Introduction
Solanum lyratum (S. lyratum), a common herbal
medicine, is widely used in China for the treatment of malaria,
icterus, cholecystitis, gonorrhea, rheumatoid arthritis, leucorrhea
and cancer (1). Various compounds
including saponins, organic acids, terpenoids, polyphenols,
flavonoids, sterols, coumarins and polysaccharides have been
identified in S. lyratum since the 20th century (2,3). The
anticancer activity of S. lyratum has received attention,
with numerous studies investigating the phenomenon (4–8). Previous
studies indicated that water extract from S. lyratum induces
apoptosis in the human gastric cancer SGC-7901 cell line and HeLa
cells, and the mechanism may be associated with changes in the
expression level and activity of the apoptotic proteins B-cell
lymphoma-extra-large (Bcl-xl), BH3 interacting domain death agonist
(Bid), p53, caspase-3 and caspase-9 (4,5). An
additional study reported that extracts of S. lyratum
induced cytotoxicity and apoptosis of the human colon
adenocarcinoma Colo 205 cell line, which may be associated with
cyclin-dependent kinase 1 (Cdk1), p27, p53, cyclin B1, cyclin E,
caspase-3, caspase-8, procaspase-9, B-cell lymphoma-2 (Bcl-2),
Bcl-2-like protein 4 (Bax) and cytochrome c activity (6). Additionally, there are studies regarding
the cytotoxicity activity of S. lyratum against different
cancer cell lines (7,8). However, the aforementioned studies
mainly focus on the anticancer activity of the crude extracts of
S. lyratum, thus the results cannot explain the active
anticancer constituent of S. lyratum. Thus, an investigation
into the anticancer activity and mechanism of the monomeric
compounds isolated from S. lyratum is required.
Therefore, the presented study explored the
anticancer activity and mechanism of the sesquiterpenoids isolated
from S. lyratum, which may be useful for the interpretation
of the anticancer effect of S. lyratum and the development
of novel therapies from this plant.
Materials and methods
Plant material
S. lyratum Thunb was obtained from
Tongrentang Pharmaceutical Group (Beijing, China) in 2013 and a
voucher specimen (voucher no. YP20130913) was stored in Nanjing
University of Chinese Medicine (Nanjing, China) for future
reference.
Chemicals and reagents
Analytical grade chloroform, ethyl acetate, n-butyl
alcohol, cyclohexane, acetone, methanol and silica-gel were
obtained from Qingdao Haiyang Chemical Co., Ltd. (Qingdao, China)
and Sephadex LH-20 and preparative thin layer chromatography (TLC)
were obtained from H&E Co., Ltd. (Beijing, China). Fetal bovine
serum (FBS) and RPMI-1640 media were purchased from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). The Cell
Counting Kit (CCK)-8 and DAPI were purchased from Beyotime
Institute of Biotechnology (Shanghai, China). Primary antibodies
against Bcl-2 (cat. no. AB112; 1:1,000), survivin (cat. no. AS792;
1:1,000), Bax (cat. no. AB026; 1:500), cleaved-caspase
(c-caspase)-3 (cat. no. AC033; 1:1,000), β-actin (cat. no. AF0003;
1:1,000) and horseradish peroxidase (HRP)-conjugated secondary
antibody (cat. no. A0192; 1:1,000) were purchased from Beyotime
Institute of Biotechnology (Shanghai, China), while antibodies
against second mitochondria-derived activator of caspase (Smac;
cat. no. 15108; 1:1,000) and c-caspase-9 (cat. no. 9501; 1:1,000)
were purchased from Cell Signaling Technology (Beverly, MA, USA).
BeyoECL Plus reagent was purchased from Beyotime Institute of
Biotechnology.
Extraction and isolation
The air-dried whole plant (60 kg) of S.
lyratum was finely cut using scissors and extracted by
refluxing 4 times with 120 l ethyl alcohol. After filtration, the
residues were used for the next extraction. The ethyl alcohol
solvent was evaporated under reduced pressure to produce a crude
extract (4.0 l), which was then suspended in water and successfully
partitioned into chloroform, ethyl acetate and n-butyl alcohol. The
chloroform fraction (238.6 g) was subject to column chromatography
(CC) over silica gel (100–200 mesh) and eluted with
cyclohexane-acetone (95:5-50:50) to produce 8 fractions. Fraction 3
(8.2 g) was separated using CC over silica gel (cyclohexane-ethyl
acetate) to produce compound 1 (58 mg) and a mixture, this was
additionally isolated by preparative thin layer chromatography
(TLC; chloroform-ethyl acetate) and purified on Sephadex LH-20
(chloroform-methanol) producing compounds 2 (123 mg) and 3 (73 mg).
Fraction 4 (25.4 g) was isolated by CC over silica gel
(cyclohexane-ethyl acetate) and preparative TLC
(chloroform-acetone) to provide compounds 4 (186 mg), 5 (92 mg) and
6 (57 mg). Fractions 5 (9.1 g) and 6 (8.4 g) were separated by CC
over silica gel (petroleum ether-acetone) and preparative TLC
(cyclohexane-acetone), and purified on Sephadex LH-20
(chloroform-methanol) to provide compounds 7 (93 mg), 8 (51 mg), 9
(105 mg), 10 (108 mg), 11 (61 mg) and 12 (72 mg). Fractions 2 (3.8
g) and 8 (19.2 g) were isolated by reversed-phase CC over silica
gel (methanol-water) and purified on Sephadex LH-20
(chloroform-methanol) to produce compounds 13 (87 mg), 14 (73 mg)
and 15 (68 mg). A total of 15 compounds were identified by nuclear
magnetic resonance (NMR) data and compared with the existing
literature. The purity of the compounds was verified by high
performance liquid chromatography (HPLC). The HPLC analysis was
carried out on LC-20AT (Shimadzu, Kyoto, Japan), equipped with an
auto sampler, a binary solvent delivery pump and a diode array
detector, using Empower 2 software (Waters, Milford, MA, USA). The
chromatographic separation was carried out on an Ultimate AQ
C18 (4.6×250 mm, 5 µm; Welch, Shanghai, China) at 35°C.
The mobile phase was composed of acetonitrile and water. The
injection volume and the flow rate were 10 µl and 1.0 ml/min,
respectively.
Cell culture
The MCF-7, HCT-8, A-549, SGC-7901 and BEL-7402 cells
were purchased from the American Type Culture Collection (Manassas,
VA, USA). In total, 5 types of cell were separately cultured in
RPMI-1640 medium including 10% FBS, 100 U/ml penicillin and 100
U/ml streptomycin at 37°C in 5% CO2/95% air.
CCK-8 assay
A CCK-8 assay was used to investigate the
cytotoxicity of the 15 sesquiterpenoids against cultured MCF-7,
HCT-8, A-549, SGC-7901 and BEL-7402 cells and the IC50
value, defined as the concentration that led to a 50% decrease in
the number of cells, was used to evaluate their cytotoxicity. Cells
were seeded at a density of 2×104/well into 96-well
plates with RPMI-1640 medium. Subsequent to 24 h incubation, the
cells were treated with different concentrations of fractions and
compounds. A total of 48 h following treatment, CCK-8 solution was
added and the cells were cultured at 37°C in 5% CO2/95%
air for 3 h. The optical density was then determined at 450 nm with
a microplate reader (Model 680; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Apoptosis assay
According to the CCK-8 assay results of the 15
sesquiterpenoids, solajiangxin H and lyratol D, which had high
cytotoxicity against SGC-7901 cells, IC50=4.8 and 5.9
µg/ml, respectively, were selected to study their anticancer
activities and mechanisms. Following treatment with solajiangxin H
and lyratol D at doses of 0 (control), 2 or 4 µg/ml for 48 h, the
SGC-7901 cells were washed 3 times with PBS. The washed SGC-7901
cells were resuspended in staining buffer and stained with DAPI
according to the manufacturer's protocol. Images were then captured
of the SGC-7901 cells using a fluorescence microscope (BX51;
Olympus, Tokyo, Japan).
