Introduction
Since its discovery as a Na/K-adenosine
triphosphatase (ATPase) inhibitor, ouabain has been an important
topic of research in virtually all aspects of biochemistry,
biophysics and physiology. A number of studies revealed the
existence of an endogenous analog of ouabain in the plasma and
certain mammalian tissues, including human tissues (1–3), and
increased levels of this hormone are associated with hypertension
(4). Furthermore, prolonged infusion
of ouabain in animal models induces significant increases in blood
pressure (5,6); although the mechanisms underlying the
association between the onset of hypertension and enhanced levels
of plasma ouabain remain unknown.
Previously, several studies suggested an anticancer
role for ouabain, which appears to be involved in complex
cell-signal transduction mechanisms that result in the selective
control of tumor growth (7–10). In contrast to the promising use of
ouabain in tumor growth control, studies have shown that ouabain
can increase the expression of ATP binding cassette subfamily B
member 1 (ABCB1) (11,12). ABCB1, also known as P-glycoprotein,
confers resistance to several unassociated drugs, and is therefore
a major concern in cancer chemotherapy (11,13). One
approach to circumvent this resistance may be to inhibit the
activity of ABCB1. However, due to the important physiological
actions of ABCB1 that affect several organs and tissues, including
cells from the immune system, this approach is clearly not a
reasonable alternative (13).
Cancer is a multifactorial disease, and cancer
patients often show alterations in the immune system (14). Ouabain is known to interfere with this
system (15), and ABCB1 is a protein
directly associated with several functions of the immune system
(16–19). At present, few studies have examined
the effects of ouabain on ABCB1 expression and activity in the
immune system in vivo. Therefore, the aim of the present
study was to assess the in vivo effects of ouabain on immune
cell counts in the blood and on ABCB1 activity in the thymus,
peripheral blood mononuclear cells and mesenteric lymph nodes in
order to contribute to the possible use of this glycoside in cancer
therapy.
Materials and methods
Animal preparation
All animal procedures were previously reviewed and
approved by the Animal Subject Committee of the UFRJ Health Science
Centre (Rio de Janeiro, Brazil; protocol nos. IBCCF 082/2009 and
153/13). Male Wistar rats weighing between 260–280 g and male Swiss
mice weighing between 26–32 g received food and water ad
libitum.
Treatment with ouabain
Wistar rats
Male Wistar rats were treated with daily
intraperitoneal injections of 30 µg/kg of ouabain (Sigma-Aldrich,
St. Louis, MO, USA) or its vehicle, phosphate-buffered saline
(PBS). A total of 20 rats were used, 12 for acute treatment (n=6
rats/group in ouabain and control groups) and 8 for chronic
treatment (n=4 rats/group in ouabain and control groups). Animals
were maintained under standard laboratory conditions, with room
temperature controlled (22°C), and subjected to 12 h light-dark
cycles with ad libitum access to food and water. Prior to
the first injection at 24 h and 7 and 14 days subsequent to the
injection, the rats had their blood pressure measured by a
computerized tail-cuff method. The animals were sacrificed by
barbiturate overdose (86 mg/kg intraperitoneal injection of
pentobarbital) after 24 h (acute treatment) or 14 days (chronic
treatment) of ouabain injections, and the mesenteric lymph nodes,
thymi and blood were collected. Full excisions of thymi and partial
excisions of mesentheric lymph nodes were performed, while blood
samples were collected by caudal venous puncture prior to animals
sacrifice.
Mesenteric lymph nodes and thymi were softly
dissociated, and the remaining cells were washed in PBS and
centrifuged at 200 × g. The pellet was suspended in ice-cold
RPMI-1640 medium (Sigma-Aldrich) supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Heparinized blood (200 µl) was separated for an evaluation of
hematological parameters, and 5 ml was centrifuged on a
Ficoll-Hystopaque (Sigma-Aldrich) density gradient at 200 × g to
isolate the peripheral blood mononuclear cells (PBMC). The sample
was then resuspended in the same culture medium and stored on ice
until required for the activity assays.
Swiss mice
A total of 8 mice were used in the present study, 4
in the control group and 4 in the ouabain-treated group. Animals
were maintained under standard laboratory conditions, with room
temperature controlled (22°C), and subjected to 12 h light-dark
cycles with ad libitum access to food and water. At 24 h
subsequent to the intraperitoneal injection with 300 µg/kg of
ouabain or PBS, the Swiss mice were sacrificed by barbiturate
overdose (86 mg/kg intraperitoneal injection of pentobarbital). The
mesenteric lymph nodes and thymi were immediately removed and
softly dissociated. The remaining cells were washed in PBS and
centrifuged at 200 × g. The pellet was suspended in ice-cold
RPMI-1640 culture medium supplemented with 10% FBS until required
for the activity assays.
Blood pressure measurement
Mean blood pressure was recorded in conscious,
resting Wistar rats by a non-invasive oscillometric tail-cuff
method (LE5001 Pressure Meter; Letica SA, Barcelona, Spain). One
week prior to the initiation of the experiments, the rats were
accustomed to restraint and inflation of the tail cuff in two
independent sessions, to minimize non-specific stress. All measures
were recorded in the morning, between 9 and 11 am. For each
session, at least 7 blood pressure readings were recorded.
