Introduction
Ovarian cancer is the most lethal gynecological
cancer, and the 5-year survival rate of patients is only 30–50%
(1). Failure in early diagnosis and
ineffective treatment are the major causes of mortality. Therefore,
recent research has been focused on the identification of new
methods of early diagnosis and novel strategies for therapy
(1).
Hyaluronan (HA) is a macromolecular
glycosaminoglycan, which is the major component of the
extracellular matrix and intercellular substance. HA serves
essential roles due to its viscoelasticity, hydrability and
interaction with its receptors. The presence and absence of HA
binding proteins and whether HA is connected with its cell surface
receptors decide its function. Increased HA was able to strengthen
the capability of invasion, metastasis and proliferation in ovarian
tumor models (2,3). Cluster of differentiation (CD)44 is a
cell surface transmembrane glycoprotein, which exhibits two forms:
i) Standard CD44 (CD44S, also named CD44H), which is mainly
expressed on the surface of stromal and hematopoietic cells; and
ii) variant CD44 (CD44V), which is mainly expressed on the surface
of epithelial cells and tumors (4).
Ovarian cancers express both CD44S and CD44V. CD44 is expressed in
a large number of cell types, including adhesion of cells and
stromal components, lymphocyte homing, activation of T lymphocyte
cells and signal transduction. Both CD44V and CD44S have been
reported to be important in the development and metastasis of
ovarian cancer and other gynecological tumors (4). The binding of HA to CD44 can initiate
several different signaling pathways, including human epidermal
growth factor receptor 2, c-Src kinase and extracellular
signal-regulated kinase (5–12). The activation of these pathways may
lead to increased motility, adhesion and invasion as well as tumor
growth in ovarian cancer (3,11,12). In
summary, HA and its receptor CD44 are considered to be valid
targets for the treatment of various types of cancer.
Several studies on the treatment efficacy and
cytotoxic effect of chemotherapeutic drugs have been conducted,
including paclitaxel, cisplatin and polyethylenimine (PEI) DNA
particles, which suggested that HA-conjugation could be utilized as
tumor-targeted therapy (13–15). Besides chemotherapy, numerous scholars
have paid attention to biotherapy, including hyaluronidase enzymes,
PH20 and pegylated recombinant human hyaluronidase PH20 (16,17).
Methods used to block the action of CD44 include neutralizing
antibodies, small interfering RNA, antisense RNA, complementary DNA
vaccination as well as the CD44 inhibitor silibinin. These methods
have shown therapeutic effect, although their toxicity could not be
ignored (17). Non-toxic alternative
therapies remain to be further investigated.
Nano-particles are solid colloidal particles with a
diameter of 10–500 nm, which is much smaller than that of a cell
(10–1,000 nm). Due to this great advantage, these particles are
easily absorbed by tissues and cells. As a new form of drug
delivery and release, nano-particles appear to be promising, and
have been widely studied in recent years (18). Chitosan, as a new type of
nano-particle, has been reported to be relatively good in
bioadhesivity and promotion of absorption as well as inhibition of
enzymes (18). The IM7 antibody is
one of the most important anti-CD44 monoclonal antibodies in
mediating cell-cell and cell-matrix interactions through its
affinity for HA. IM7 has been used to treat cancers; however, its
major disadvantages are its toxicity and unsustainable stimulating
effect (4).
In the present study, a new carrier system composed
of chitosan nano-particles coated with polylactic acid (PLA)
(termed PLA-chitosan-IM7) has been developed to carry IM7. The
advantages and disadvantages of treating CD44-positive cancers
in vitro and in vivo with PLA-chitosan-IM7 were
evaluated, and the results obtained may provide a new approach for
cancer treatment.
Materials and methods
Preparation of IM7 loaded with
chitosan nano-particles
An ionic crosslinking method was used to prepare
nano-particles according the method of Bodmeier (16). First, 200 µl IM7 (Cat#ab171211; Abcam,
Cambridge, UK) were added to 4 ml thiamine pyrophosphate (TPP)
solution (Cat#C8754; Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) at 1 mg/ml (pH 7–9), and then the mixture was added to 10
ml chitosan solution (Cat#740,500; Sigma-Aldrich; Merck Millipore)
(pH 4–6) at a constant rotating speed, and incubated for 10 min at
57°C. Due to molecular linkage between TPP and chitosan, the
nano-particles were prepared when the color of the solution became
homogeneously light blue. The size and zeta potential of the
nano-particles was determined with a transmission electron
microscope (TEM).
Surface coverage of chitosan
nano-particles with PLA
1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide
hydrochloride (EDC)/N-hydroxysuccinimide (NHS) was used to coat the
chitosan nano-particles with PLA (Sigma-Aldrich; Merck Millipore).