Western blot assay
Subsequent to treatment with solajiangxin H and
lyratol D at concentrations of 0 (control), 2, 4 and 8 µg/ml for 48
h, respectively, the SGC-7901 cells were collected and their total
proteins were extracted using cell lysis buffer, ultrasound and
centrifugation at 12,000 × g for 15 min at 4°C. The
concentration of total proteins was determined using an enhanced
Bicinchoninic Acid assay kit (Beyotime, Haimen, China) according to
the manufacturer's protocol. Subsequently, ~35 µg total protein was
separated by SDS-PAGE, transferred onto polyvinylidene fluoride
membranes and incubated with the primary antibodies. The protein
was then incubated with HRP-conjugated secondary antibody, and the
anti- and proapoptotic proteins were detected immediately
subsequent to incubation using BeyoECL Plus reagent. In addition,
antibody directed against β-actin was used to assess the level of
protein loading.
Statistical analysis
All data were presented as the mean ± standard
deviation. A one-way analysis of variance using SPSS 21.0 (IBM
SPSS, Armonk, NY, USA) determined whether the results showed
statistical significance between groups, and P<0.05 was
considered to indicate a statistically significant difference.
Results
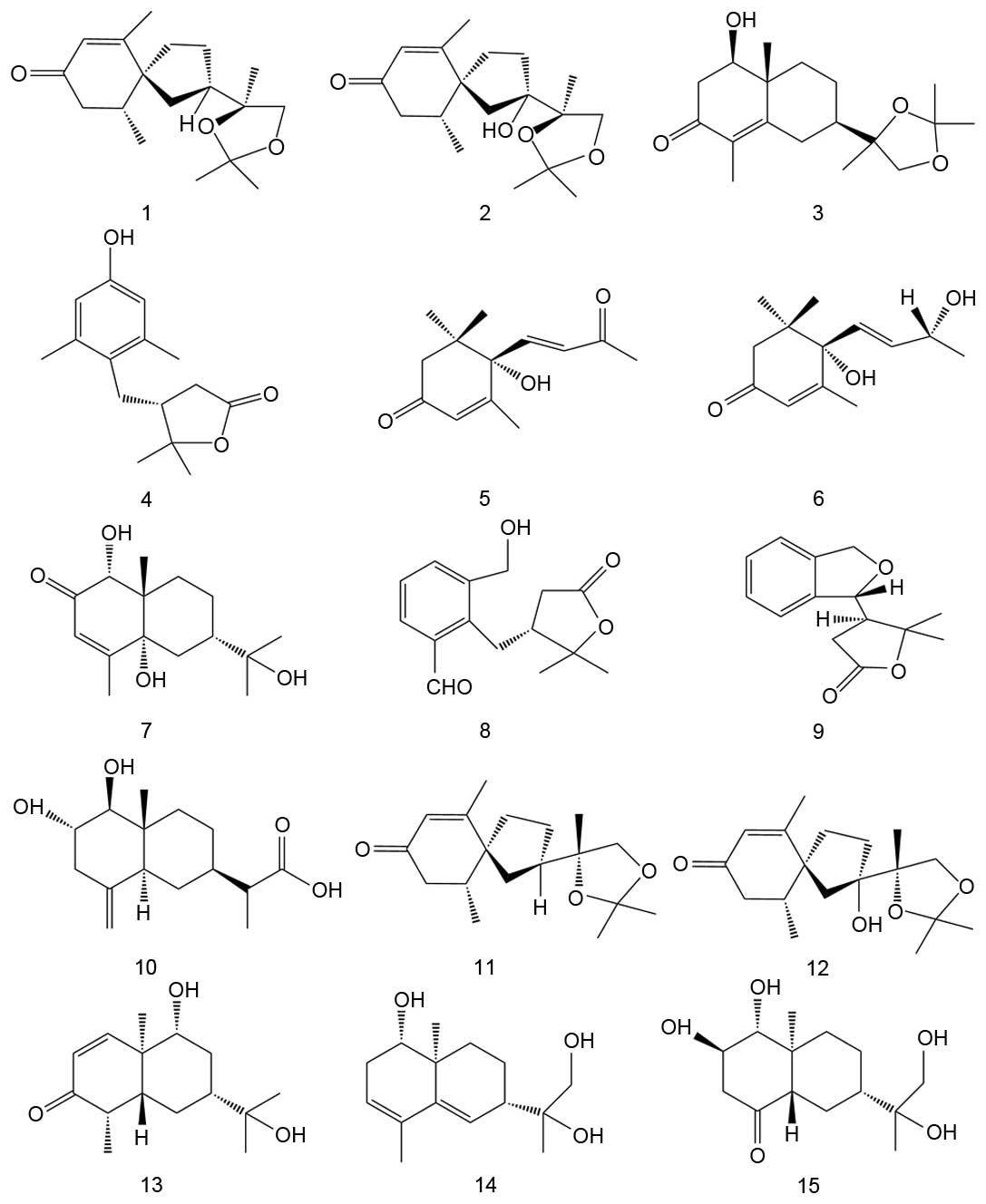
Sesquiterpenoids isolated from S.
lyratum
A total of 15 sesquiterpenoids, including compounds
1 (solajiangxin I, C18H28O3), 2
(7-hydroxylsolajiangxin I,
C18H28O4), 3 (solajiangxin H,
C18H28O4), 4 (lyratol D,
C15H20O3), 5 (dehydrovomifoliol,
C13H18O3), 6 (blumenol A,
C13H20O3), 7 (solajiangxin A,
C15H24O4), 8 (solajiangxin B,
C15H18O4), 9 (solajiangxin C,
C15H18O3), 10 (solajiangxin D,
C15H24O4), 11 (solajiangxin E,
C18H28O3), 12
(2-hydroxysolajiangxin E,
C18H28O4), 13 (lyratol A,
C15H24O3), 14 (lyratol B,
C15H24O3) and 15 (lyratol C,
C15H26O4) were successfully
isolated from S. lyratum, and their chemical structures are
shown in Fig. 1. In addition, the NMR
data are as follows:
Compound 1 (solajiangxin I,
C18H28O3) presented as a colorless
viscous oil; purity: 97.3%; 1H-NMR (400 MHz,
CDCl3) δ: 2.67 (1H, dd, J=13.0, 7.3 Hz, Ha-1),
2.05 (1H, dd, J=13.0, 11.6 Hz, Hb-1), 2.17 (1H, m, H-2),
1.80 (1H, m, Ha-3), 2.02 (1H, m, Hb-3), 1.86 (1H, m, Ha-4), 1.97
(1H, m, Hb-4), 5.98 (1H, s, H-7), 2.68 (1H, dd, J=16.8, 4.8
Hz, Ha-9), 2.61 (1H, dd, J=16.8, 4.3 Hz, Hb-9), 2.28 (1H, m,
H-10), 3.61 (1H, d, J=8.7, Ha-12), 3.74 (1H, d,
J=8.7, Hb-12), 1.31 (3H, s, H-13), 1.90 (3H, s, H-14), 1.13
(3H, d, J=7.1, H-15), 1.50 (3H, s, H-2′), 1.49 (3H, s,
H-3′); 13C-NMR (100 MHz, CDCl3) δ: 35.5
(C-1), 46.3 (C-2), 28.1 (C-3), 33.7 (C-4), 50.6 (C-5), 165.6 (C-6),
124.6 (C-7), 197.6 (C-8), 43.6 (C-9), 39.8 (C-10), 82.1 (C-11),
72.9 (C-12), 24.7 (C-13), 21.6 (C-14), 16.5 (C-15), 109.6 (C-1′),
26.2 (C-2′), 27.7 (C-3′) (9).