Activity assay by flow cytometry
Rhodamine (Rho)123 (Sigma-Aldrich) was used to
measure ABCB1 activity in lymphocytes from PBMCs, mesenteric lymph
nodes and in thymocytes, as previously described (20). The gates used to select the
lymphocytes from PBMC, mesenteric lymph nodes and thymocytes were
performed using forward and side scattering, according to previous
descriptions (21,22).
Statistical analysis
Each experiment was performed with at least 4
animals in each group. Data are expressed as the mean ± standard
error of the mean and were analyzed using two-way analysis of
variance (blood pressure analyses) or an unpaired Student's
t-test. Values of P<0.05 were considered to indicate a
statistically significant difference.
Results
Acute ouabain treatment
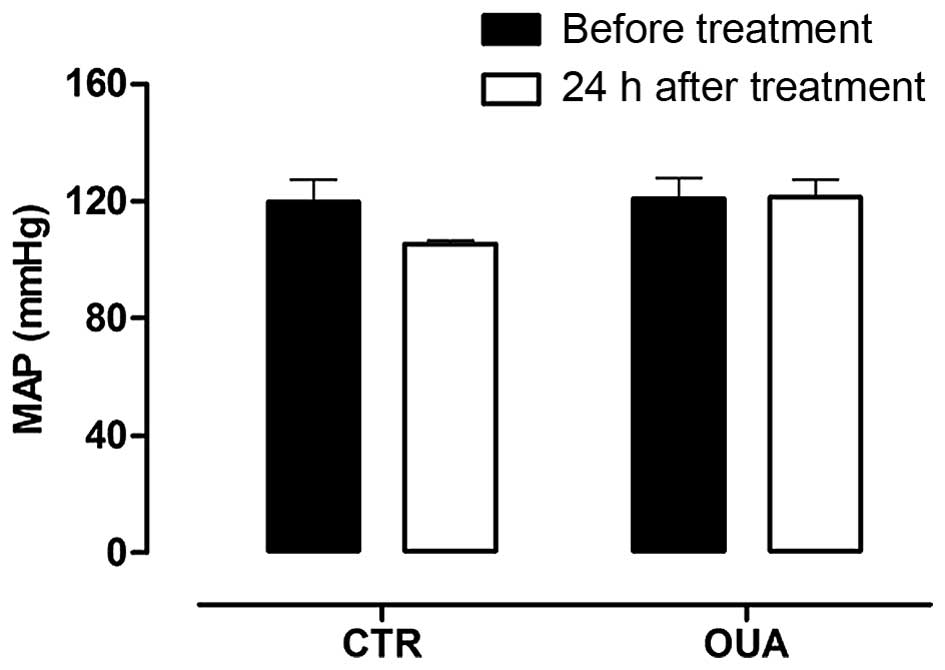
As expected, the mean arterial pressure (MAP) of
Wistar rats injected with a single dose of ouabain was similar to
the MAP obtained prior to the administration of either ouabain or
PBS (prior to injection, 121±7 mmHg; 24 h following intraperitoneal
ouabain injection, 122±6 mmHg) (Fig.
1). However, significant alterations were identified in the
basophil and monocyte populations, with basophil populations being
increased by 55% and monocyte populations being decreased by almost
45% in blood samples from animals treated with ouabain (Table I). No alterations in red cells
characteristics were observed (data not shown).
 | Table I.White blood cell count of Wistar rats
acutely treated with ouabain. |
Table I.
White blood cell count of Wistar rats
acutely treated with ouabain.
| Cells | Control | Ouabain |
|---|
| Leukocytes,
×103/µl |
3.18±0.20 |
3.60±0.39 |
| Basophils, % |
1.55±0.23 |
2.41±0.23a |
| Eosinophils, % |
2.34±0.26 |
2.25±0.32 |
| Neutrophils, % |
21.90±2.08 |
18.75±3.28 |
| Lymphocytes, % |
73.22±2.16 |
76.05±3.61 |
| Monocytes, % |
0.99±0.19 |
0.54±0.15b |
Ouabain has been shown to induce alterations in the
expression and activity of ABCB1 in vitro (11,12).