First, 0.4 mg EDC (final concentration, 2 mM) (Cat#22,890; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and 0.6 mg NHS (final
concentration, 5 mM) (Cat#24,500; Thermo Fisher Scientific, Inc.)
were added to 1 ml PLA, and then the mixture was added to the
nano-particles solution. The reaction components were mixed
thoroughly and allowed to react for 15 min at room temperature.
Investigation of drug loading rate and
stability
An spectrophotometer was used to detect the optical
density (OD) of IM7 prior and subsequent to being loaded with
nano-particles, and the loading rate was calculated as follows:
Drug loading rate = amount of doxorubicin (Dox; Sigma-Aldrich;
Merck Millipore) encapsulated / total weight of nano-particles. In
addition, the release rate of PLA-chitosan-IM7 was observed for 0,
1, 2, 5, 6 and 7 days at neutral (pH 7.4) and acidic (pH 5.0)
environments, and was calculated as follows: Release rate = amount
of Dox encapsulated / Total Dox added.
Anti-proliferative effect of
PLA-chitosan-IM7 on an ovarian cancer line
The human ovarian cancer cell line HO-8910PM was
purchased from Peking Union Medical College (Beijing, China) and
cultured with RPMI 1640 medium with 10% fetal bovine serum (Thermo
Fisher Scientific, Inc.) and 1% penicillin/streptomycin. Then, MTT
assay was used to observe the suppressing effect of
PLA-chitosan-IM7 on HO-8910M cells. Briefly, HO-8910PM cells were
cultured for 24 h and then divided into three groups: i) Control
group without any treatment; ii) IM7 group treated with IM7 (final
concentration, 20 ng/ml); and iii) PLA group treated with
PLA-chitosan-IM7 (final concentration of IM7, 20 ng/ml). The
stimulation time was 0, 12, 24, 36, 48 and 72 h. Lastly, MTT assay
(Cat#30-1010K; American Type Culture Collection, Manassas, VA, USA)
was employed to observe the viability and proliferation of
HO-8910PM as follows: Each group was adjusted to a density of
2×105 cells/ml and plated into 96-well culture plates.
The plates were incubated for 6 h, and 10 µl MTT reagent was added
until a purple precipitate was visible. Next, 10 µl detergent
reagent (dimethyl sulfoxide) was added and incubated at room
temperature in the dark for 2 h. The optical density was measured
at 570 nm using a spectrophotometer.
Animal studies
In total, 15 female BALB/c nude mice (6–8 weeks old;
body weight, 20–22 g) were obtained from Vital River (Beijing,
China) and housed under pathogen-free conditions with a 12 h
light-dark cycle. Food and water were provided ad libitum
throughout the study. The mice were subcutaneously injected with
2×106 HO-8910PM cells for 72 h to successfully establish
an ovarian mouse model. The 30 mice with ovarian cancer were
divided into three groups: i) PLA group, which was treated with
PLA-chitosan-IM7 at 100 ng/g; ii) IM7 group, which was treated with
IM7 antibody at 100 ng/g; and iii) positive control group, which
was treated with Dox at 50 ng/g. Animal models of in vivo
fluorescence were established according to the protocol of
D-luciferin (Cat#LUCNA; Gold BioTechnology, Inc., St. Louis, MO,
USA). Each group received 15 mg/ml D-luciferin, and an in
vivo imaging system (Xenogen IVIS spectrum; Caliper Life
Sciences, Hopkinton, MA, USA) was used to evaluate the targeting
specificity and treatment efficacy of IM7 loaded with PLA-chitosan
nano-particles. In addition, when the mean volumes of tumors were
between 150 and 200 mm3, mice were randomly divided in
three groups (10 mice/group) prior to treatment. The tumor volume
and body weight in each group were matched, at 180 ± 10
mm3 and 20 ± 2 g, respectively.
A total of 35 days subsequent to PLA-chitosan-IM7
treatment, all mice were sacrificed by CO2, and the
tumors were removed and weighted. All the animals experiments
conducted in the present study were approved by the Animal Ethics
Committee of General Hospital of PLA (Beijing, China).
Statistical analysis
All data were analyzed with SPSS 12.0 software (SPSS
Inc., Chicago, IL, USA). Statistical analysis was performed using
Student's t-test and P<0.05 was considered to indicate a
statistically significant difference.