Compound 2 (7-hydroxylsolajiangxin I,
C18H28O4) presented as a colorless
viscous oil; purity: 98.1%; 1H-NMR (400 MHz,
DMSO-d6) δ: 1.88 (1H, d, J=13.9 Hz, Ha-1),
1.79 (1H, d, J=13.9 Hz, Hb-1), 1.56 (1H, m, Hb-3), 1.73 (1H,
m, Ha-3), 1.84 (1H, m, Ha-4), 1.98 (1H, m, Hb-4), 5.66 (1H, s,
H-7), 2.78 (1H, dd, J=16.2, 5.0 Hz, Ha-9), 2.21 (1H, dd,
J=16.2, 4.1 Hz, Hb-9), 2.46 (1H, m, H-10), 3.81 (1H, d,
J=8.5, Ha-12), 4.13 (1H, d, J=8.5, Hb-12), 1.51 (3H,
s, H-13), 1.86 (3H, s, H-14), 1.13 (3H, d, J=7.2, H-15),
1.20 (3H, s, H-2′), 1.39 (3H, s, H-3′), 4.67 (1H, s, C2-OH);
13C-NMR (100 MHz, DMSO-d6) δ: 43.2
(C-1), 84.1 (C-2), 36.6 (C-3), 33.8 (C-4), 50.6 (C-5), 166.4 (C-6),
124.8 (C-7), 198.9 (C-8), 43.1 (C-9), 40.3 (C-10), 85.5 (C-11),
72.7 (C-12), 22.3 (C-13), 20.1 (C-14), 16.5 (C-15), 109.6 (C-1′),
26.4 (C-2′), 27.3 (C-3′) (9).
Compound 3 (solajiangxin H,
C18H28O4) presented as a colorless
viscous oil; purity: 95.1%; 1H-NMR (400 MHz,
CDCl3) δ: 3.78 (1H, dd, J=12.4, 5.1 Hz, H-1),
2.47 (1H, dd, J=16.1, 12.3 Hz, Ha-2), 2.57 (1H, dd,
J=16.1, 5.2 Hz, Hb-2), 1.77 (1H, br t, J=14.5 Hz,
Ha-6), 2.97 (1H, br d, J=14.5 Hz, Hb-6), 1.52 (1H, m, H-7),
1.91 (1H, m, Ha-8), 1.43 (1H, m, Hb-8), 1.31 (1H, m, Ha-9), 2.07
(1H, m, Hb-9), 3.70 (1H, d, J=8.3, Ha-12), 3.63 (1H, d,
J=8.3, Hb-12), 1.31 (3H, s, H-13), 1.10 (3H, s, H-14), 1.68
(3H, s, H-15), 1.41 (3H, s, H-2′), 1.46 (3H, s, H-3′);
13C-NMR (100 MHz, CDCl3) δ: 74.1 (C-1), 42.0
(C-2), 197.5 (C-3), 129.4 (C-4), 161.5 (C-5), 29.3 (C-6), 46.4
(C-7), 22.7 (C-8), 37.1 (C-9), 41.9 (C-10), 82.7 (C-11), 72.4
(C-12), 22.6 (C-13), 16.1 (C-14), 11.3 (C-15), 26.6 (C-2′), 27.3
(C-3′) (9).
Compound 4 (lyratol D,
C15H20O3) presented as a pale
yellow lamellar crystal; purity: 98.2%; 1H-NMR (400 MHz,
CDCl3) δ: 2.31 (1H, dd, J=18.3, 3.6, Ha-3), 2.43
(1H, dd, J=18.3, 10.9, Hb-3), 2.54 (1H, m, H-4), 2.43 (1H,
dd, J=14.1, 5.0, Ha-6), 2.79 (1H, dd, J=14.1, 11.3,
Hb-6), 6.43 (1H, s, H-9), 6.43 (1H, s, H-11), 1.46 (3H, s, H-13),
1.59 (3H, s, H-14), 2.56 (3H, s, H-15), 2.26 (3H, s, H-16);
13C-NMR (100 MHz, CDCl3) δ: 175.5 (C-2), 34.1
(C-3), 46.6 (C-4), 86.6 (C-5), 27.3 (C-6), 127.2 (C-7), 137.9
(C-8), 115.4 (C-9), 153.5 (C-10), 115.8 (C-11), 137.8 (C-12), 21.7
(C-13), 27.7 (C-14), 20.3 (C-15), 20.3 (C-16) (10).
Compound 5 (dehydrovomifoliol,
C13H18O3) presented as a pale
yellow oil; purity: 93.0%; 1H-NMR (400 MHz,
CDCl3) δ: 2.37 (1H, d, J=17.0 Hz, H-2a), 2.51
(1H, d, J=17.0 Hz, H-2b), 5.98 (1H, s, H-4), 6.94 (1H, d,
J=15.4 Hz, H-7), 6.44 (1H, d, J=15.4 Hz, H-8), 2.33
(3H, s, H-10), 1.89 (3H, s, H-11), 1.08 (3H, s, H-12), 1.01 (3H, s,
H-13); 13C-NMR (100 MHz, CDCl3) δ: 41.6
(C-1), 49.3 (C-2), 197.3 (C-3), 127.8 (C-4), 160.1 (C-5), 79.5
(C-6), 144.8 (C-7), 130.5 (C-8), 196.8 (C-9), 28.2 (C-10), 18.7
(C-11), 24.5 (C-12), 22.9 (C-13) (11).
Compound 6 (blumenol A,
C13H20O3) presented as a pale
yellow oil; purity: 91.2%; 1H-NMR (400 MHz,
CDCl3) δ: 2.36 (1H, d, J=17.3 Hz, H-2a), 2.57
(1H, d, J=17.3 Hz, H-2b), 5.98 (1H, s, H-4), 5.80 (1H, d,
J=15.5 Hz, H-7), 5.81 (1H, dd, J=15.5, 5.3 Hz, H-8),
4.58 (1H, m, H-9), 1.32 (3H, d, J=6.7, H-10), 1.08 (3H, s,
H-11), 1.05 (3H, s, H-12), 1.85 (3H, s, H-13); 13C-NMR
(100 MHz, CDCl3) δ: 41.6 (C-1), 49.5 (C-2), 198.3 (C-3),
127.4 (C-4), 162.1 (C-5), 78.5 (C-6), 128.6 (C-7), 135.4 (C-8),
68.8 (C-9), 23.2 (C-10), 22.3 (C-11), 23.8 (C-12), 18.9 (C-13)
(11).
Compound 7 (solajiangxin A,
C15H24O4) presented as a white
powder; purity: 917.5%; 1H-NMR (400 MHz,
DMSO-d6) δ: 3.30 (1H, br s, H-1), 5.58 (1H, s,
H-3), 1.67 (1H, dd, J=13.1, 3.7 Hz, Ha-6), 1.14 (1H, dd,
J=13.1, 6.2 Hz, Hb-6), 1.78 (1H, m, H-7), 1.54 (1H, br d,
J=12.6 Hz, Ha-8), 1.46 (1H, m, Hb-8), 2.17 (1H, br t,
J=13.4 Hz, Ha-9), 1.18 (1H, m, Hb-9), 1.17 (3H, s, H-12),
1.19 (3H, s, H-13), 0.86 (3H, s, H-14), 1.90 (3H, s, H-15), 6.61
(1H, br s, C1-OH), 5.32 (1H, s, C5-OH), 4.11 (1H, s, C11-OH);
13C-NMR (100 MHz, CDCl3) δ: 79.9 (C-1), 197.5
(C-2), 122.7 (C-3), 164.5 (C-4), 73.3 (C-5), 29.1 (C-6), 42.3
(C-7), 20.1 (C-8), 28.5 (C-9), 40.7 (C-10), 70.8 (C-11), 27.1
(C-12), 27.5 (C-13), 20.2 (C-14), 19.0 (C-15) (12).