Therefore, the present study tested whether a single in vivo
administration of ouabain could result in alterations in ABCB1
protein expression in lymph nodes and thymi. ABCB1 activity is only
observed in 5–10% of thymocytes, as it is restricted to the most
immature subset, the double negative cluster of differentiation
(CD)4−/CD8− subpopulation, and to the fully
mature subset, the CD4+ and CD8+ T cells
(23). Therefore, the number of cells
presenting ABCB1 activity in the thymus was determined by the
percentage of cells with low Rho content, which were modulated by
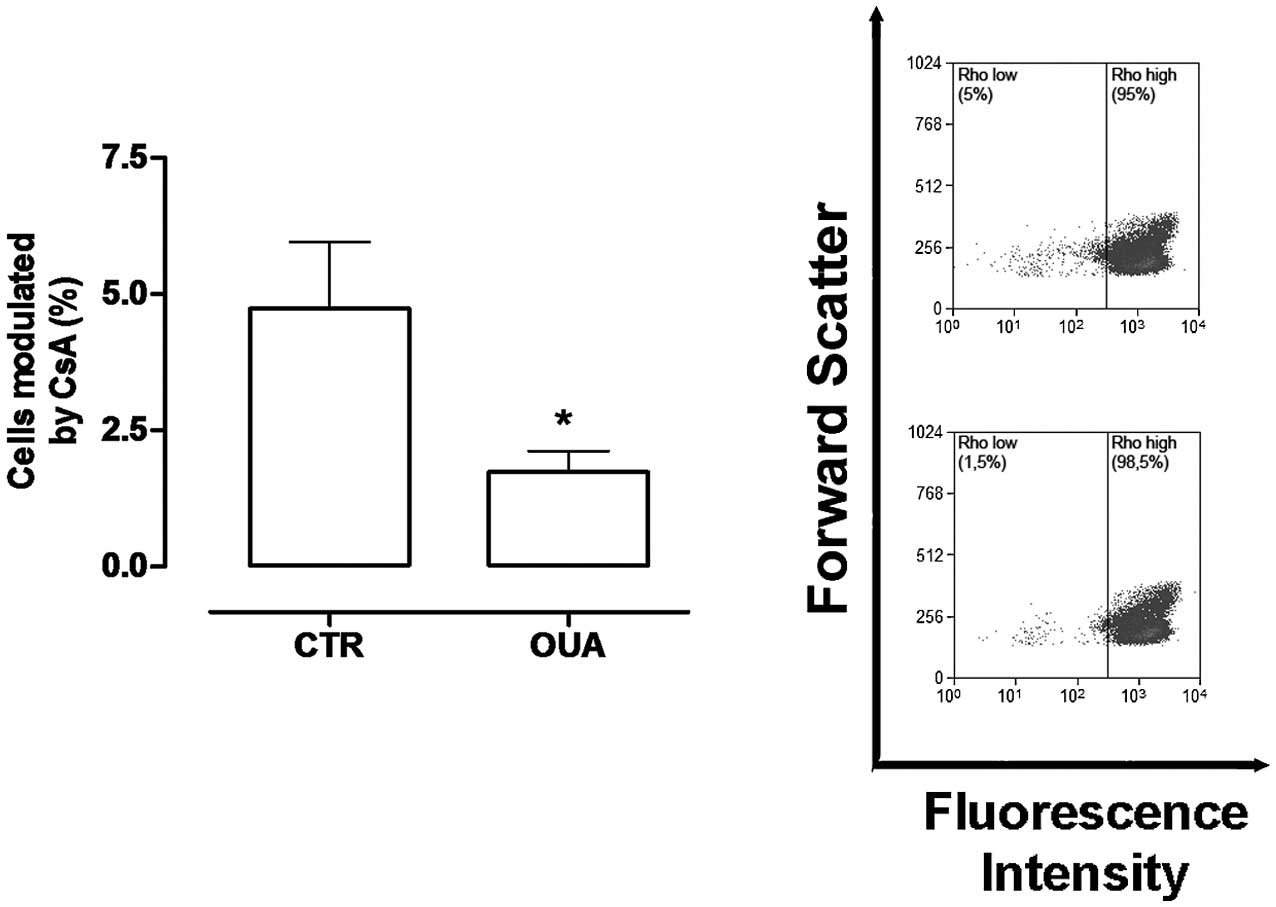
the ABCB1 inhibitor cyclosporin A (CsA) (Fig. 2A). However, as the majority of mature
lymphocytes demonstrate ABCB1 activity, the gates used to analyze
the percentage of cells modulated by CsA were drawn by dividing the
population into two halves, using the incubation with Rho123 as a
reference (Fig. 2B).