Results
Preparation of PLA-chitosan-IM7
nano-particles
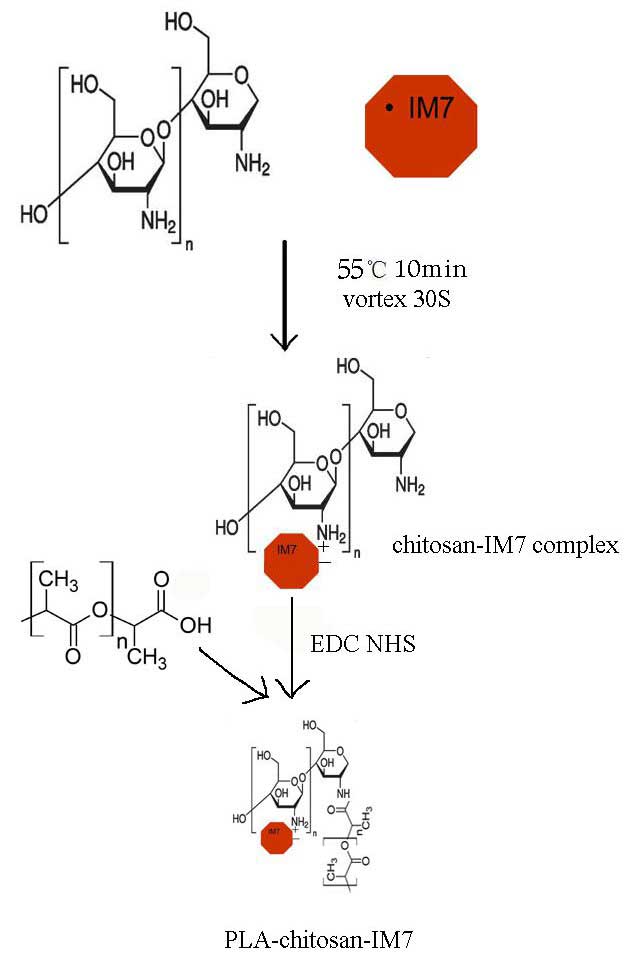
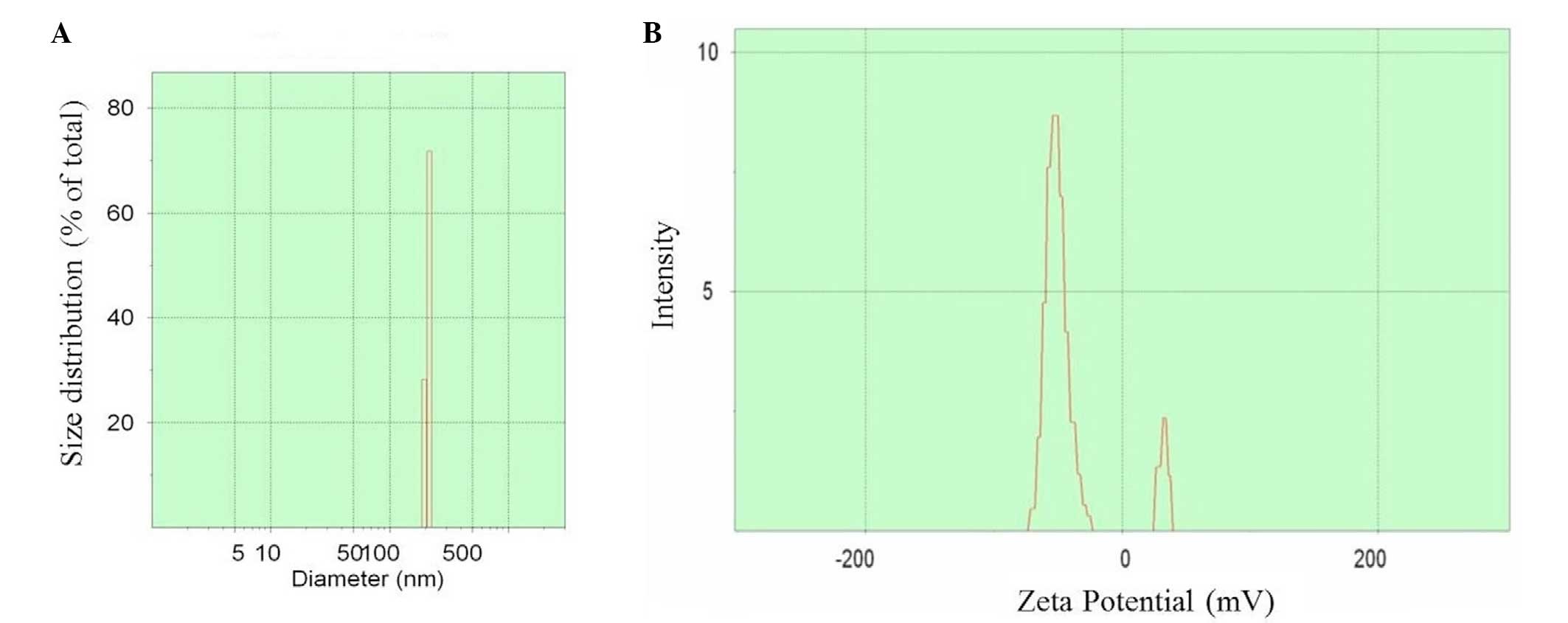
The preparation process is shown in Fig. 1. Upon preparation, TEM was used to
observe the size and zeta potential of the nano-particles, and it
was noticed t§hat the diameter was 100–500 nm (mean value, 350 nm)
(Fig. 2A) and the zeta potential
varied between −75 and +45 mV (Fig.