Compound 8 (solajiangxin B,
C15H18O4) presented as a colorless
viscous oil; purity: 90.6%; 1H-NMR (400 MHz,
CDCl3) δ: 2.56 (1H, dd, J=15.6, 11.0 Hz, Ha-3),
2.01 (1H, dd, J=15.6, 6.1 Hz, Hb-3), 2.41 (1H, m, H-4), 3.12
(2H, br d, J=6.6 Hz, H-6), 7.51 (1H, br d, J=7.7 Hz,
H-9), 7.31 (1H, br t, J=7.7 Hz, H-10), 7.63 (1H, br d,
J=7.6 Hz, H-11), 1.39 (3H, s, H-13), 1.54 (3H, s, H-14),
4.70 (2H, s, H-15), 10.19 (1H, s, H-16); 13C-NMR (100
MHz, CDCl3) δ: 175.6 (C-2), 33.6 (C-3), 46.7 (C-4), 87.4
(C-5), 26.3 (C-6), 139.2 (C-7), 134.1 (C-8), 136.5 (C-9), 127.9
(C-10), 134.1 (C-11), 140.3 (C-12), 21.7 (C-13), 26.5 (C-14), 62.0
(C-15), 193.6 (C-16) (12).
Compound 9 (solajiangxin C,
C15H18O3) presented as a colorless
viscous oil; purity: 91.4%; 1H-NMR (400 MHz,
CDCl3) δ: 2.58 (1H, dd, J=17.5, 10.1 Hz, Ha-3),
2.67 (1H, dd, J=17.5, 9.0 Hz, Hb-3), 3.17 (1H, m, H-4), 5.88
(1H, d, J=9.2 Hz, H-6), 7.06 (1H, br d, J=7.6 Hz,
H-9), 7.13 (1H, br t, J=7.6 Hz, H-10), 7.05 (1H, br d,
J=7.6 Hz, H-11), 1.10 (3H, s, H-13), 1.35 (3H, s, H-14),
4.69 (1H, d, J=12.1 Hz, Ha-15), 4.82 (1H, d, J=12.1
Hz, Hb-15), 2.47 (3H, s, H-16); 13C-NMR (100 MHz,
CDCl3) δ: 176.3 (C-2), 32.5 (C-3), 51.4 (C-4), 70.4
(C-5), 79.4 (C-6), 135.0 (C-7), 137.6 (C-8), 132.8 (C-9), 128.7
(C-10), 129.3 (C-11), 138.4 (C-12), 26.8 (C-13), 29.0 (C-14), 64.3
(C-15), 21.0 (C-16) (12).
Compound 10 (solajiangxin D,
C15H24O4) presented as a white
powder; purity: 90.2%; 1H-NMR (400 MHz,
DMSO-d6) δ: 3.17 (1H, d, J=9.2 Hz, H-1),
3.18 (1H, m, H-2), 1.84 (1H, br t, J=12.5 Hz, Ha-3), 2.30
(1H, dd, J=12.5, 5.1 Hz, Hb-3), 1.53 (1H, br d,
J=11.4 Hz, H-5), 1.18 (1H, m, Ha-6), 1.51 (1H, m, Hb-6),
2.01 (1H, m, H-7), 1.17 (1H, m, Ha-8), 1.38 (1H, m, Hb-8), 1.74
(1H, m, Ha-9), 1.26 (1H, m, Hb-9), 1.41 (1H, m, H-11), 1.12 (3H, d,
J=7.1 Hz, H-13), 0.49 (3H, s, H-14), 4.30 (1H, s, Ha-15),
4.70 (1H, s, Hb-15), 5.81 (1H, br s, C1-OH), 4.80 (1H, br s,
C2-OH), 11.35 (1H, br s, COOH); 13C-NMR (100 MHz,
DMSO-d6) δ: 84.3 (C-1), 69.8 (C-2), 43.4 (C-3),
147.5 (C-4), 47.1 (C-5), 27.4 (C-6), 44.2 (C-7), 23.3 (C-8), 36.2
(C-9), 39.1 (C-10), 39.5 (C-11), 177.4 (C-12), 13.9 (C-13), 11.3
(C-14), 107.5 (C-15) (13).
Compound 11 (solajiangxin E,
C18H28O3) presented as a colorless
viscous oil; purity: 94.0%; 1H-NMR (400 MHz,
CDCl3) δ: 1.85 (1H, dd, J=13.1, 7.7 Hz, Ha-1),
1.57 (1H, dd, J=13.1, 11.4 Hz, Hb-1), 2.04 (1H, m, H-2),
1.76 (1H, m, Ha-3), 1.60 (1H, m, Hb-3), 1.61 (1H, m, Ha-4), 1.78
(1H, m, Hb-4), 5.67 (1H, s, H-7), 2.43 (1H, dd, J=16.6, 4.8
Hz, Ha-9), 2.08 (1H, dd, J=16.6, 4.2 Hz, Hb-9), 1.93 (1H, m,
H-10), 3.74 (1H, d, J=8.3 Hz, Ha-12), 3.84 (1H, d,
J=8.3 Hz, Hb-12), 1.32 (3H, s, H-13), 1.94 (3H, s, H-14),
1.00 (3H, d, J=7.1 Hz, H-15), 1.41 (3H, s, H-2′), 1.40 (3H,
s, H-3′); 13C-NMR (100 MHz, CDCl3) δ: 37.1
(C-1), 47.3 (C-2), 28.6 (C-3), 34.1 (C-4), 50.2 (C-5), 166.0 (C-6),
125.8 (C-7), 199.3 (C-8), 43.1 (C-9), 38.6 (C-10), 82.1 (C-11),
73.2 (C-12), 24.8 (C-13), 20.7 (C-14), 15.9 (C-15), 109.1 (C-1′),
26.8 (C-2′), 27.0 (C-3′) (13).
Compound 12 (2-hydroxysolajiangxin E,
C18H28O4) presented as a colorless
viscous oil; purity: 92.3%; 1H-NMR (400 MHz,
DMSO-d6) δ: 2.01 (1H, d, J=14.1 Hz, Ha-1),
1.65 (1H, d, J=14.1 Hz, Hb-1), 1.94 (1H, m, Ha-3), 1.55 (1H,
m, Hb-3), 1.80 (1H, m, Ha-4), 1.76 (1H, m, Hb-4), 5.68 (1H, s,
H-7), 2.48 (1H, dd, J=16.3, 5.0 Hz, Ha-9), 2.13 (1H, dd,
J=16.3, 4.1 Hz, Hb-9), 2.51 (1H, m, H-10), 3.54 (1H, d,
J=8.7 Hz, Ha-12), 4.07 (1H, d, J=8.7 Hz, Hb-12), 1.18
(3H, s, H-13), 1.83 (3H, s, H-14), 0.81 (3H, d, J=7.0 Hz,
H-15), 1.23 (3H, s, H-2′), 1.35 (3H, s, H-3′), 4.49 (1H, s, C1-OH);
13C-NMR (100 MHz, DMSO-d6) δ: 44.3
(C-1), 84.2 (C-2), 35.0 (C-3), 33.8 (C-4), 49.2 (C-5), 166.1 (C-6),
124.7 (C-7), 198.5 (C-8), 43.6 (C-9), 39.4 (C-10), 84.6 (C-11),
72.1 (C-12), 22.8 (C-13), 20.6 (C-14), 15.9 (C-15), 108.4 (C-1′),
27.0 (C-2′), 27.3 (C-3′) (13).