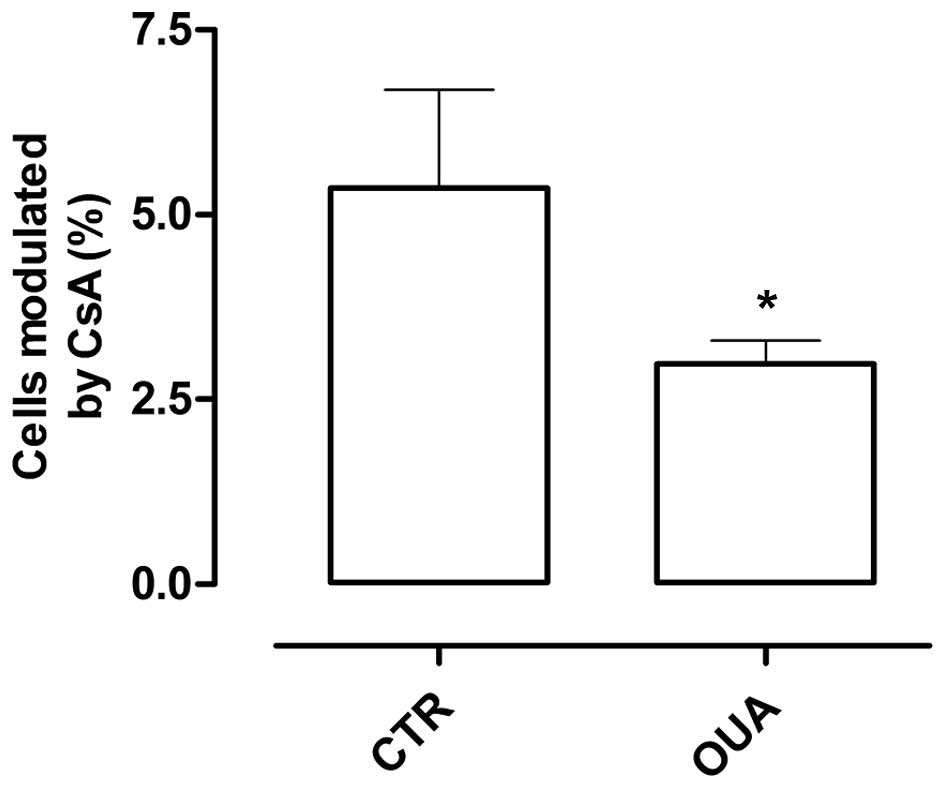
Fig. 2A shows that the
percentage of thymocytes with ABCB1 activity in control rats was
almost 5%, which was obtained by corroborating previous results
(23); however, in the thymocytes of
rats pretreated with ouabain, this activity decreased by ~50% of
the control. Treatment with ouabain did not alter the number of
cells modulated with CSA in PBMCs or mesenteric lymph nodes (data
not shown).
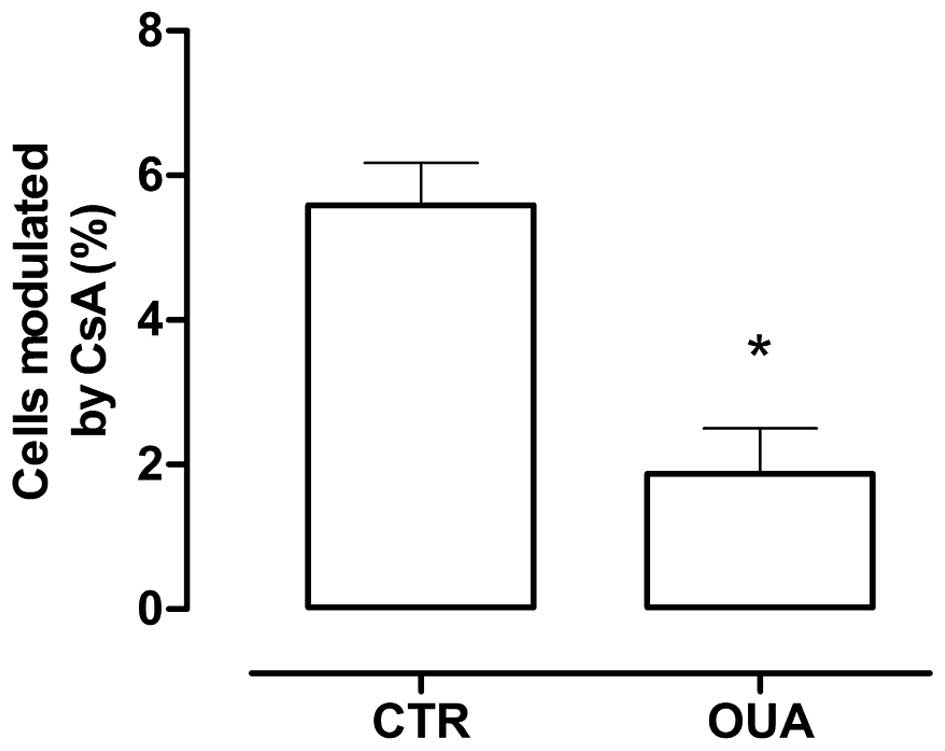
To evaluate whether the observed effect in
thymocytes was species dependent, the same experiments were
performed in mice. Due to the difficulties in obtaining and
separating blood cells from the mice, only lymph nodes and thymi
were tested. As shown in Fig. 3, the
same results were obtained, with an approximate 50% reduction of
ABCB1 activity in the thymi and no difference in lymph nodes.