2B).
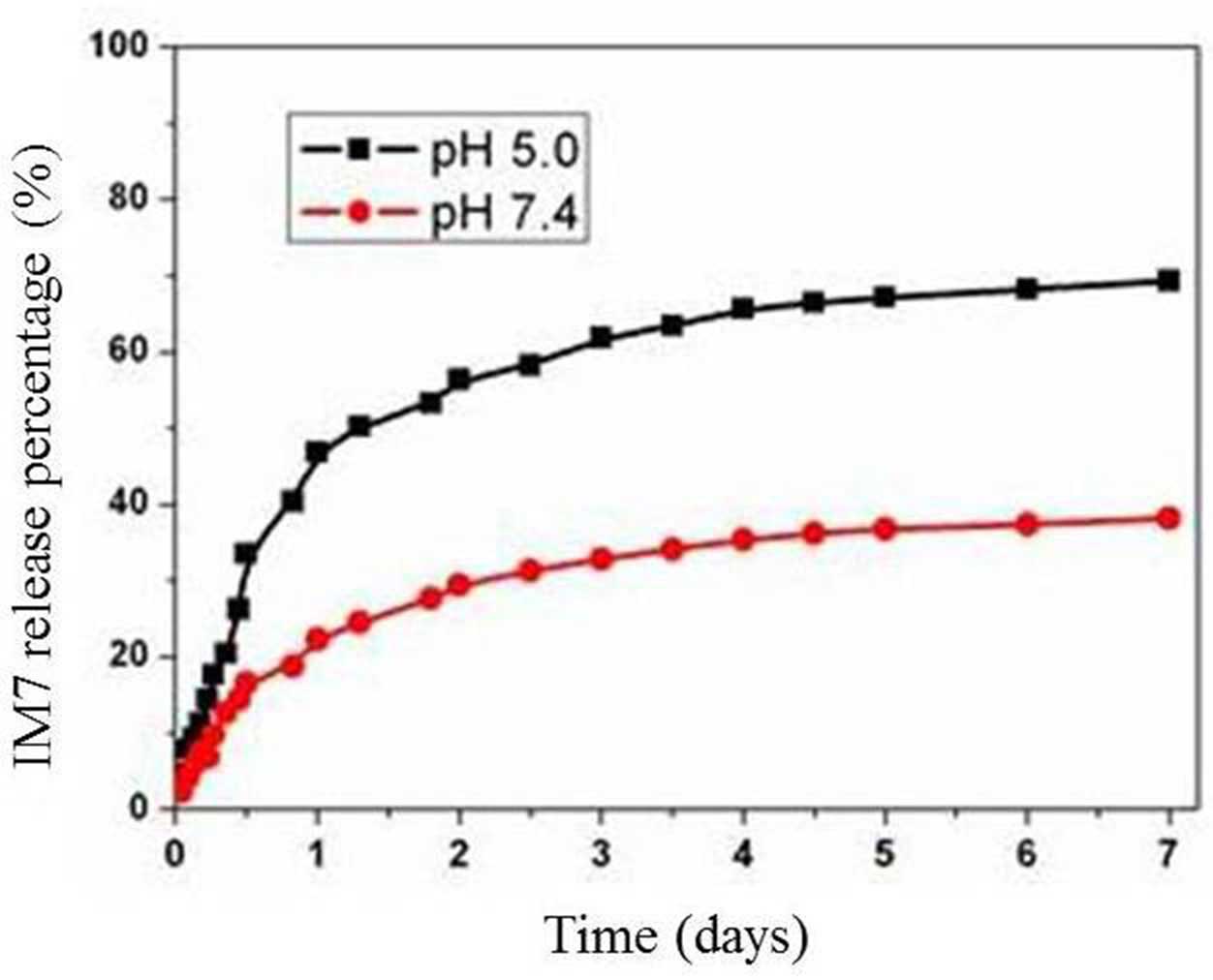
Determination of the loading rate and
stability of PLA-chitosan-IM7
Spectrophotometry was used to determine the OD of
IM7 prior and subsequent to being loaded, and the loading rate was
calculated, which was 52%. In addition, the stability of
PLA-chitosan-IM7 was observed for 0, 1, 2, 5, 6 and 7 days at
neutral (pH 7.4) and acidic (pH 5.0) environments. The results
indicated that the release of PLA-chitosan-IM7 in acidic
environments was slightly faster than that in neutral environments.