Compound 13 (lyratol A,
C15H24O3) presented as a colorless
viscous oil; purity: 95.1%; 1H-NMR (400 MHz,
CDCl3) δ: 7.17 (1H, d, J=9.6 Hz, H-1), 5.70 (1H,
d, J=9.6, H-2), 2.54 (1H, m, H-4), 1.39 (1H, m, H-5), 1.26
(1H, m, Ha-6), 1.70 (1H, m, Hb-6), 1.45 (1H, m, H-7), 1.91 (1H, m,
Ha-8), 1.33 (1H, m, Hb-8), 3.27 (1H, dd, J=11.0, 4.5 Hz,
H-9), 1.12 (3H, s, H-12), 1.20 (3H, s, H-13), 1.05 (3H, d,
J=6.8 Hz, H-14), 0.96 (3H, s, H-15); 13C-NMR (100
MHz, CDCl3) δ: 156.5 (C-1), 126.4 (C-2), 201.5 (C-3),
41.5 (C-4), 46.8 (C-5), 23.1 (C-6), 46.6 (C-7), 31.0 (C-8), 74.5
(C-9), 41.4 (C-10), 72.1 (C-11), 27.0 (C-12), 26.9 (C-13), 12.5
(C-14), 11.2 (C-15) (14).
Compound 14 (lyratol B,
C15H24O3) presented as a colorless
viscous oil; purity: 94.6%; 1H-NMR (400 MHz,
CDCl3) δ: 3.39 (1H, dd, J=11.2, 4.3 Hz, H-1),
2.26 (1H, m, Ha-2), 2.27 (1H, m, Hb-2), 5.14 (1H, br d,
J=4.6 Hz, H-3), 5.34 (1H, br s, H-6), 2.25 (1H, m, H-7),
1.92 (1H, m, Ha-8), 1.44 (1H, m, Hb-8), 1.12 (1H, m, Ha-9), 2.04
(1H, m, Hb-9), 3.49 (1H, d, J=11.0 Hz, Ha-12), 3.58 (1H, d,
J=11.0 Hz, Ha-12), 1.08 (3H, s, H-13), 1.89 (3H, s, H-14),
0.99 (3H, s, H-15); 13C-NMR (100 MHz, CDCl3)
δ: 75.1 (C-1), 31.5 (C-2), 122.4 (C-3), 131.3 (C-4), 143.4 (C-5),
121.1 (C-6), 43.8 (C-7), 19.0 (C-8), 33.8 (C-9), 37.5 (C-10), 74.6
(C-11), 67.3 (C-12), 21.4 (C-13), 19.6 (C-14), 16.3 (C-15)
(14).
Compound 15 (lyratol C,
C15H26O4) presented as a colorless
prismatic crystal; purity: 92.5%; 1H-NMR (400 MHz,
C5D5N) δ: 3.44 (1H, d, J=9.1 Hz, H-1),
4.00 (1H, m, H-2), 3.04 (1H, dd, J=12.4, 5.5 Hz, Ha-3), 2.55
(1H, dd, J=12.4, 11.7 Hz, Hb-3), 1.83 (1H, dd,
J=10.3, 3.6 Hz, H-5), 1.64 (1H, m, Ha-6), 1.88 (1H, m,
Hb-6), 2.21 (1H, m, H-7), 1.53 (1H, m, Ha-8), 2.04 (1H, m, Hb-8),
2.73 (1H, m, Ha-9), 1.24 (1H, m, Ha-9), 4.10 (1H, d, J=10.8
Hz, Ha-12), 3.83 (1H, d, J=10.8 Hz, Hb-12), 1.50 (3H, s,
H-13), 1.28 (3H, s, H-14), 4.83 (1H, s, Ha-15), 4.94 (1H, s,
Hb-15); 13C-NMR (100 MHz, C5D5N)
δ: 84.3 (C-1), 71.9 (C-2), 44.0 (C-3), 148.5 (C-4), 48.9 (C-5),
25.0 (C-6), 45.6 (C-7), 21.8 (C-8), 38.7 (C-9), 39.8 (C-10), 74.6
(C-11), 68.7 (C-12), 21.6 (C-13), 11.5 (C-14), 107.6 (C-15)
(2).
Cytotoxic potential of
sesquiterpenoids from S. lyratum
The anti-proliferation effect of sesquiterpenoids
on human breast adenocarcinoma MCF-7, human intestinal
adenocarcinoma HCT-8, human lung carcinoma A-549, human gastric
cancer SGC-7901 and human hepatocarcinoma BEL-7402 cells were
studied using a CCK-8 assay, which has commonly been used to
investigate the cytotoxicity of compounds (15). The results are presented in Table I. The IC50 values of the 15
sesquiterpenoids identified were between 4.8 and 56.4 µg/ml.
Compounds 3 (solajiangxin H) and 4 (lyratol D) had high
cytotoxicity against SGC-7901 cells (IC50=4.8 and 5.9
µg/ml, respectively), so they were selected for additional study
into their anticancer mechanisms by DAPI staining and western blot
analysis.
 | Table I.Cytotoxicity of 15 sesquiterpenoids
against cultured MCF-7, HCT-8, A-549, SGC-7901 and BEL-7402
cells. |
Table I.
Cytotoxicity of 15 sesquiterpenoids
against cultured MCF-7, HCT-8, A-549, SGC-7901 and BEL-7402
cells.