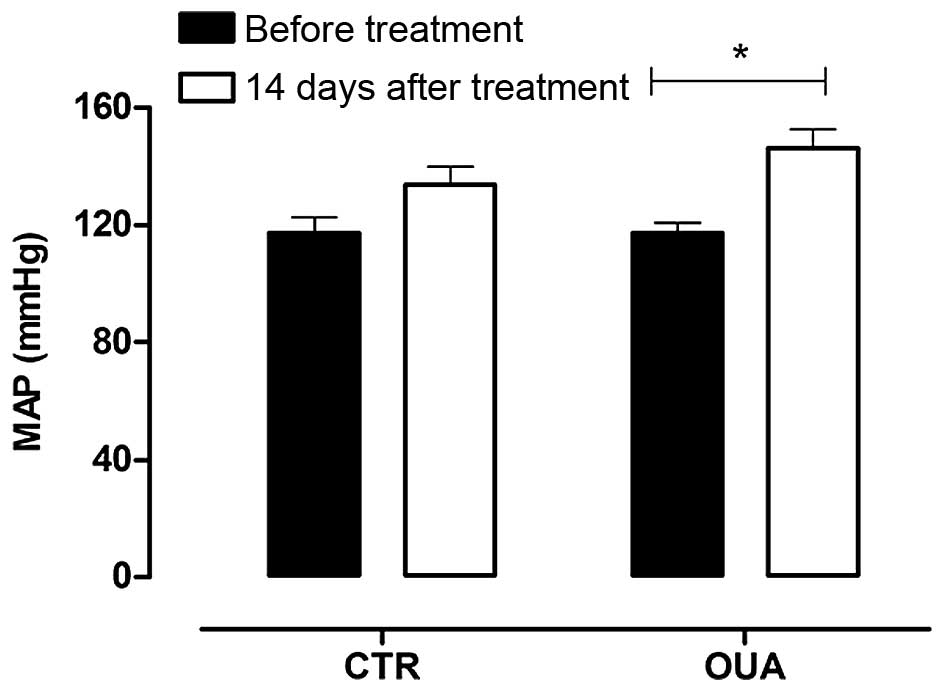
Chronic ouabain treatment
Since a chronic treatment with ouabain may lead to
hypertension and, to the best of our knowledge, no studies assess
ABCB1 activity in chronic ouabain-treated animals, the present
study also tested the effects of chronic ouabain treatment on ABCB1
activity in the lymph nodes and thymi of rats. The rats were
treated daily with intraperitoneal injections of 30 mg/kg ouabain.
The MAP was measured on days 0, 7 and 14. As shown in Fig. 4, after 14 days the MAP of
ouabain-treated rats was significantly elevated. The animals were
then sacrificed and the ABCB1 activity was measured. Fig. 5 shows that the effect observed in the
thymus with acute ouabain treatment remained present following
chronic treatment. In addition, the ABCB1 activity of mesenteric
lymph nodes was also diminished after chronic treatment with
ouabain.
Discussion
To the best of our knowledge, the present study is
the first to evaluate the effects of acute ouabain treatment in the
blood. The dose of ouabain used (30 µg/kg) has been previously
shown to induce hypertension when infused or released
subcutaneously for long periods of time in Wistar rats (6,24).
Corroborating with the results of other studies (6,24), the
current study found that ouabain increased the MAP after 14 days of
continuous daily treatment, but did not promote any alteration in
the blood pressure of Wistar rats after 24 h. However, the acute
administration of ouabain was found to induce significant
alterations to the basophil and monocyte counts in the peripheral
blood.
Basophils are granulocytes known to participate in
allergic reactions and parasite infections. This type of cell
expresses major histocompatibility complex class II, CD80 and CD86,
and has the potential to capture and process antigens and present
peptides to effector cells, which promote the development of T
helper 2 cells via early secretion of interleukin (IL)-4 during
allergic immune responses (25). The
differentiation of bone marrow precursors in basophils requires
IL-3, and these differentiated cells are capable of producing
histamine, IL-4, IL-13 and leukotriene C4 (26), which is an important substrate
described for another member of the ABC superfamily, ABCC1
(27). Basophilia has a strong
association with certain malignancies, particularly acute and
chronic myeloid leukemia (26).
Monocytes are mononuclear cells present in the
blood, which, after activation, migrate from the blood to the
tissues and usually differentiate into macrophages. Monocytes are
important for the initiation, amplification and shutdown of the
immune response through the production of cytokines, recognition of
pathogen-associated molecular patterns, antigen presentation and
phagocytosis (28–30). Ebner et al (31) demonstrated that monocytes can
differentiate in dendritic cells after stimulation with IL-3 and
IL-4.
The actions of ouabain are extremely diverse and may
vary according to the target organ or tissue. Certain studies have
shown important implications of the ouabain hormone in cell
proliferation, muscle contraction and cell survival in distinct
cell types (32–34). In the immune system, ouabain modulates
the expression of several important receptors in lymphocytes and
monocytes (35,36), impairs lymphocyte proliferation
induced by mitogens (36) and induces
cytokine secretion in PBMC (37,38). In
addition, the in vivo administration of ouabain has been
shown to reduce the amount of mature B lymphocytes in the spleen
and prevent the inflammation induced by several substances in mice
(39,40).
Although no studies make direct associations between
ouabain and IL-3, certain studies have shown that ouabain
interferes with other interleukin pathways (41,42) and
that IL-3 activates Na+/K+-ATPase, which is
the main target of ouabain, in macrophages (43). Therefore, the possibility that ouabain
regulates the synthesis or secretion of IL-3, which could result in
augmented number of basophils and reduced number of monocytes in
peripheral circulation, cannot be ruled out. In addition, it has
been reported that ouabain affects the activation of monocytes and
modulates their functions, acting as an immunomodulator of these
cells (44).
At present, the consequences of the observed
alterations in leukocyte counts that were induced by short-term
treatment with ouabain remain unclear; however, these alterations
could potentially affect the immune response of cancer patients.