PLA-chitosan-IM7 was stable at 3 days in any type of environment
(Fig. 3).
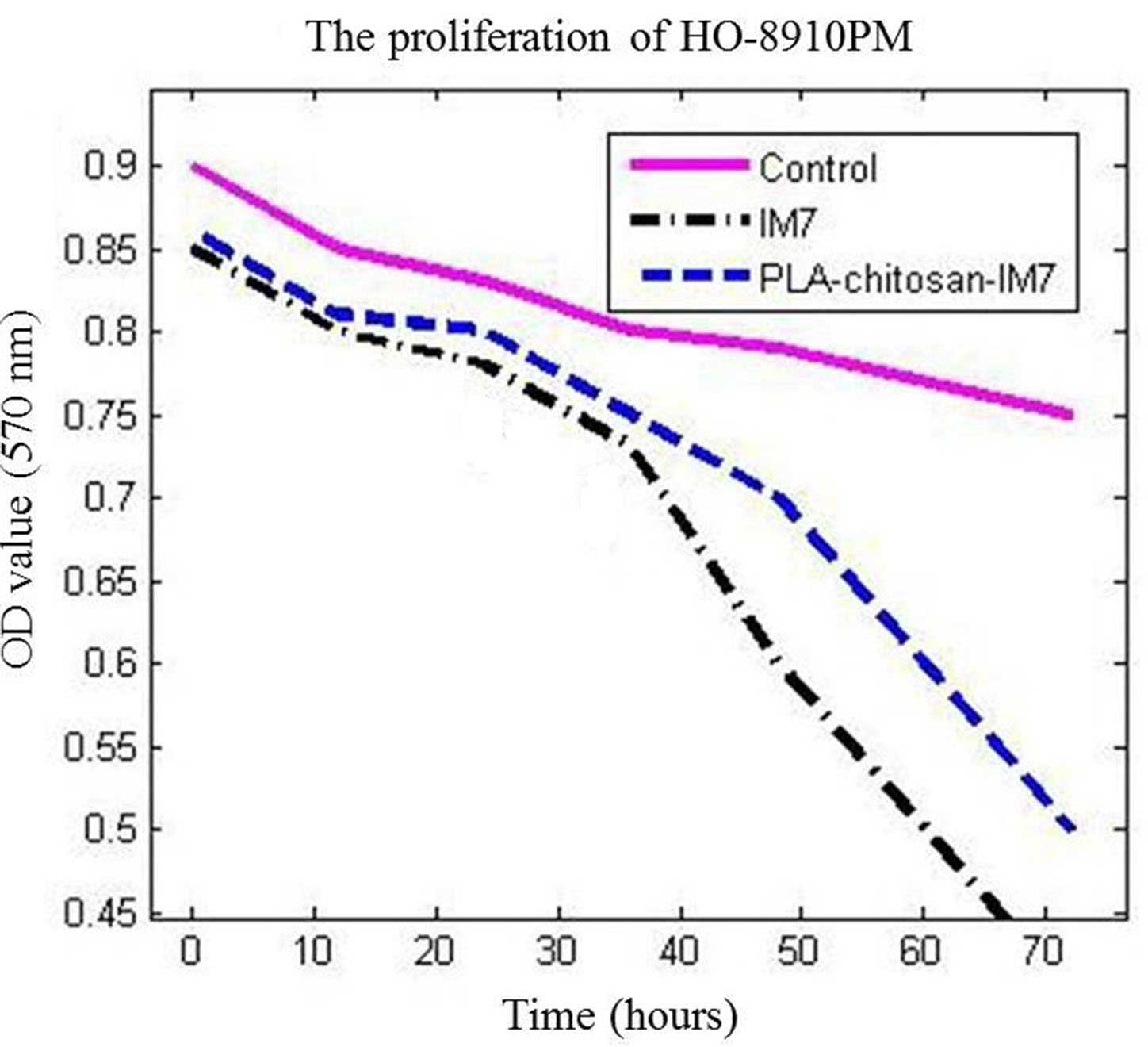
Suppressing effect of PLA-chitosan-IM7
on an ovarian cancer cell line
MTT assay was used to analyze the influence of
PLA-chitosan-IM7 nano-particles on the human ovarian cell line
HO-8910PM. The results indicated that PLA-chitosan-IM7 and IM7
could suppress the proliferation of cancer cells (P=0.0156 vs. the
control group). In addition, the survival time of the IM7 group was
markedly lower than that of the other two groups, suggesting that
application of IM7 alone had increased toxicity compared with
PLA-chitosan-IM7 (Fig. 4).