|
|
| IC50
(µg/ml) |
|---|
|
|
|
|
|---|
| No. | Compound | MCF-7 | HCT-8 | A-549 | SGC-7901 | BEL-7402 |
|---|
| 1 | Solajiangxin I | 18.9±0.8 | 23.8±1.3 | 17.6±0.7 | 18.6±0.7 | 23.5±3.1 |
| 2 |
7-Hydroxylsolajiangxin I | 43.1±1.3 | 21.3±1.4 | 16.4±1.0 | 19.3±1.1 | 32.1±0.9 |
| 3 | Solajiangxin H | 15.4±0.9 | 19.6±2.3 | 28.3±0.5 | 4.8±0.3 | 18.4±1.4 |
| 4 | Lyratol D | 33.6±1.8 | 18.5±0.7 | 19.3±0.8 | 5.9±0.2 | 41.5±0.8 |
| 5 |
Dehydrovomifoliol | 19.3±2.0 | 22.4±1.0 | 20.3±1.2 | 10.5±1.4 | 51.0±1.2 |
| 6 | Blumenol A | 56.4±0.7 | 16.5±4.1 | 28.2±1.1 | 13.5±1.6 | 25.4±0.5 |
| 7 | Solajiangxin A | 38.9±1.5 | 40.1±5.4 | 30.3±1.8 | 18.9±2.4 | 41.2±1.3 |
| 8 | Solajiangxin B | 28.6±2.9 | 23.6±2.6 | 18.2±0.9 | 21.3±2.5 | 32.5±2.4 |
| 9 | Solajiangxin C | 35.1±1.5 | 27.3±3.8 | 25.8±2.1 | 18.4±3.0 | 12.5±0.4 |
| 10 | Solajiangxin D | 23.4±1.6 | 18.6±1.1 | 29.2±0.9 | 14.1±1.5 | 31.4±1.7 |
| 11 | Solajiangxin E | 31.2±2.3 | 25.7±2.4 | 17.8±1.1 | 15.3±2.6 | 28.9±0.7 |
| 12 |
2-Hydroxysolajiangxin E | 26.3±0.7 | 18.4±2.9 | 16.9±1.6 | 12.4±1.8 | 25.4±1.6 |
| 13 | Lyratol A | 31.5±1.4 | 18.4±0.9 | 11.3±0.5 | 9.6±1.4 | 25.7±2.4 |
| 14 | Lyratol B | 42.8±1.8 | 15.1±1.2 | 12.4±0.8 | 8.7±1.0 | 18.9±3.1 |
| 15 | Lyratol C | 35.8±2.1 | 23.4±1.5 | 13.6±3.2 | 11.3±2.3 | 31.2±0.8 |
Effect of solajiangxin H and lyratol D
on apoptosis in SGC-7901 cells
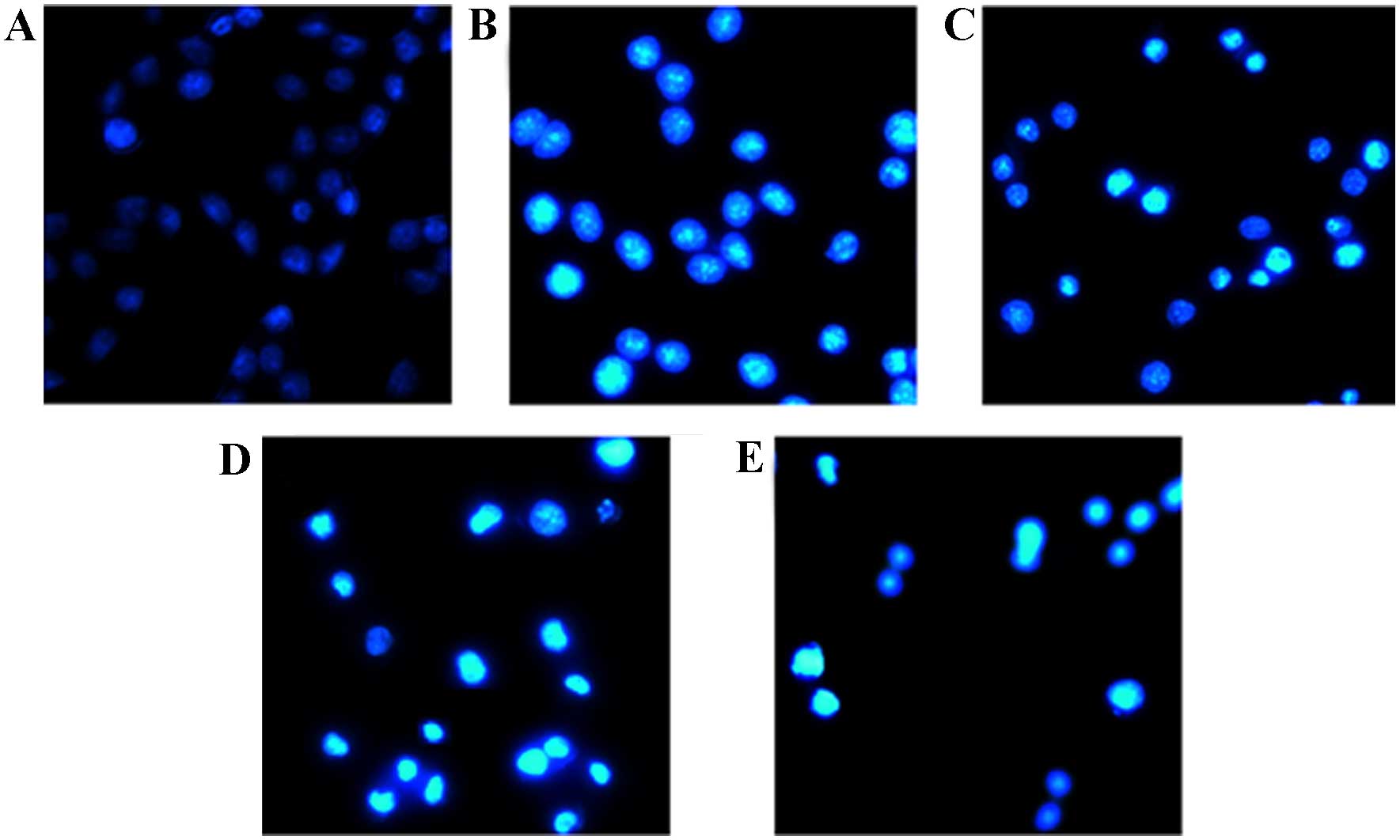
DAPI staining (16)
was used to study whether the cytotoxic effects of solajiangxin H
and lyratol D on SGC-7901 cells were associated with apoptosis. As
depicted in Fig. 2, subsequent to
treatment with solajiangxin H and lyratol D at doses of 0
(control), 2 and 4 µg/ml for 48 h, respectively, evident
condensation of the nucleus of the SGC-7901 cells was induced,
which can be regarded as a characteristic marker of apoptosis.
Therefore, the cytotoxic effects of solajiangxin H and lyratol D on
SGC-7901 cells were associated with apoptosis.
Effect of solajiangxin H and lyratol D
on apoptotic proteins in SGC-7901 cells
A western blot assay was used to study the
mechanism of apoptosis induced by solajiangxin H and lyratol D. The
present study examined the expression changes of apoptotic
proteins, including Bcl-2, survivin, Bax, Smac, c-caspase-3 and
c-caspase-9 subsequent to treatment with solajiangxin H or lyratol
D at concentrations of 2, 4 or 8 µg/ml for 48 h, respectively. As
Figs. 3 and 4 demonstrate, the results indicated that
solajiangxin H and lyratol D may significantly (P<0.01)
downregulate the expression of the antiapoptotic proteins Bcl-2 and
survivin, and upregulate the expression of the proapoptotic
proteins Bax, Smac, c-caspase-3 and c-caspase-9 (P<0.01), in a
dose-dependent manner (17).