Since basophilia has been shown to be associated with a bad
prognosis (reduced survival) in myelodysplastic syndromes (45) and also in patients with solid tumors
(46), the present observations
suggest that additional studies are required to assess the use of
ouabain for treatment in cancer patients.
Ouabain is known to modulate the expression of two
ATPases associated with cancer resistance, ABCB1 and ABCC1
(47). Therefore, the present study
aimed to assess whether ouabain could alter the expression or
activity of ABCB1 in PBMC and thymocytes in vivo. Indeed,
acute and chronically ouabain-treated Wistar rats displayed
significant decrease in ABCB1 activity in lymphocytes from the
thymus. Despite being more resistant to ouabain compared with rats
(48), which explains the greater
dose (300 µg/kg) used, Swiss mice acutely treated with the ABCB1
glycoside also presented similar results, indicating that this
effect is not species-dependent.
ABCB1 is associated with the transport of several
substrates, including lipids, hormones and cytokines (19,49,50). In
thymocytes, the expression of this protein is restricted to the
double negative (CD4−/CD8−) thymocytes and to
the simple positive populations CD4+ and
CD8+, representing the most immature subset and the
mature thymocyte populations, respectively (23). Thus, the observation that ouabain
induced a significant decrease in the percentage of thymocytes with
ABCB1 activity raises at least three hypotheses: i) That thymocytes
with ABCB1 are simply undergoing protein downregulation in the
plasma membrane; ii) that thymocyte maturation is being impaired,
leading to an accumulation of double positive cells
(CD4+/CD8+), which do not exhibit ABCB1
activity; or iii) that the thymocytes with decreased ABCB1 activity
after the ouabain injection could be undergoing apoptosis.
Although, at present, the significance of the present observations
is not understood, the findings suggest that ouabain could
contribute to immunodeficiencies in cancer patients, which argues
against its use in cancer treatment.
As the immune system is directly associated with the
development of cancer, and since acute and chronic treatment with
ouabain were able to alter the activity of ABCB1 in immature
lymphocytes and the percentage of monocytes and basophils in the
peripheral blood, the current results suggest that additional
studies are required to assess the use of ouabain as an adjuvant
anticancer therapy, and may also contribute to elucidating the
mechanisms of development of hypertension triggered by ouabain.
Acknowledgements
The present study was supported by grants from the
Cancer Foundation (Rio de Janeiro, Brazil) and the National Council
for Scientific and Technological Development (Brasília,
Brazil).
References
|
1
|
Tymiak AA, Norman JA, Bolgar M, DiDonato
GC, Lee H, Parker WL, Lo LC, Berova N, Nakanishi K and Haber E:
Physicochemical characterization of a ouabain isomer isolated from
bovine hypothalamus. Proc Natl Acad Sci USA. 90:8189–8193. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ludens JH, Clark MA, Robinson FG and
DuCharme DW: Rat adrenal cortex is a source of a circulating
ouabainlike compound. Hypertension. 19:721–724. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hamlyn JM, Blaustein MP, Bova S, DuCharme
DW, Harris DW, Mandel F, Mathews WR and Ludens JH: Identification
and characterization of a ouabain-like compound from human plasma.
Proc Natl Acad Sci USA. 88:6259–6263. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Manunta P, Stella P, Rivera R, Ciurlino D,
Cusi D, Ferrandi M, Hamlyn JM and Bianchi G: Left ventricular mass,
stroke volume, and ouabain-like factor in essential hypertension.
Hypertension. 34:450–456. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Manunta P, Hamilton BP and Hamlyn JM:
Structure-activity relationships for the hypertensinogenic activity
of ouabain: Role of the sugar and lactone ring. Hypertension.
37:472–477. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang BS, Huang X, Harmsen E and Leenen
FH: Chronic central versus peripheral ouabain, blood pressure and
sympathetic activity in rats. Hypertension. 23:1087–1090. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Winnicka K, Bielawski K, Bielawska A and
Surazyński A: Antiproliferative activity of derivatives of ouabain,
digoxin and proscillaridin A in human MCF-7 and MDA-MB-231 breast
cancer cells. Biol Pharm Bull. 31:1131–1140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Newman RA, Yang P, Pawlus AD and Block KI:
Cardiac glycosides as novel cancer therapeutic agents. Mol Interv.
8:36–49. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tailler M, Senovilla L, Lainey E, Thépot
S, Métivier D, Sébert M, Baud V, Billot K, Fenaux P, Galluzzi L, et
al: Antineoplastic activity of ouabain and pyrithione zinc in acute
myeloid leukemia. Oncogene. 31:3536–3546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pezzani R, Rubin B, Redaelli M, Radu C,
Barollo S, Cicala MV, Salvà M, Mian C, Mucignat-Caretta C, Simioni
P, et al: The antiproliferative effects of ouabain and everolimus
on adrenocortical tumor cells. Endocr J. 61:41–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brouillard F, Tondelier D, Edelman A and
Baudouin-Legros M: Drug resistance induced by ouabain via the
stimulation of MDR1 gene expression in human carcinomatous
pulmonary cells. Cancer Res. 61:1693–1698. 2001.PubMed/NCBI
|
|
12
|
Riganti C, Campia I, Polimeni M,
Pescarmona G, Ghigo D and Bosia A: Digoxin and ouabain induce
P-glycoprotein by activating calmodulin kinase II and
hypoxia-inducible factor-1alpha in human colon cancer cells.