Animal studies
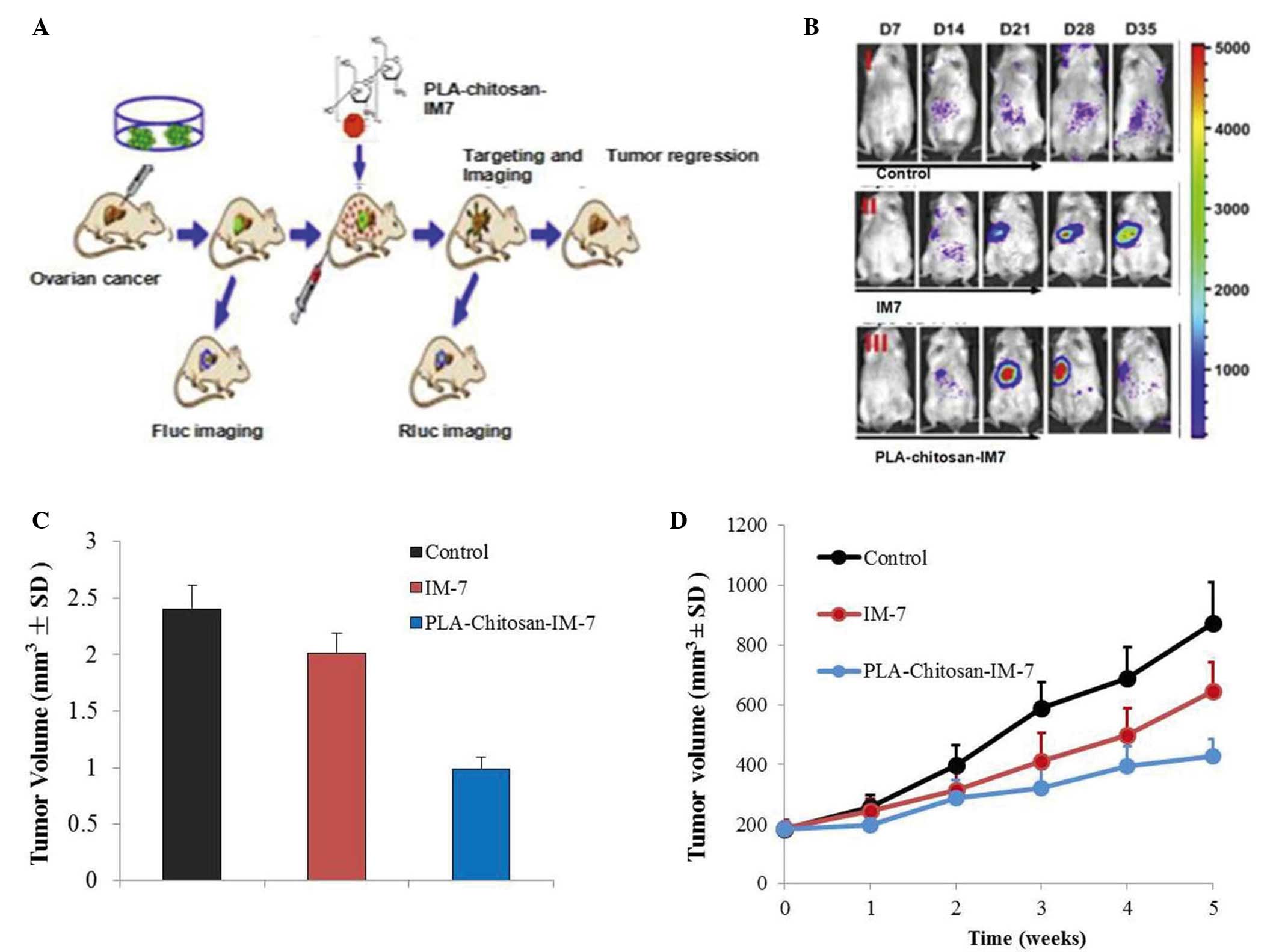
An in vivo imaging system and a subcutaneous
tumor model were employed to investigate the effect of
PLA-chitosan-IM7 nano-particles on mice with ovarian cancer.
Fluorescence animal models in vivo were successfully
established (Fig. 5A), and the
results indicated that PLA-chitosan-IM7 nano-particles could target
the tumor cells and suppress their proliferation (Fig. 5B). A total of 35 days subsequent to
PLA-chitosan-IM7 treatment, all animals were sacrificed, and the
tumor tissues were removed and weighted. PLA-chitosan-IM7 could
inhibit tumor growth effectively (Fig.
5C). Regarding the animals' behavior, the hair and mental state
of the mice in the PLA-chitosan-IM7 group were relatively better
than those of mice in the other groups. As for the subcutaneous
tumor model, similar results were obtained (Fig. 5D).