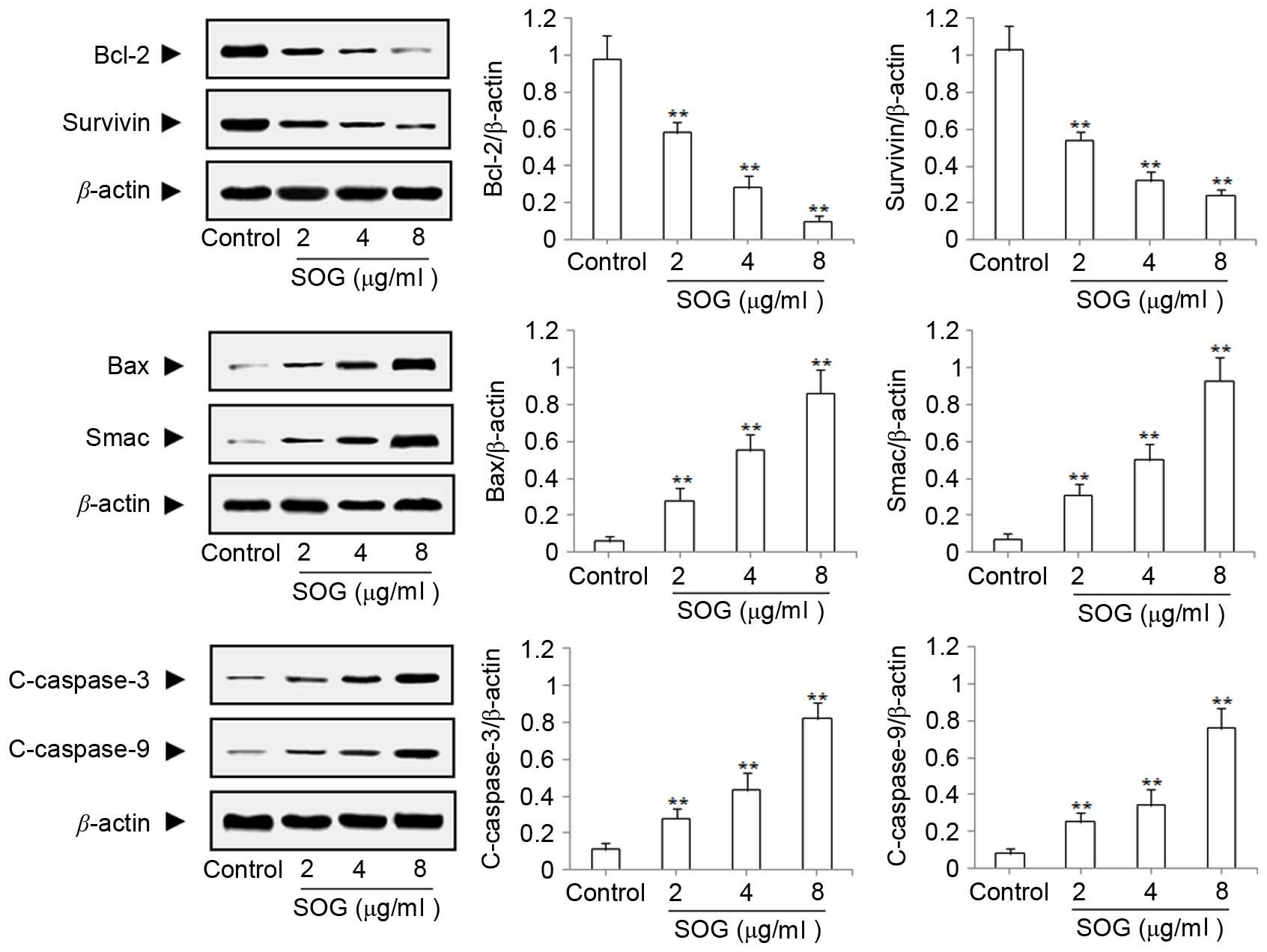 | Figure 3.Effects of SOG on the antiapoptotic
proteins Bcl-2 and survivin and the proapoptotic proteins Bax,
Smac, c-caspase-3 and c-caspase-9 in SGC-7901 cells. SGC-7901 cells
were treated with SOG at concentrations of 0 (control), 2, 4 and 8
µg/ml for 48 h, and their total proteins were analyzed by western
blot assay. **P<0.01, compared with control. SOG, solajiangxin
H; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-like protein 4; Smac,
second mitochondria-derived activator of caspase; c-caspase,
cleaved caspase. |
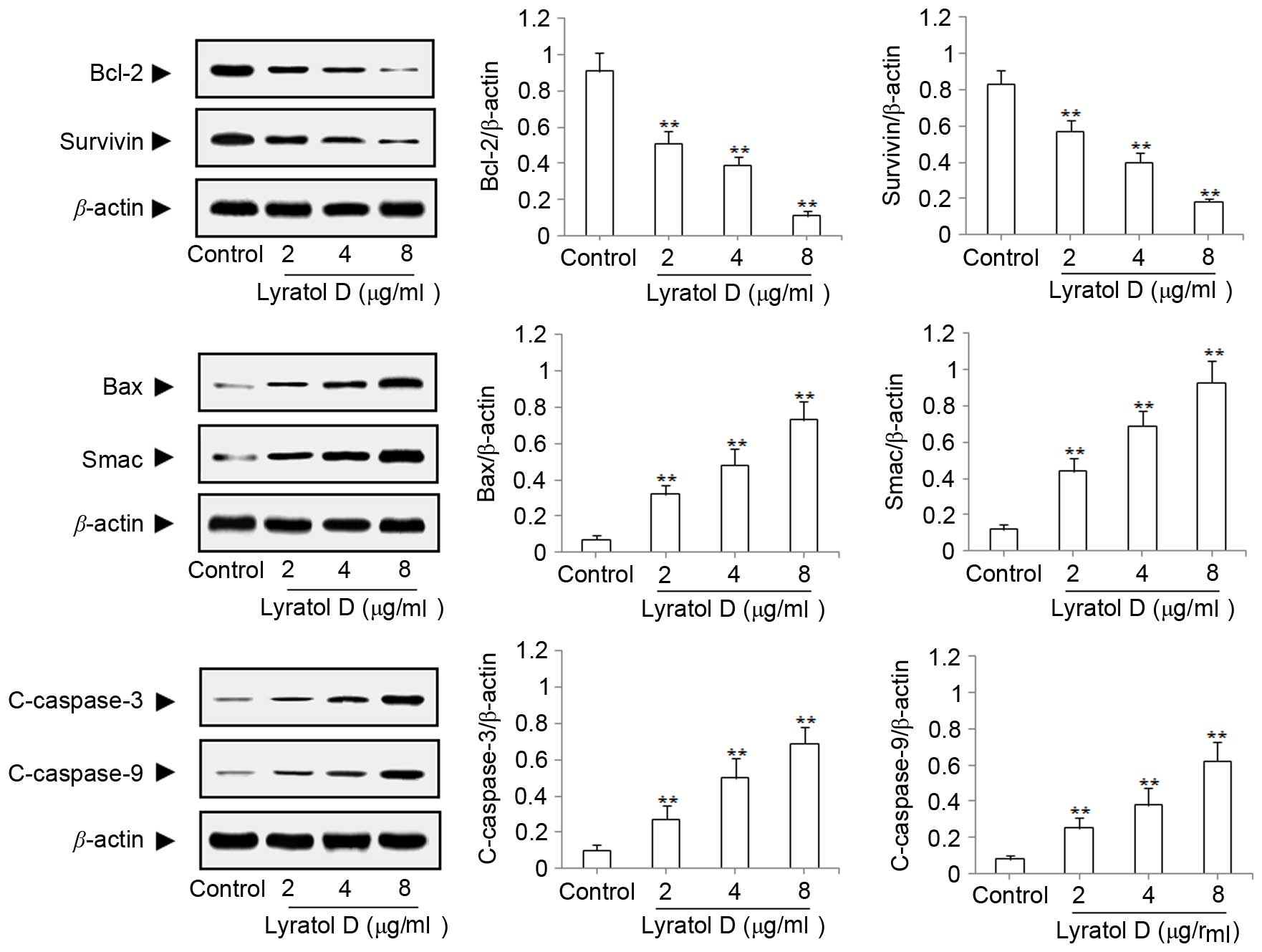 | Figure 4.Effects of lyratol D on the
antiapoptotic proteins Bcl-2 and survivin, and the proapoptotic
proteins Bax, Smac, c-caspase-3 and c-caspase-9 in SGC-7901 cells.
The SGC-7901 cells were treated with lyratol D at concentrations of
0 (control), 2, 4 and 8 µg/ml for 48 h, respectively, and total
protein content was analyzed by western blot assay. **P<0.01,
compared with control. Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-like
protein 4; Smac, second mitochondria-derived activator of caspase;
c-caspase, cleaved caspase. |
Discussion
In the present study, 15 sesquiterpenoids were
isolated from S. lyratum, and their antiproliferation
effects on MCF-7, HCT-8, A-549, SGC-7901 and BEL-7402 cell lines
were investigated using a CCK-8 assay. According to the results of
CCK-8 assay, solajiangxin H and lyratol D, which had strong
cytotoxicity against SGC-7901 cells (IC50=4.8 and 5.9
µg/ml, respectively), were selected to study their anticancer
mechanisms by DAPI staining and western blot assay. These
techniques demonstrated that the cytotoxicity of solajiangxin H and
lyratol D against SGC-7901 cells was associated with apoptosis.