Toxicol Appl Pharmacol. 240:385–392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cordon-Cardo C, O'Brien JP, Boccia J,
Casals D, Bertino JR and Melamed MR: Expression of the multidrug
resistance gene product (P-glycoprotein) in human normal and tumor
tissues. J Histochem Cytochem. 38:1277–1287. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Visser KE, Eichten A and Coussens LM:
Paradoxical roles of the immune system during cancer development.
Nat Rev Cancer. 6:24–37. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rodrigues-Mascarenhas S, Da Silva de
Oliveira A, Amoedo ND, Affonso-Mitidieri OR, Rumjanek FD and
Rumjanek VM: Modulation of the immune system by ouabain. Ann N Y
Acad Sci. 1153:153–163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Honig SM, Fu S, Mao X, Yopp A, Gunn MD,
Randolph GJ and Bromberg JS: FTY720 stimulates multidrug
transporter- and cysteinyl leukotriene-dependent T cell chemotaxis
to lymph nodes. J Clin Invest. 111:627–637. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenbraun MD, Mosley RL, Teitelbaum DH
and Miller RA: Altered development of intestinal intraepithelial
lymphocytes in P-glycoprotein-deficient mice. Dev Comp Immunol.
24:783–795. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gupta S, Kim CH, Tsuruo T and Gollapudi S:
Preferential expression and activity of multidrug resistance gene 1
product (P-glycoprotein), a functionally active efflux pump, in
human CD8+ T cells: A role in cytotoxic effector function. J Clin
Immunol. 12:451–458. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Raghu G, Park SW, Roninson IB and
Mechetner EB: Monoclonal antibodies against P-glycoprotein, an MDR1
gene product, inhibit interleukin-2 release from PHA-activated
lymphocytes. Exp Hematol. 24:1258–1264. 1996.PubMed/NCBI
|
|
20
|
Valente RC, Capella LS, Nascimento CR,
Braga F, Echevarria-Lima J, Lopes AG and Capella MAM: ABCB1
(P-glycoprotein) but not ABCC1 (MRP1) is downregulated in
peripheral blood mononuclear cells of spontaneously hypertensive
rats. Pflugers Arch. 456:359–368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shapiro HM, Schildkraut ER, Curbelo R,
Turner RB, Webb RH, Brown DC and Block MJ: Cytomat-R: A
computer-controlled multiple laser source multiparameter flow
cytophotometer system. J Histochem Cytochem. 25:836–844. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hoffman RA, Kung PC, Hansen WP and
Goldstein G: Simple and rapid measurement of human T lymphocytes
and their subclasses in peripheral blood. Proc Natl Acad Sci USA.
77:4914–4917. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leite DF, Echevarria-Lima J, Salgado LT,
Capella MA, Calixto JB and Rumjanek VM: In vivo and in vitro
modulation of MDR molecules in murine thymocytes. Int
Immunopharmacol. 6:204–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J, Hamlyn JM, Karashima E, Raina H,
Mauban JR, Izuka M, Berra-Romani R, Zulian A, Wier WG and Blaustein
MP: Low-dose ouabain constricts small arteries from
ouabain-hypertensive rats: Implications for sustained elevation of
vascular resistance. Am J Physiol Heart Circ Physiol.
297:H1140–H1150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakanishi K: Basophils as APC in Th2
response in allergic inflammation and parasite infection. Curr Opin
Immunol. 22:814–820. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cromheecke JL, Nguyen KT and Huston DP:
Emerging role of human basophil biology in health and disease. Curr
Allergy Asthma Rep. 14:4082014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cole SP: Targeting multidrug resistance
protein 1 (MRP1, ABCC1): Past, present and future. Annu Rev
Pharmacol Toxicol. 54:95–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tacke F, Ginhoux F, Jakubzick C, van
Rooijen N, Merad M and Randolph GJ: Immature monocytes acquire
antigens from other cells in the bone marrow and present them to T
cells after maturing in the periphery. J Exp Med. 203:583–597.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schiff DE, Kline L, Soldau K, Lee JD,
Pugin J, Tobias PS and Ulevitch RJ: Phagocytosis of gram-negative
bacteria by a unique CD14-dependent mechanism. J Leukoc Biol.
62:786–794. 1997.PubMed/NCBI
|
|
30
|
Akira S and Hemmi H: Recognition of
pathogen-associated molecular patterns by TLR family. Immunol Lett.
85:85–95. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ebner S, Hofer S, Nguyen VA, Fürhapter C,
Herold M, Fritsch P, Heufler C and Romani N: A novel role for IL-3:
Human monocytes cultured in the presence of IL-3 and IL-4
differentiate into dendritic cells that produce less IL-12 and
shift Th cell responses toward a Th2 cytokine pattern. J Immunol.