Discussion
HA and its receptor CD44 appear to be valid targets
in numerous studies that had been designed to treat human ovarian
cancers. However, their toxicity is their major issue, which limits
the widespread use of these approaches (8). Therefore, it is important and meaningful
to identify a new approach for cancer treatment. Nano-particles are
easily absorbed by tissues and cells, and have been widely studied
as a drug carrier in recent years. In addition, nano-particles have
provided a good platform for cancer gene therapy based on their
unique properties, including diverse surface chemistry, appropriate
size scale and organ-specific pharmacokinetics (19). Chitosan has been long studied as a
special drug delivery system, and one of the most important
characteristics is to be developed to ionic gelatinization with
polyanion (20,21). Cai et al developed a gold-PEI
nano-carrier with good transfection efficiency to deliver
anti-Epstein-Barr virus (RBV) microRNA (miR)-BART7-3p and elicit
its potential therapeutic effect. In vitro and in
vivo data revealed that cell proliferation and tumor growth
were effectively suppressed by anti-EBV-miR-BART7-3p transported by
nano-particles, and the expression of relevant genes was modulated
accordingly, indicating the feasibility of utilizing nano-particles
to deliver anti-miR and silence endogenous EBV-miR-BART7-3p
(22).
In the present study, chitosan coated with PLA was
used as the delivery system for IM7 in order to avoid its toxicity.
Firstly, IM7 was added into a TPP solution (pH 7–9), and the
mixture was then added into a chitosan solution (pH 4–6). The
nano-particles that carried the anti-CD44 antibody were prepared by
reaction between TPP and chitosan. Upon preparation, TEM was used
to detect the diameter and zeta potential of the particles, and it
was observed that the former was 100–500 nm (mean value, 350 nm)
(Fig. 2A) and the latter was between
−75 and +45 mV, which requires further research. In addition, the
stability of the anti-CD44 antibody IM7 carried with nano-particles
was evaluated in acidic and neutral environments. The release rate
was noticed to be slow and steady (Fig.
3). Secondly, the anti-tumor effect of PLA-chitosan-IM7 was
assessed in vitro and in vivo. The human ovarian cell
line HO-8910PM was used as the target cell, and it was observed
that PLA-chitosan-IM7 could suppress the proliferation of cancer
cells (Fig. 4). Then, an in
vivo imaging system was used to investigate the anti-tumor
effect of PLA-chitosan-IM7, and it was observed that
PLA-chitosan-IM7 have relative organ-specific and improved
anti-tumor growth effect (Fig. 5B and
C). Additionally, the hair and mental state of the mice in the
group treated with PLA-chitosan-IM7 were relatively better than
those of the mice in the other groups.
Large efforts have been undertaken to improve the
therapeutic efficacy of bioactive molecules, including cancer
therapeutics. Nano-preparations and targeting the drug-loaded
nano-carriers to the disease site to generate so-called ‘magic
bullets’ are examples of the remarkable efforts that have been
undertaken to design and develop strategies to improve the cellular
uptake of therapeutic molecules and their delivery to an organelle
of interest (23). The present study
demonstrated that PLA-chitosan-IM7 displays a good prospect in
clinical application. However, future studies are required on its
specificity, degradation and immune tolerance. The current study
provided a new aspect of enhancing the effectiveness of cancer
treatment while reducing its toxicity, which is of great use in
tumor therapy, particularly in tumor biotherapy.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bourguignon LY, Zhu H, Zhou B, Diedrich F,
Singleton PA and Hung MC: Hyaluronan promotes Cd44V3-Vav2
interaction with Grb2-p185 (HER2) and induces Racl and Ras
signaling during ovarian tumor cell migration and growth. J Biol
Chem. 276:48679–48692. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carpenter PM and Dao AV: The role of
hyaluronan in mesothelium-induced motility of ovarian carcinoma
cells. Anticancer Res. 23:3985–3990. 2003.PubMed/NCBI
|
|
4
|
Cannistra SA, Abu-Jawdeh G, Niloff J,
Strobel T, Swanson L, Andersen J and Ottensmeier C: CD44 variant
expression is a common feature of epithelial ovarian cancer: Lack
of association with standard prognostic factors. J Clin Oncol.
13:1912–1921. 1995.PubMed/NCBI
|
|
5
|
Ourguignon LY, Zhu H, Zhou B, Diedrich F,
Singleton PA and Hung MC: Hyaluronan promotes Cd44V3-Vav2
interaction with Grb2-p185(HER2) and induces Racl and Ras signaling
during ovarian tumor cell migration and growth. J Biol Chem.