Previous studies revealed that apoptotic proteins,
including Bcl-2, survivin, Bax, Smac, caspase-3 and caspase-9,
serve important roles in mitochondria-mediated apoptosis (17,18). The
associations between the proteins are complex. For example, the
antiapoptotic Bcl-2 protein can suppress the release of the
proapoptotic Smac protein from the mitochondria to the cytoplasm,
but the proapoptotic Bax protein can inhibit the activity of Bcl-2
protein (19,20). The active form of caspase-3,
c-caspase-3, is an important signal for apoptosis, and the
activation of caspase-3 is associated with the activation of
caspase-9 into c-caspase-9 (21). The
antiapoptotic survivin protein can inhibit the c-caspase-9 to
activate caspases-3, but Smac can inhibit the activity of survivin
(22). Thus, changes in expression
level and activity of the apoptotic proteins Bcl-2, survivin, Bax,
Smac, caspase-3 and caspase-9 are directly associated with
apoptosis. In the present study, changes in the expression levels
of apoptotic proteins, including Bcl-2, survivin, Bax, Smac,
c-caspase-3 and c-caspase-9, subsequent to treatment with
solajiangxin H and lyratol D was investigated. The results
indicated that solajiangxin H and lyratol D significantly
downregulates the expression of the antiapoptotic proteins Bcl-2
and survivin, and upregulates the expression levels of the
proapoptotic proteins Bax, Smac, c-caspase-3 and c-caspase-9 in
dose-dependent manners.
In conclusion, the present study demonstrated that
15 sesquiterpenoids isolated from S. lyratum exhibit
cytotoxicity against MCF-7, HCT-8, A-549, SGC-7901 and BEL-7402
cells. The results of studies on the anticancer mechanisms of
solajiangxin H and lyratol D in SGC-7901 cells indicated that the
anticancer mechanisms may be associated with mitochondria-mediated
apoptosis. However, additional studies on mechanisms of these
sesquiterpenoids are required as they may be candidates for
treatment of cancer.
Acknowledgements
The present study was supported by the Foundation
of the Priority Academic Program Development of Jiangsu Higher
Education Institutions and the National Natural Science Foundation
of China (grant no. 81202954).
References
|
1
|
Sun LX, Bi KS and Wang MW: Progress in the
studies of Solanum lyratum Thunb. J Shenyang Pharm Univ.
23:251–255. 2006.
|
|
2
|
Ren Y, Zhang DW and Dai SJ: Chemical
constituents from Solanum lyratum. Chin J Nat Med. 7:203–205. 2009.
View Article : Google Scholar
|
|
3
|
Yang L, Feng F and Gao Y: Chemical
constituents from herb of Solanum lyratum. Zhongguo Zhong Yao Za
Zhi. 34:1805–1808. 2009.(In Chinese). PubMed/NCBI
|
|
4
|
Wan FS, Wu J, Li H, Tu S and Yu LH: Study
on apoptosis of human stomach SGC-7901 cells induced by extracts of
Solanum lyratum. Zhong Yao Cai. 32:245–249. 2009.(In Chinese).
PubMed/NCBI
|
|
5
|
Yu LH, Xu BH, Wu J, Li H, Tu S and Wan FS:
Influence of Solanum lyratum Thunb etract on apoptosis and the
expression of bcl-xl/bid genes in human stomach cancer SGC-7901
cells. Chin Tradit Pat Med. 30:1744–1748. 2008.
|
|
6
|
Hsu SC, Lu JH, Kuo CL, Yang JS, Lin MW,
Chen GW, Su CC, Lu HF and Chung JG: Crude extracts of Solanum
lyratum induced cytotoxicity and apoptosis in a human colon
adenocarcinoma cell line (Colo 205). Anticancer Res. 28:1045–1054.
2008.PubMed/NCBI
|
|
7
|
Ren J, Feng GN, Wang MW and Sun LX: The
primary study on the anti-tumor effect of tatal saponin of Solanum
lyratum Thunb. Cance Res Prevent Treat. 33:262–264. 2006.(In
Chinese).
|
|
8
|
Sun LX, Ren J, Wang MW and Bi KS:
Preliminary study on the anti-tumor effect of the extracts of
Solanum lyratum Thunb. J Shenyang Pharm Univ. 22:210–212. 2005.
|
|
9
|
Li GS, Yao F, Zhang L, Yue XD and Dai SJ:
New sesquiterpenoids derivatives from Solanum lyratum and their
cytotoxicies. J Asian Nat Prod Res. 16:129–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ren Y, Shen L, Zhang DW and Dai SJ: Two
new sesquiterpenoids from Solanum lyratum with cytotoxic
activities. Chem Pharm Bull (Tokyo). 57:408–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yue XD, Yue XD, Yao F, Zhang L, Li GS and
Dai SJ: Sesquiterpenoids from Solanum lyratum. Zhongguo Zhong Yao
Za Zhi. 39:453–456. 2014.(In Chinese). PubMed/NCBI
|
|
12
|
Yao F, Song QL, Zhang L, Li GS and Dai SJ:
Solajiangxins A-C, three new cytotoxic sesquiterpenoids from
Solanum lyratum. Fitoterapia. 89:200–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yao F, Song QL, Zhang L, Li GS and Dai SJ:
Three new cytotoxic sesquiterpenoids from Solanum lyratum.
Phytochem Lett. 6:453–456. 2013. View Article : Google Scholar
|
|
14
|
Dai SJ, Shen L and Ren Y: Two new
eudesmane-type sesquiterpenoids from Olanum lyratum. Nat Prod Res.
23:1196–1200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie B, Zhou JP, Shu GS, Liu DC, Zhou J,
Chen J and Yuan L: Restoration of klotho gene expression induces
apoptosis and autophagy in gastric cancer cells: Tumor suppressive
role of klotho in gastric cancer. Cancer Cell Int. 13:182013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baharara J, Namvar F, Ramezani T, Mousavi
M and Mohamad R: Silver nanoparticles biosynthesized using Achillea
biebersteinii flower extract: Apoptosis induction in MCF-7 cells
via caspase activation and regulation of Bax and Bcl-2 gene
expression. Molecules. 20:2693–2706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng W, Wu JG, Jiang YB, Liu YJ, Sun T, Wu
N and Wu CJ: Antitumor activity of
4-O-(2′-O-acetyl-6′-O-p-coumaroyl-β-D-glucopyranosyl)-p-coumaric
acid against lung cancers via mitochondrial-mediated apoptosis.
Chem Biol Interact. 233:8–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi YG: A structural view of
mitochondria-mediated apoptosis. Nat Struct Biol. 8:394–401. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng YB, Lin YH and Wu XW: TRAIL-induced
apoptosis requires Bax-dependent mitochondrial release of
Smac/DIABLO. Genes Dev. 16:33–45. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Du C, Fang M, Li Y, Li L and Wang X: Smac,
a mitochondrial protein that promotes cytochrome c-dependent
caspase activation by eliminating IAP inhibition. Cell. 102:33–42.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang XJ, Mei WL, Tan GH, Wang CC, Zhou
SL, Huang FR, Chen B, Dai HF and Huang FY: Strophalloside induces
apoptosis of SGC-7901 cells through the mitochondrion-dependent
caspase-3 pathway. Molecules. 20:5741–5728. 2015.
|
|
22
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, surviving, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|