermore, consistent with previous studies, CDK6
regulation by specific miRNAs is essential in tumor development and
progression.
It has also been shown that cell cycle inhibitors
block the proliferation of adult stem cells in multiple tissue
types. For example, p21Cip1 and p27Kip1 may
control self-renewal of neural, intestinal and hematopoietic
progenitors (26,27). Consistent with those studies, the
present study demonstrated that ectopic miR-143 expression
inhibited cyclin D1 expression and induced p21Cip1 and
p27Kip1 expression.
In summary, the present study reports for the first
time an important link between miR-143 and CDK6 in NPC progression.
miR-143 regulates NPC cell proliferation by downregulating CDK6.
These results suggest that miR-143 is an anti-oncogene and may
represent a potential therapeutic target for NPC.
Acknowledgements
The present study was supported by a grant from the
Science and Technology Planning Project of Guangdong Province
(Guangzhou, China; grant no. 2012B031800480).
References
|
1
|
Chen HK, Liu XQ, Lin J, Chen TY, Feng QS
and Zeng YX: Mutation analysis of KLF6 gene in human nasopharyngeal
carcinomas. Ai Zheng. 21:1047–1050. 2002.(In Chinese). PubMed/NCBI
|
|
2
|
Lo KW and Huang DP: Genetic and epigenetic
changes in nasopharyngeal carcinoma. Semin Cancer Biol. 12:451–462.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheung ST, Huang DP, Hui AB, Lo KW, Ko CW,
Tsang YS, Wong N, Whitney BM and Lee JC: Nasopharyngeal carcinoma
cell line (C666-1) consistently harbouring Epstein-Barr virus. Int
J Cancer. 83:121–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Macfarlane LA and Murphy PR: MicroRNA:
Biogenesis, function and role in cancer. Curr Genomics. 11:537–561.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim VN and Nam JW: Genomics of microRNA.
Trends Genet. 22:165–173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Olena AF and Patton JG: Genomic
organization of microRNAs. J Cell Physiol. 222:540–545.
2010.PubMed/NCBI
|
|
9
|
Godlewski J, Nowicki MO, Bronisz A,
Williams S, Otsuki A, Nuovo G, Raychaudhury A, Newton HB, Chiocca
EA and Lawler S: Targeting of the Bmi-1 oncogene/stem cell renewal
factor by microRNA-128 inhibits glioma proliferation and
self-renewal. Cancer Res. 68:9125–9130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cho WC: OncomiRs: The discovery and
progress of microRNAs in cancers. Mol Cancer. 6:602007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhong W, He B, Zhu C, Xiao L, Zhou S and
Peng X: MiR-143 inhibits migration of human nasopharyngeal
carcinoma cells by negatively regulating GLI3 gene. Nan Fang Yi Ke
Da Xue Xue Bao. 33:1057–1061. 2013.(In Chinese). PubMed/NCBI
|
|
12
|
Peng X, Guo W, Liu T, Wang X, Tu X, Xiong
D, Chen S, Lai Y, Du H, Chen G, et al: Identification of miRs-143
and −145 that is associated with bone metastasis of prostate cancer
and involved in the regulation of EMT. PLoS One. 6:e203412011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang CJ, Zhou ZG, Wang L, Yang L, Zhou B,
Gu J, Chen HY and Sun XF: Clinicopathological significance of
microRNA-31, −143 and −145 expression in colorectal cancer. Dis
Markers. 26:27–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takagi T, Iio A, Nakagawa Y, Naoe T,
Tanigawa N and Akao Y: Decreased expression of microRNA-143 and
−145 in human gastric cancers. Oncology. 77:12–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Noguchi S, Mori T, Hoshino Y, Maruo K,
Yamada N, Kitade Y, Naoe T and Akao Y: MicroRNA-143 functions as a
tumor suppressor in human bladder cancer T24 cells. Cancer Lett.
307:211–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deftereos G, Corrie SR, Feng Q, Morihara
J, Stern J, Hawes SE and Kiviat NB: Expression of mir-21 and
mir-143 in cervical specimens ranging from histologically normal
through to invasive cervical cancer. PLoS One. 6:e284232011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Medema RH, Kops GJ, Bos JL and Burgering
BM: AFX-like Forkhead transcription factors mediate cell-cycle
regulation by Ras and PKB through p27kip1. Nature. 404:782–787.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kozar K and Sicinski P: Cell cycle
progression without cyclin D-CDK4 and cyclin D-CDK6 complexes. Cell
Cycle. 4:388–391. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: A changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Wu J, Lin Y, Zhu Y, Xu X, Xu X,
Liang Z, Li S, Hu Z, Zheng X and Xie L: MicroRNA-320c inhibits
tumorous behaviors of bladder cancer by targeting Cyclin-dependent
kinase 6. J Exp Clin Cancer Res. 33:692014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Wang L, Li B, Huo M, Mu M, Liu J
and Han J: miR-145 downregulates the expression of cyclin-dependent
kinase 6 in human cervical carcinoma cells. Exp Ther Med.
8:591–594. 2014.PubMed/NCBI
|
|
22
|
Kikkawa N, Kinoshita T, Nohata N, Hanazawa
T, Yamamoto N, Fukumoto I, Chiyomaru T, Enokida H, Nakagawa M,
Okamoto Y and Seki N: microRNA-504 inhibits cancer cell
proliferation via targeting CDK6 in hypopharyngeal squamous cell
carcinoma. Int J Oncol. 44:2085–2092. 2014.PubMed/NCBI
|
|
23
|
Honeywell DR, Cabrita MA, Zhao H,
Dimitroulakos J and Addison CL: miR-105 inhibits prostate tumour
growth by suppressing CDK6 levels. PLoS One. 8:e705152013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu YF, Li YQ, Guo R, He QM, Ren XY, Tang
XR, Jia WH, Kang TB, Zeng MS, Sun Y, et al: Identification of
miR-143 as a tumour suppressor in nasopharyngeal carcinoma based on
microRNA expression profiling. Int J Biochem Cell Biol. 61:120–128.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang W, Wu S, Shi Y, Miao Y, Luo X, Ji M,
Yao K and He J: c-MYB regulates cell growth and DNA damage repair
through modulating MiR-143. FEBS Lett. 589:555–564. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choudhury AR, Ju Z, Djojosubroto MW,
Schienke A, Lechel A, Schaetzlein S, Jiang H, Stepczynska A, Wang
C, Buer J, et al: Cdkn1a deletion improves stem cell function and
lifespan of mice with dysfunctional telomeres without accelerating
cancer formation. Nat Genet. 39:99–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fasano CA, Dimos JT, Ivanova NB, Lowry N,
Lemischka IR and Temple S: shRNA knockdown of Bmi-1 reveals a
critical role for p21-Rb pathway in NSC self-renewal during
development. Cell Stem Cell. 1:87–99. 2007. View Article : Google Scholar : PubMed/NCBI
|