276:48679–48692. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bourguignon LY, Zhu H, Chu A, Iida N,
Zhang L and Huang MC: Interaction between the adhesion receptor,
CD44, and the oncogene product, p185HER2, promotes human ovarian
tumor cell activation. J Biol Chem. 272:27913–27918. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ourguignon LY, Zhu H, Shao L and Chen YW:
CD44 interaction with c-Src kinase promotes cortactin-mediated
cytoskeleton function and hyaluronic acid-dependdent ovarian tumor
cell migration. J Biol Chem. 276:7327–7336. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bourguignon LY, Peyrollier K, Gilad E and
Brightman A: Hyaluronan-CD44 interaction with neural
Wiskott-Aldrich syndrome protein (N-WASP) promotes actin
polymerization and ErbB2 activation leading to beta-catenin nuclear
translocation, transcriptional up-regulation, and cell migration in
ovarian tumor cells. J Biol Chem. 282:1265–1280. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bourugnon LY, Gilad E and Peyrollier K:
Heregulin-mediated ErbB2-ERK signaling activates hyaluronan
synthases leading to CD44-dependent ovarian tumor cell growth and
migration. J Biol Chem. 282:19426–19441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu D and Bourguignon LY: Interaction
between CD44 and the repeat domain of ankyrin promotes hyaluronic
acid-mediated ovarian tumor cell migration. J Cell physiol.
183:182–195. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tzuman YC, Sapoznik S, Granot D, Nevo N
and Neeman M: Peritoneal adhesion and angiogenesis in ovarian
carcinoma are inversely regulated by hyaluronan: The role of
gonadoptropins. Neoplasia. 12:51–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ween MP, Hummitzsch K, Roggers RJ, Oehler
MK and Ricciardelli C: Versican induces a pro-metastatic ovarian
cancer cell behavior which can be inhibited by small hyaluronan
oligosaccharides. Clin Exp Metastasis. 28:113–125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee H, Lee K and Park TG: Hyaluronic
acid-paclitaxel conjugate micelles: Syhthesis, characterization,
and antitumor activity. Bioconjug Chem. 19:1319–1325. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cohen MS, Cai S, Xie Y and Forrest ML: A
novel intralymphatic nanocarrier delivery system for cisplatin
therapy in breast cancer with improved tumor efficacy and lower
systemic toxicity in vivo. Am J Surg. 198:781–786. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Banzato A, Bibisse S, Rondina M, Renier D,
Bettella F, Esposito G, Quintieri L, Meléndez-Alafort L, Mazzi U,
Zanovello P and Rosato A: A paclitaxel-hyaluronan bioconjugate
targeting ovarian cancer affords a potent in vivo therapeutic
activity. Clin Cancer Res. 14:3598–3606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thompson CB, Shepard HM, O'Connor PM,
Kadhim S, Jiang P, Osgood RJ, Bookbinder LH, Li X, Sugarman BJ,
Connor RJ, et al: Enzymatic depletion of tumor hyaluronan induces
antitumor responses in preclinical animal models. Mol Cancer Ther.
9:3052–3064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guedan S, Rojas JJ, Gros A, Mercade E,
Cascallo M and Alemany R: Hyaluronidase expression by an oncolytic
adenovrirus enhances its intratumoral spread and suppresses tumor
growth. Mol Ther. 18:1275–1283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SJ, Min HS, Ku SH, Son S, Kwon IC, Kim
SH and Kim K: Tumor-targeting glycol chitosan nanoparticles as a
platform delivery carrier in cancer diagnosis and therapy.
Nanomedicine Lond). 9:1697–1713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y, Zhu X, Zhang X, Liu B and Huang L:
Nanoparticles modified with tumor-targeting scFv deliver siRNA and
miRNA for cancer therapy. Mol Ther. 18:1650–1656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun X, Zeng L, Chen C, Huang Y, Han F,
Xiao W, Liu S and Lu T: Comparing treatment outcomes of different
chemotherapy sequences during intensity modulated radiotherapy for
advanced N-stage nasopharyngeal carcinoma patients. Radiat Oncol.
8:2652013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Elbakry A, Zaky A, Liebl R, Rachel R,
Goepferich A and Breunig M: Layer-by-layer assembled gold
nanoparticles for siRNA delivery. Nano Lett. 9:2059–2064. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai L, Li J, Zhang X, Lu Y, Wang J, Lyu X,
Chen Y, Liu J, Cai H, Wang Y and Li X: Gold nano-particles (AuNPs)
carrying anti-EBV-miR-BART7-3p inhibit growth of EBV-positive
nasopharyngeal carcinoma. Oncotarget. 6:7838–7850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Biswas S and Torchilin VP:
Nanopreparations for organelle-specific delivery in cancer. Adv
Drug Deliv Rev. 66:26–41. 2014. View Article : Google Scholar : PubMed/NCBI
|