168:6199–6207. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tian J, Li X, Liang M, Liu L, Xie JX, Ye
Q, Kometiani P, Tillekeratne M, Jin R and Xie Z: Changes in sodium
pump expression dictate the effects of ouabain on cell growth. J
Biol Chem. 284:14921–14929. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schoner W and Scheiner-Bobis G: Endogenous
and exogenous cardiac glycosides: Their roles in hypertension, salt
metabolism and cell growth. Am J Physiol Cell Physiol.
293:C509–C536. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
De Rezende Corrêa G, dos Santos A Araujo,
Fontes C Frederico Leite and de Araujo E Giestal: Ouabain induces
an increase of retinal ganglion cell survival in vitro: The
involvement of protein kinase C. Brain Res. 1049:89–94. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Valente RC, Nascimento CR, Araujo EG and
Rumjanek VM: Mcd14 expression in human monocytes is downregulated
by ouabain via transactivation of epithelial growth factor receptor
and activation of p38 mitogen-activated protein kinase.
Neuroimmunomodulation. 16:228–236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pires V, Harab RC, Olej B and Rumjanek VM:
Ouabain effects on activated lymphocytes: Augmentation of CD25
expression on TPA-stimulated cells and of CD69 on PHA- end
TPA-stimulated cells. Int J Immunopharmacol. 19:143–148. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Matsumori A, Ono K, Nishio R, Igata H,
Shioi T, Matsui S, Furukawa Y, Iwasaki A, Nose Y and Sasayama S:
Modulation of cytokine production and protection against lethal
endotoxemia by the cardiac glycoside ouabain. Circulation.
96:1501–1506. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Foey AD, Crawford A and Hall ND:
Modulation of cytokine production by human mononuclear cells
following impairment of Na, K-ATPase activity. Biochim Biophys
Acta. 1355:43–49. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
de Vasconcelos DI, Leite JA, Carneiro LT,
Piuvezam MR, de Lima MR, de Morais LC, Rumjanek VM and
Rodrigues-Mascarenhas S: Anti-inflammatory and antinociceptive
activity of ouabain in mice. Mediators Inflamm. 2011:9129252011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
De Paiva LS, Costa KM, Canto FB, Cabral
VR, Fucs R, Nobrega A and Rumjanek VM: Modulation of mature B cells
in mice following treatment with ouabain. Immunobiology.
216:1038–1043. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Echevarria-Lima J and Rumjanek VM: Effect
of ouabain on the immune system. Curr Hypertens Rev. 2:83–95. 2006.
View Article : Google Scholar
|
|
42
|
De Sá Lima L, Kawamoto EM, Munhoz CD,
Kinoshita PF, Orellana AM, Curi R, Rossoni LV, Avellar MC and
Scavone C: Ouabain activates NFκB through an NMDA signaling pathway
in cultured cerebellar cells. Neuropharmacology. 73:327–336. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vairo G and Hamilton JA: Activation and
proliferation signals in murine macrophages: Stimulation of Na+,
K+-ATPase activity by hemopoietic growth factors and other agents.
J Cell Physiol. 134:13–24. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Teixeira MP and Rumjanek VM: Ouabain
affects the expression of activation markers, cytokine production
and endocytosis of human monocytes. Mediators Inflamm.
2014:7603682014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wimazal F, Germing U, Kundi M, Noesslinger
T, Blum S, Geissler P, Baumgartner C, Pfeilstoecker M, Valent P and
Sperr WR: Evaluation of the prognostic significance of eosinophilia
and basophilia in a larger cohort of patients with myelodysplastic
syndromes. Cancer. 116:2372–2381. 2010.PubMed/NCBI
|
|
46
|
Bishara S, Griffin M, Cargill A, Bali A,
Gore ME, Kaye SB, Shepherd JH and Van Trappen PO: Pre-treatment
white blood cell subtypes as prognostic indicators in ovarian
cancer. Eur J Obstet Gynecol Reprod Biol. 138:71–75. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Delou JM, Lopes AG and Capella M:
Unveiling the role of multidrug resistance proteins in
hypertension. Hypertension. 54:210–216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dostanic I, Paul RJ, Lorenz JN, Theriault
S, Van Huysse JW and Lingrel JB: The alpha2-isoform of Na-K-ATPase
mediates ouabain-induced hypertension in mice and increased
vascular contractility in vitro. Am J Physiol Heart Circ Physiol.
288:H477–H485. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ueda K, Okamura N, Hirai M, Tanigawara Y,
Saeki T, Kioka N, Komano T and Hori R: Human P-glycoprotein
transports cortisol, aldosterone, and dexamethasone, but not
progesterone. J Biol Chem. 267:24248–24252. 1992.PubMed/NCBI
|
|
50
|
Van Helvoort A, Smith AJ, Sprong H,
Fritzsche I, Schinkel AH, Borst P and Van Meer G: MDR1
P-glycoprotein is a lipid translocase of broad specificity, while
MDR3 P-glycoprotein specifically translocates phosphatidylcholine.
Cell. 87:507–517. 1996. View Article : Google Scholar : PubMed/NCBI
|